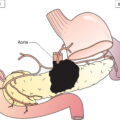
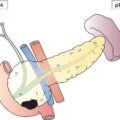
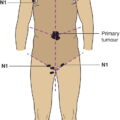
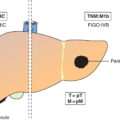
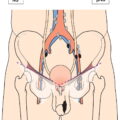
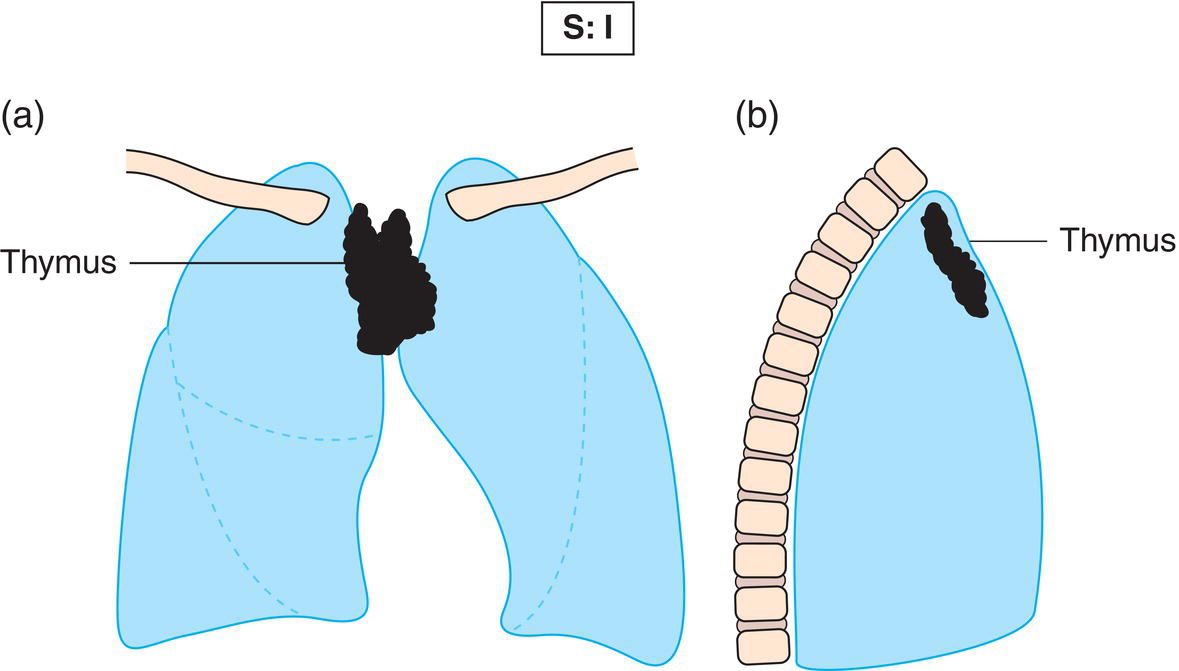
