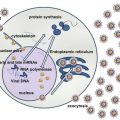
Fig. 1
(a) Top: cytology on a fine-needle aspiration. Note the 2 mitotic figures. Botton: H&E stain of a lymph node biopsy showing BL. (b) Top: CD20, middle Ki67 immunohistochemistry. Bottom EBER in situ hybridization
4 Pathophysiology of BL
4.1 BL and MYC Rearrangements
BL is derived from post-germinal B-cells. Translocations involving the MYC oncogene are the cytogenetical hallmarks of BL and are found in over 90 % of the cases [4]. In about 80 % of BL, the t(8;14)(q24;q32) involve MYC on chromosome 8 and the immunoglobulin heavy-chain locus (IgH) on chromosome 14. Less frequent translocations include t(2;8)(p12;q24) and t(8;22)(q24;q11) involving MYC and the kappa and lambda immunoglobulin light-chain loci (IgL), respectively. As a result, MYC is transcriptionally activated in B-cells leading to cell proliferation. The translocations are thought to be facilitated by AID, an enzyme responsible for somatic hypermutations and class-switch recombination in B-cells during the germinal center reaction [5]. Mechanisms associated with defective apoptosis in BL include frequent abrogation of p53 function by mutations of the TP53 gene [6] and inhibition of BIM [7].
4.2 BL and EBV
EBV was isolated from endemic BL tissue in 1964 [8] and was later epidemiologically associated with the development of BL [9]. The distribution of EBV is worldwide and over 90 % of the adult population is infected. Acquisition of EBV occurs around 2 years of age in sub-Saharan Africa and during adolescence and young adulthood in the northern hemisphere [10]. EBV-infected individuals remain lifelong carriers of the virus. EBV infects epithelial cells (usually of the oropharynx) where it establishes a lytic infection and B-cells where it establishes a persisting latent infection [11]. B-cells infected in vitro with EBV are transformed and proliferate indefinitely as lymphoblastoid cell lines (LCL). During latency, the viral genome is maintained as an episome in the nuclei of B-cells and only a handful of viral genes are expressed. These can interfere with normal cellular pathways. Latent membrane proteins (LMP) 1 and 2 are expressed at the cellular membrane of B-cells infected with EBV and drive their proliferation and differentiation by mimicking the signals that normal B-cells receive during the germinal center reaction (LMP1 acts as a constitutively active CD40 signal [12] and LMP2 as a constitutively active B-cell receptor signal [13]). Other intracellular latent genes include EBNA1, EBNA2, EBNA3A-C, EBNA-LP, and virally encoded small RNA transcripts (EBER). The expression pattern of the EBV latent genes is tightly regulated. After naive B-cells become infected with EBV, LMP1 and LMP2 drive B-cell proliferation and their differentiation [14]. Infected B-cells expressing the full repertoire of EBV latent genes are highly immunogenic and give rise to a strong cytotoxic T-cell response that eventually opposes B-cell proliferation. Simultaneously, the EBV latent program switches to a restricted pattern, ultimately leading to infected B-cells acquiring a memory phenotype and harboring EBV with a very restricted pattern of gene expression. These cells are the reservoir of EBV infection and persist during the lifetime of the infected individual. Occasionally, EBV-infected memory B-cells are driven to differentiate into plasma cells, triggering the lytic program [15]. There are three distinct types of EBV latency programs occurring in EBV-associated lymphomas [16]. In immunodeficiency-associated lymphomas (posttransplant lymphoproliferative disorders and HIV-associated lymphomas in severely immunocompromised patients), type III latency is observed and closely resembles that observed in vitro in LCL, where most of the latent genes are expressed and drive the B-cell proliferation. In EBV-associated Hodgkin’s lymphoma, a more restricted type II latency is observed, where LMP1 and LMP2 are expressed and contribute to the survival of malignant cells. EBV is universally associated with eBL, and a restricted-type I latency is observed, similar to that observed in EBV-infected memory B-cells in healthy carriers [17]. A minority of sBL and iBL are also associated with EBV infection and a type I latency. During type I latency, only EBNA1 is expressed. Although eBL has been linked to EBV infection for over 40 years, the precise oncogenic role of the virus has remained elusive. EBNA1 is not directly oncogenic but may exert an antiapoptotic role that contributes to the malignant phenotype [16]. Nevertheless, the universal presence of EBV in eBL cells, as well as experimental evidence, points to a pathogenic role of EBV in the development of eBL. EBV may cooperate with MYC in BL and may provide at least part of the antiapoptotic machinery required for cells that overexpress deregulated MYC.