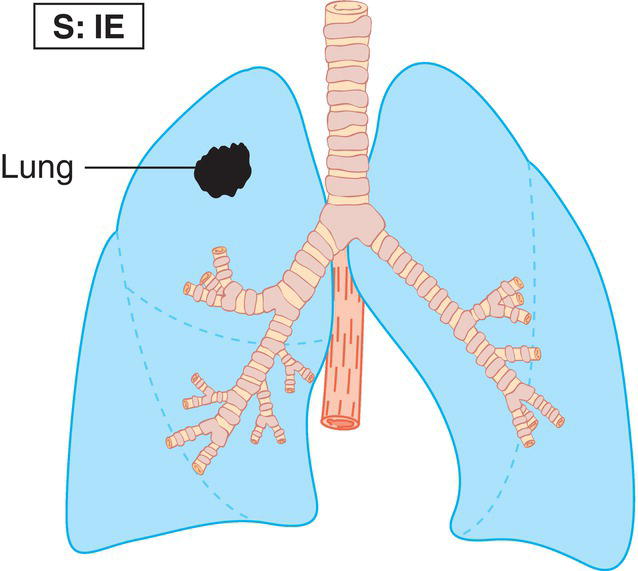
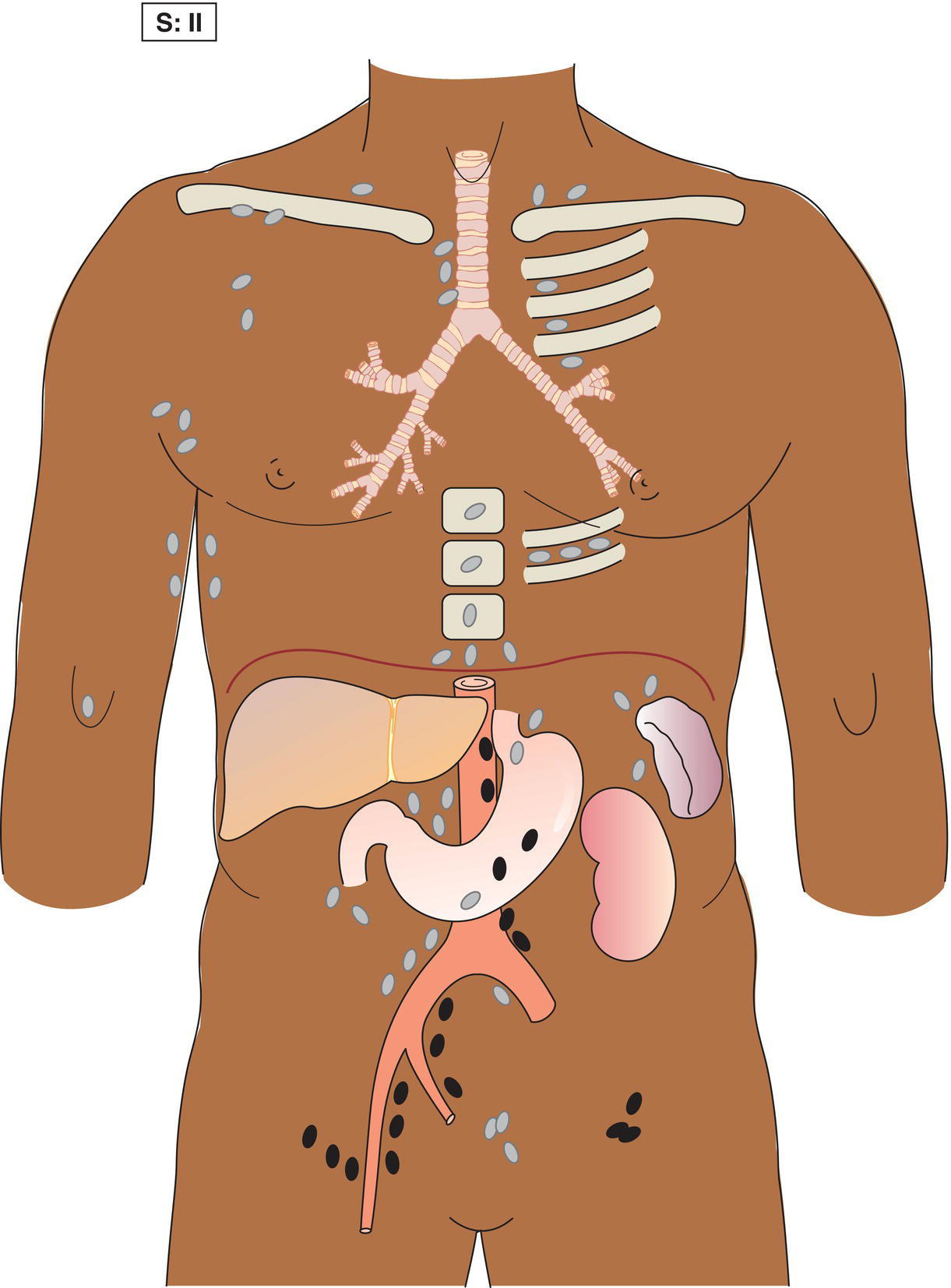
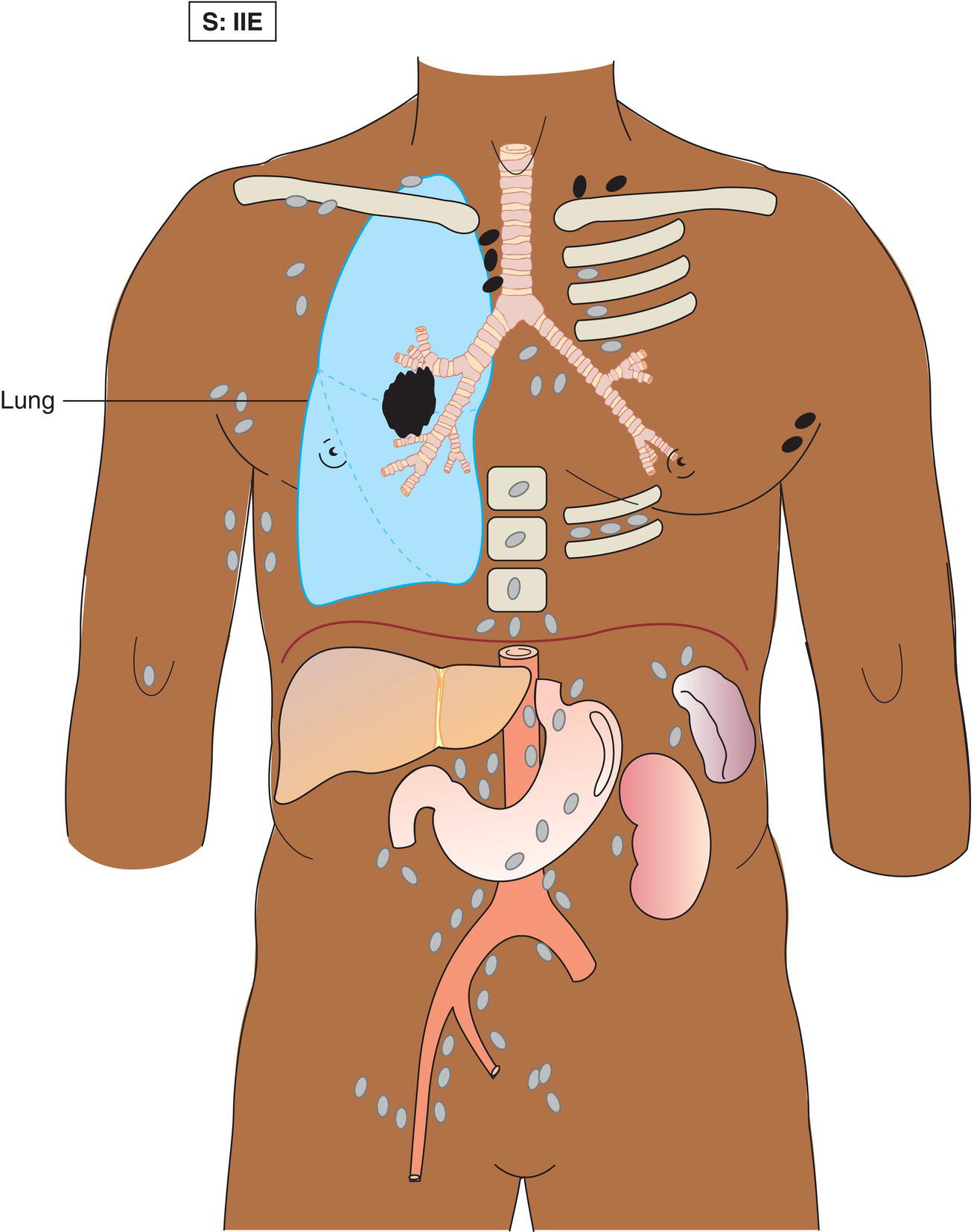
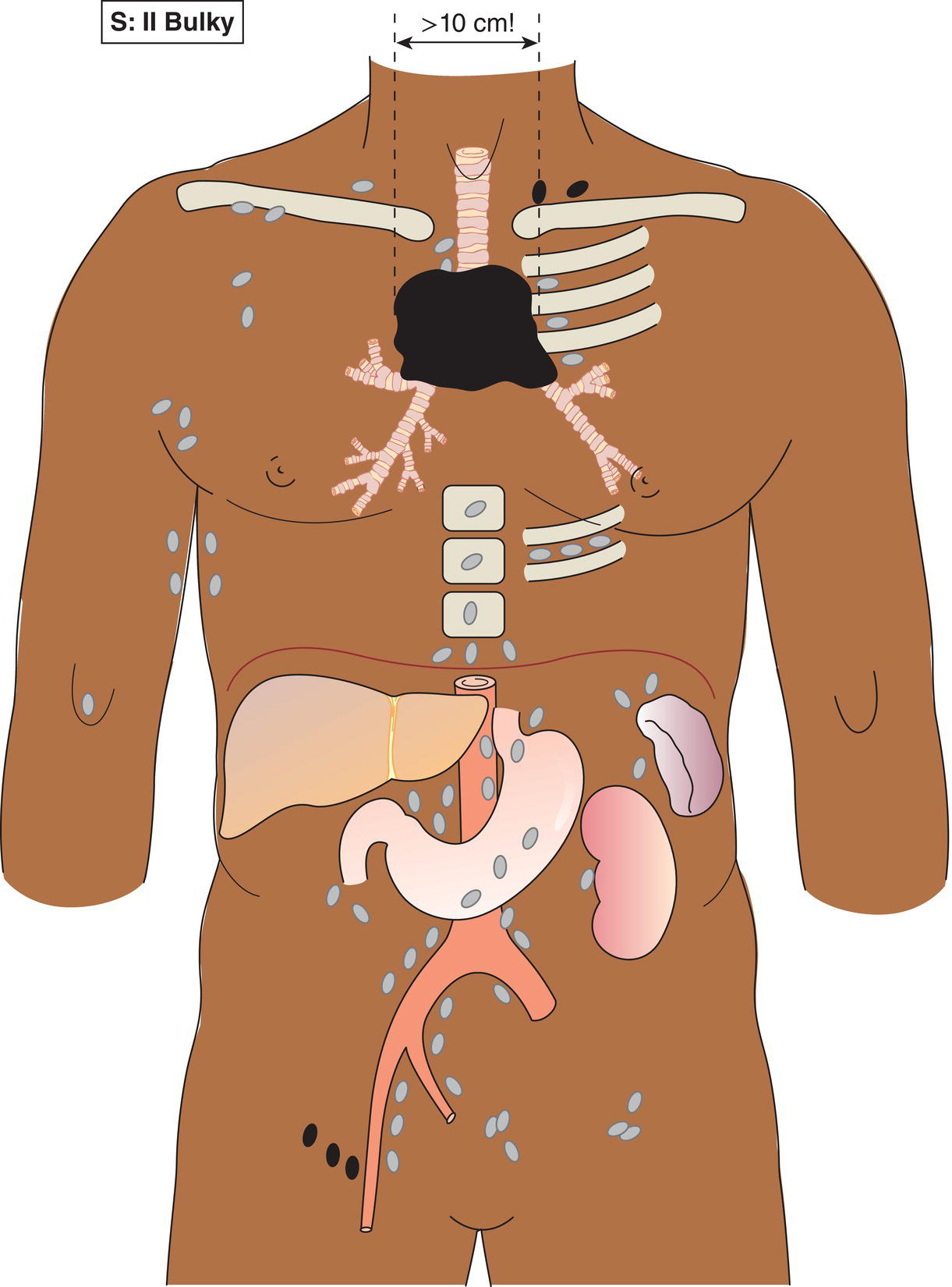
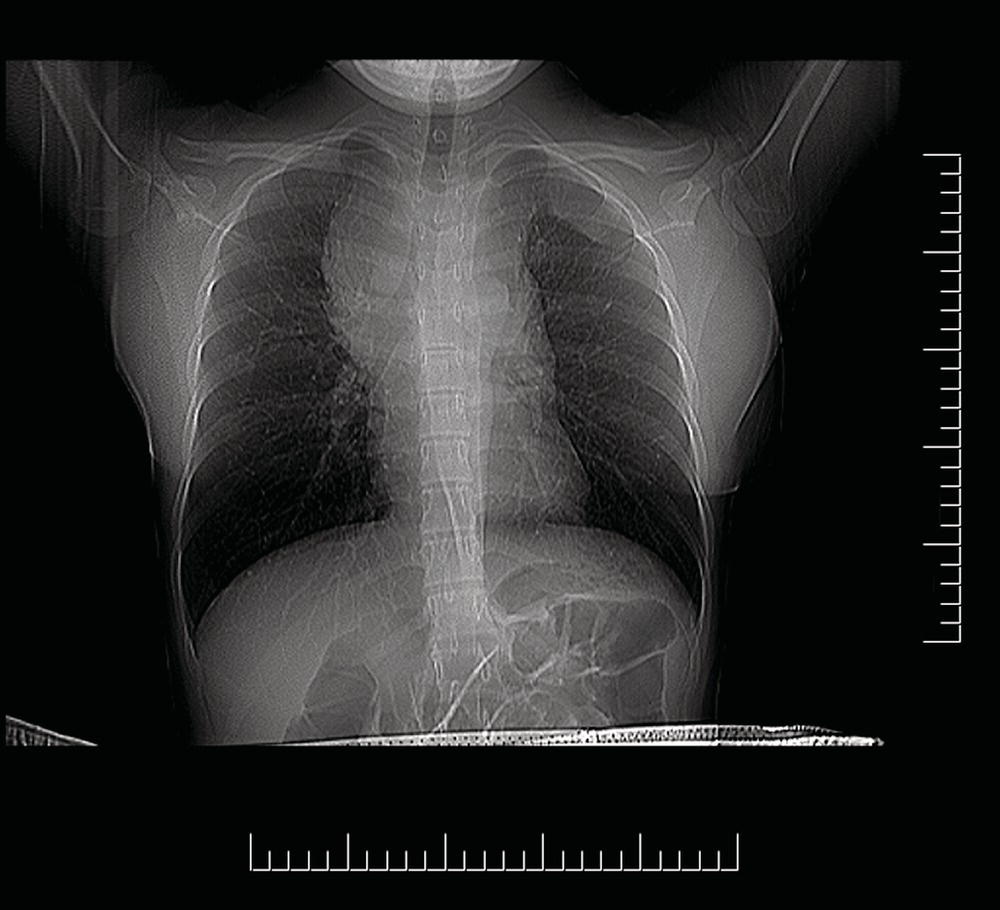