4.3 eBL and Malaria
There is strong epidemiological evidence associating malaria with eBL [18]. Extensive infection with Plasmodium sp. may serve as a cofactor to EBV in the development of BL in equatorial regions. Chronic infection with Plasmodium sp. leads to polyclonal B-cell activation, favoring the emergence of the MYC rearrangements characteristic of BL. Moreover, Plasmodium sp. contributes to immunodepression and plasmodial factors can lead to reactivation of the EBV lytic cycle [19].
4.4 eBL and HIV Infection
HIV infection also leads to chronic polyclonal B-cell activation and may contribute to BL development [20], as is the case for malaria.
5 Diagnosis and Initial Workup
Evaluation of BL in high-income countries relies on histological examination of surgical biopsies of involved tissue and staging using bone marrow and cerebrospinal fluid (CSF) examination and thoracic and abdominal computed tomography. Complete biological workup with complete blood count and blood chemistry with creatinine, electrolytes, uric acid, and lactate dehydrogenase (LDH) is mandatory given the high proliferative rate and the high risk of spontaneous and therapy-induced tumor lysis syndrome.
6 Treatment
BL was one of the first lymphomas to be cured solely by chemotherapy. Despite its very aggressive presentation, BL is a highly curable form of NHL. Improved outcome has been achieved over the last two decades with the use of intensive chemotherapy following the protocols developed by the LMB and the BFM cooperative groups. These protocols rely on an initial cytoreduction, followed by a combination of intensive chemotherapy, and include high-dose methotrexate and intrathecal chemotherapy to address the frequent CNS involvement [21]. Chemotherapy induces profound neutropenia, anemia, and thrombocytopenia that require antibacterial therapy for frequent neutropenic fever and transfusions of packed red blood cells and platelets. Several courses of chemotherapy are required and the entire treatment period lasts approximately 4 to 6 months with frequent hospitalizations. Overall survival and cure extends from 70 % to over 90 % depending on age and initial stage.
7 Challenges in Low-Income and Developing Countries
Low income of parents of affected children, poor or absent health insurance policies, sparse hospital infrastructure, and lack of appropriate health networks constitute tremendous challenges to the care of eBL in low-income countries [22–24]. Recommended diagnostic procedures, supportive care, and intensive chemotherapy required to guarantee the curability of this otherwise highly treatable lymphoma are unrealistic in equatorial Africa. Intense efforts have been made by medical researchers in affected countries and have led to the development of strategies that can be adapted to the various situations. The cost-effectiveness of treating pediatric cancer, particularly BL in low-income countries, has been evaluated and found to be favorable [25]. Current overall survival with such approaches is in the range of 50 % in children [26]. Therapy for adults is more complicated and has not been extensively studied in these regions.
Diagnostic pathology and hematopathology is not readily available in equatorial Africa (0.1–1.3 pathologists per million population versus 62 in the USA) [27]. Diagnosis of BL on surgically removed biopsies is generally not feasible. Cytopathological evaluation of fine-needle aspirates (FNA) is almost universally available and has a good diagnostic value [28]. Even if eBL is by far the most frequent NHL in children in these regions and its clinical presentation is typical, a FNA is recommended for the diagnosis. In recent protocols undertaken in equatorial Africa, FNA was used for diagnosis of eBL in over 75 % of the cases [26]. In some cases, patients are treated solely on the basis of a clinical diagnosis highly suggestive of eBL. Staging includes CSF evaluation imaging procedures that are usually limited to chest X-ray and abdominal ultrasonography. Complete staging should be performed when feasible, to determine the St Jude stage as some protocols use risk-adapted chemotherapy. As expected, most studies have found poorer outcomes for more advanced disease.
Chemotherapy strategies for children with eBL in low-income countries have evolved over the years (Table 1). Initial attempts based on protocols used in high-income countries were developed around the use of cyclophosphamide, vincristine, and intravenous methotrexate, together with steroids and intrathecal methotrexate. Such protocols have excessive toxicities in these settings and are difficult to implement, as they require prolonged hospitalization, are often complicated by infection, and need transfusions which are generally not available. Furthermore, such approaches are costly, in the region of 500 USD per patient, several orders of magnitude above the estimated per capita health care expenditures in these countries [29, 30]. Lately, even simpler protocols have been tested. Hesseling et al. developed a short-course (28-day) schedule of cyclophosphamide monotherapy, associated with intrathecal methotrexate. This approach has been evaluated in Malawi [26] and in several other countries with minor modifications (treatment adaptation according to disease risk) [31]. Complete responses are over 80 %. Relapses or primary refractory diseases occur in around in approximately 30 % of the patients, yielding a 1-year EFS of approximately 50 %. As fewer than 5 % of the patients’ relapse after 1 year, the cure rate is approximately 50 %. The cost of this approach is less than 50 USD per patient.
Table 1
Chemotherapy strategies for children with eBL in low-income countries
Protocol | Phase | Drugs
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|
|---|