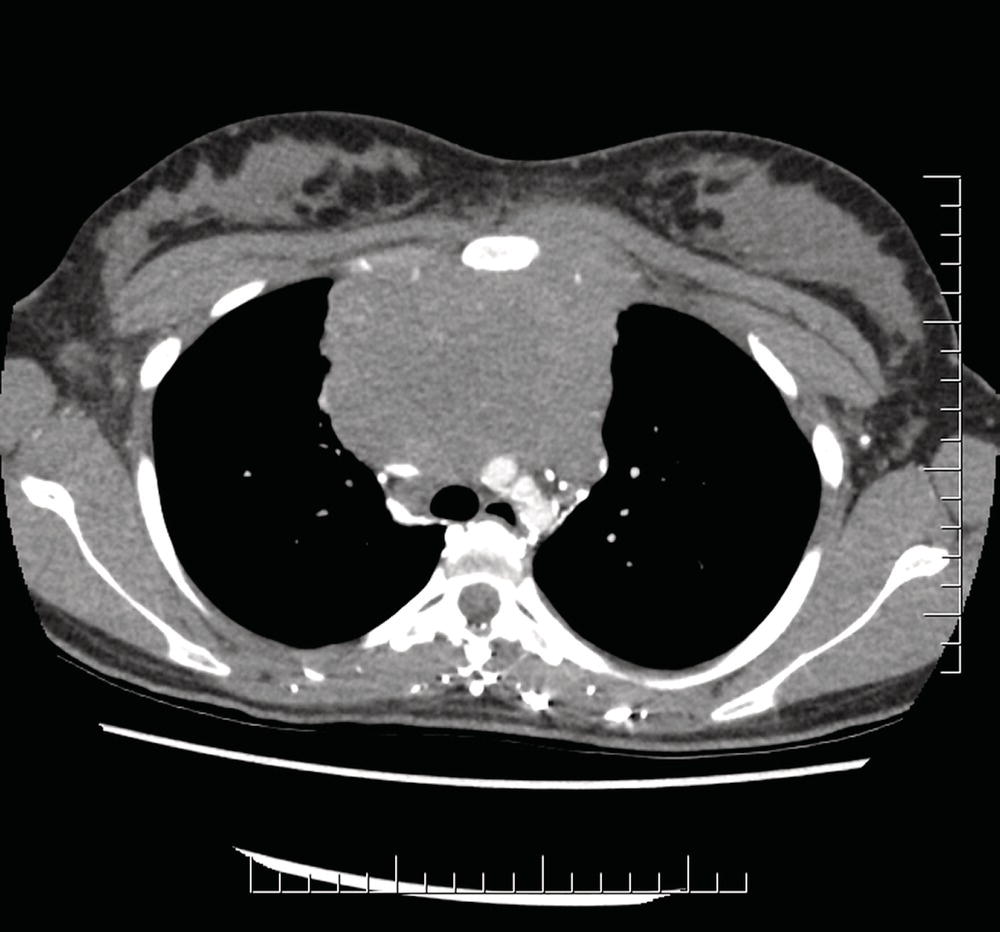
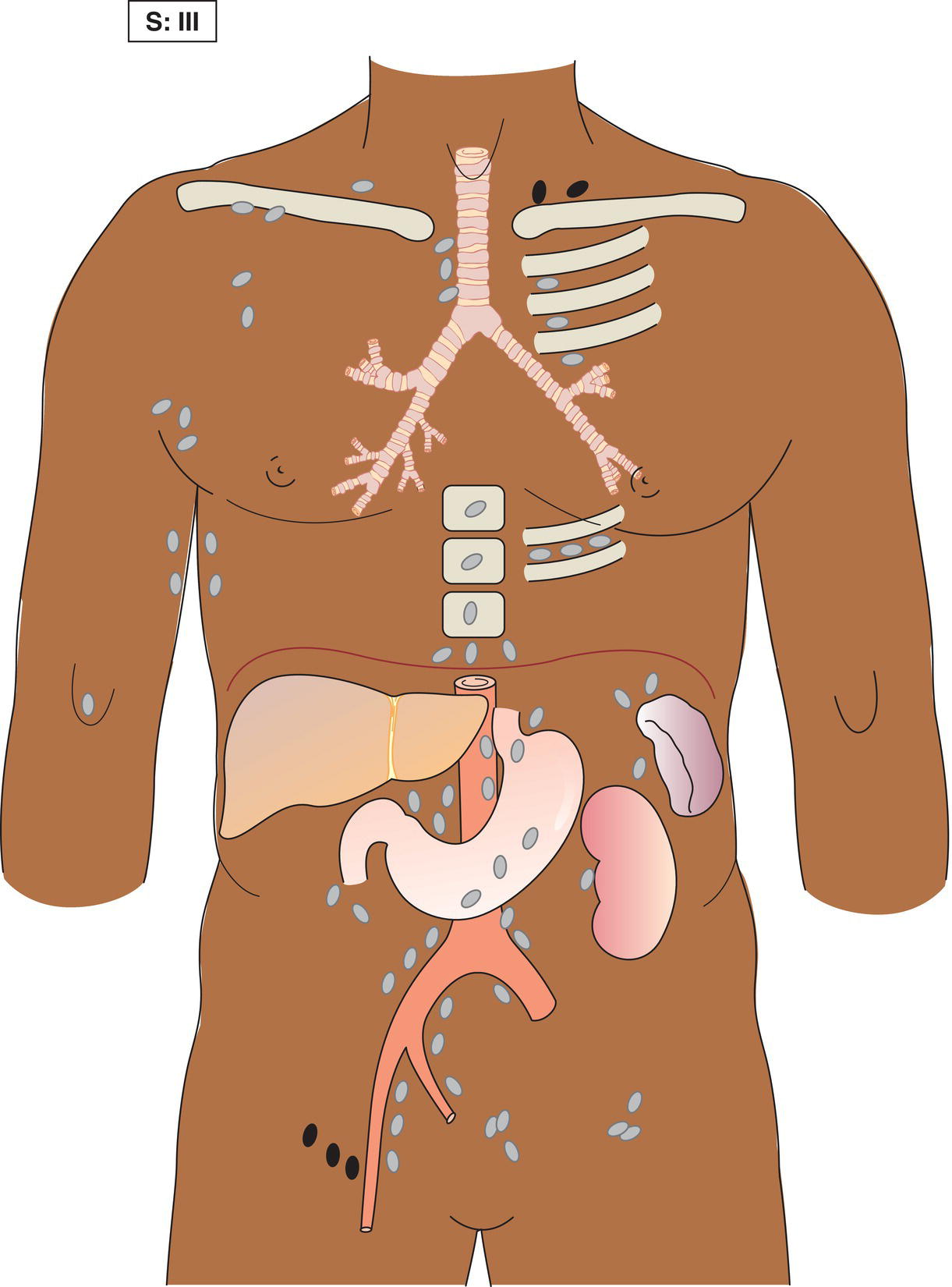
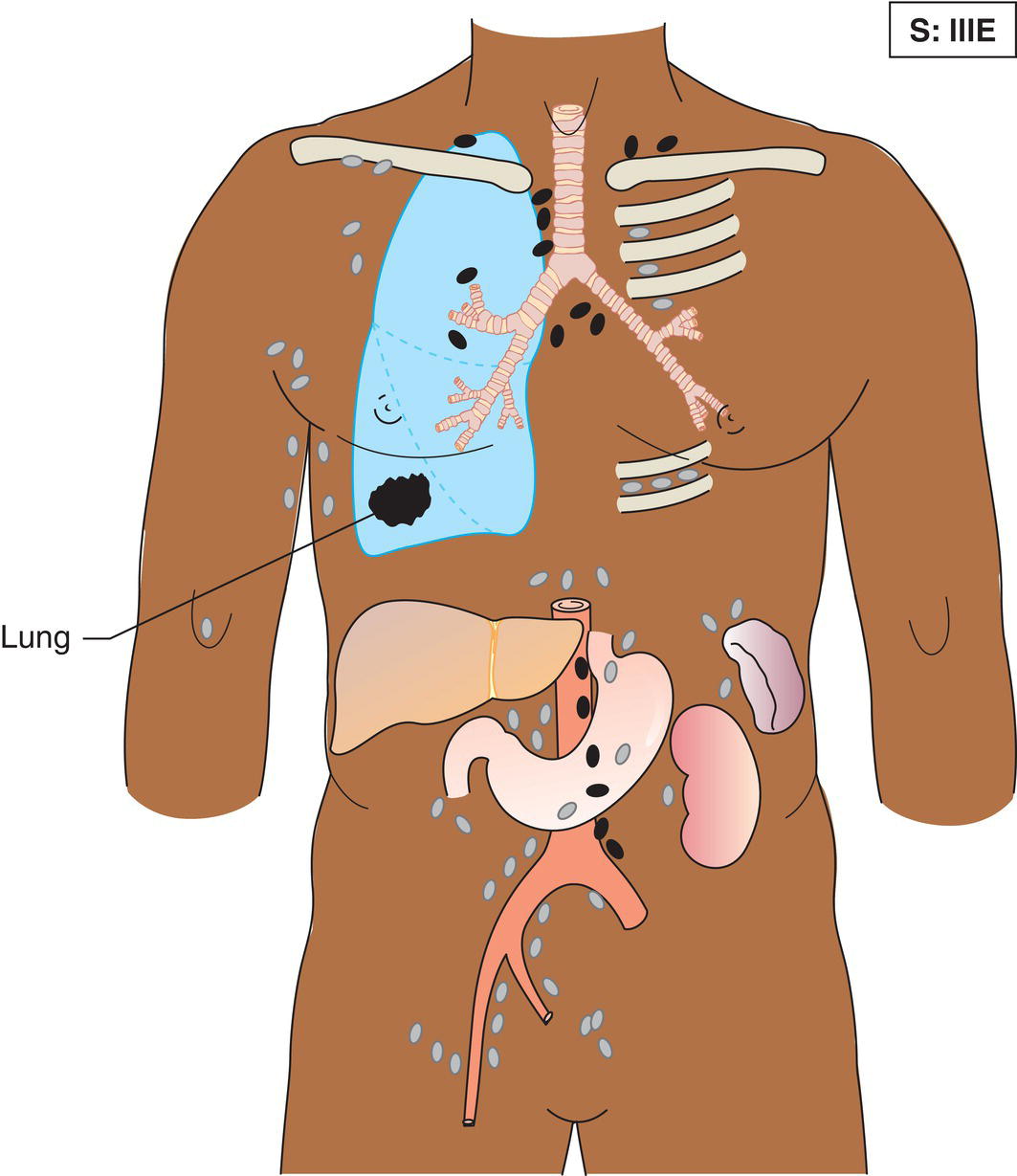
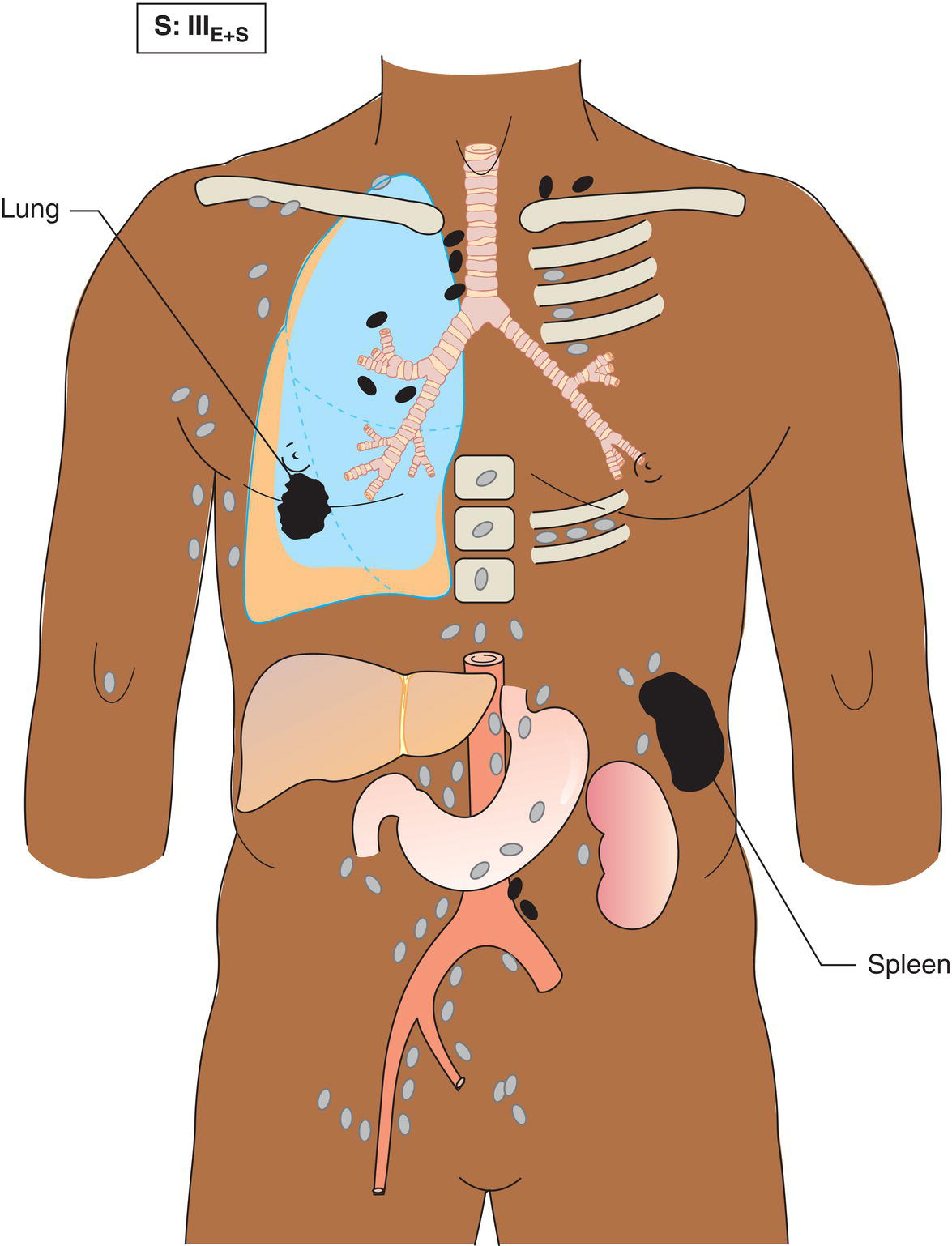
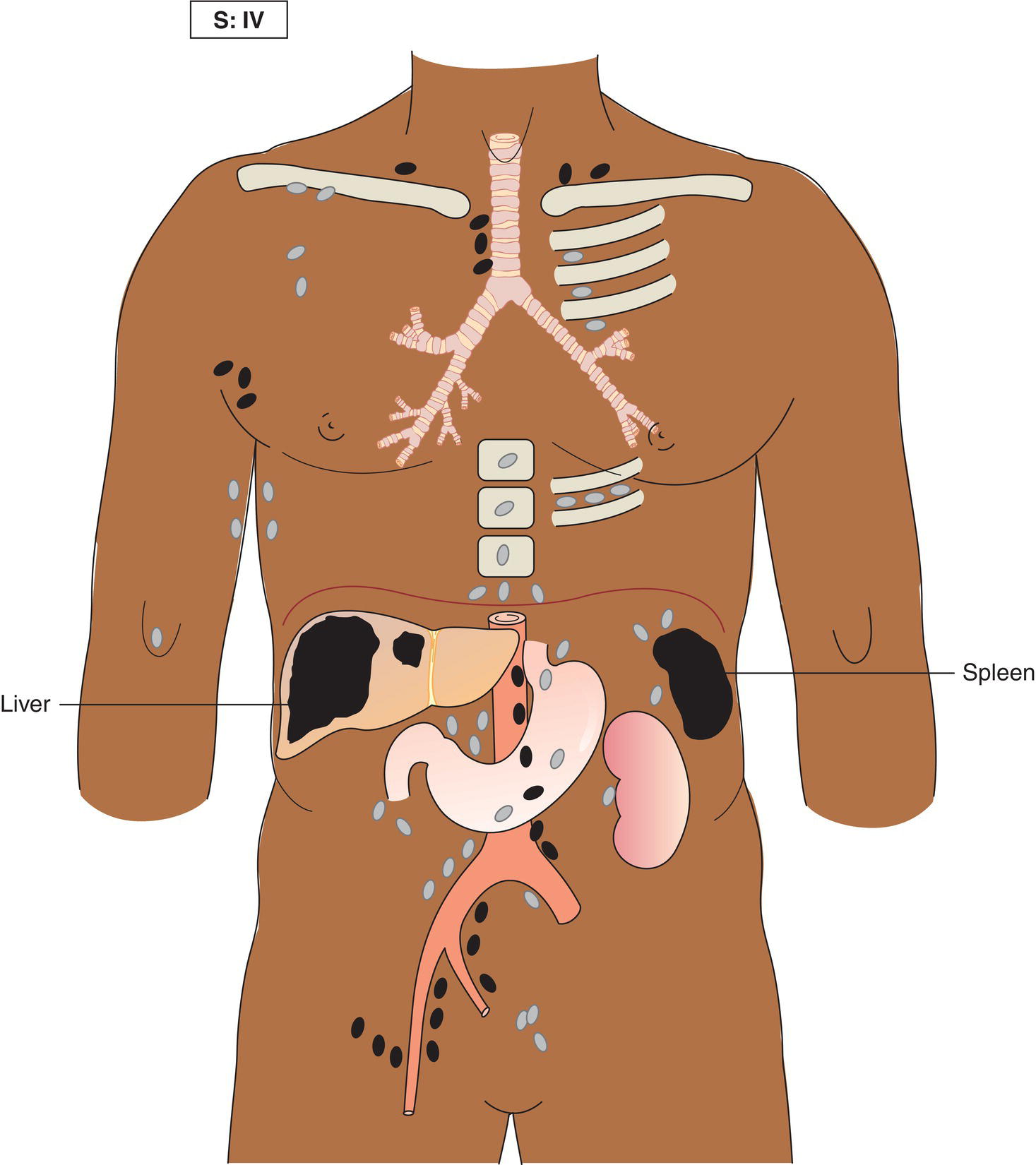
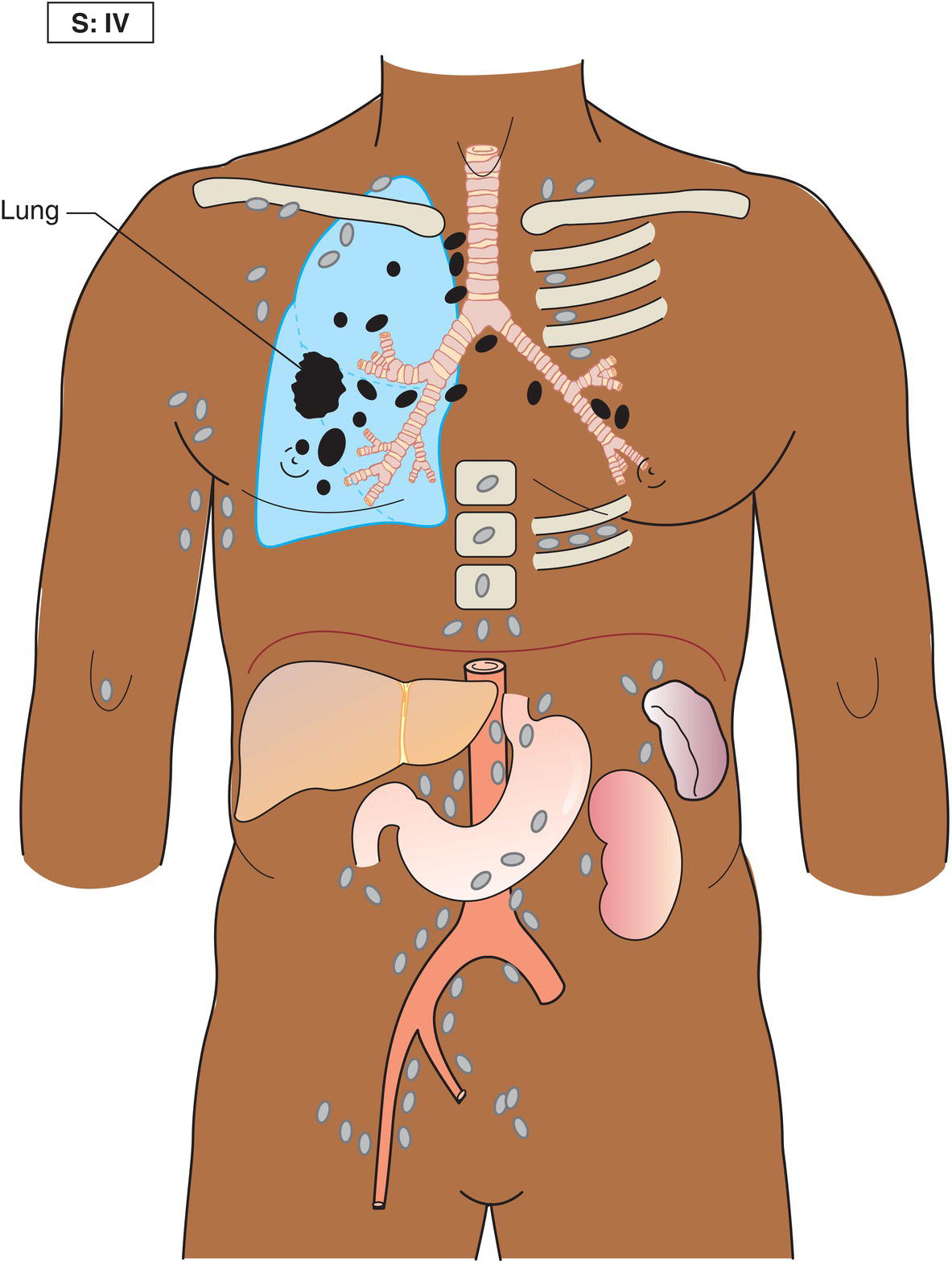
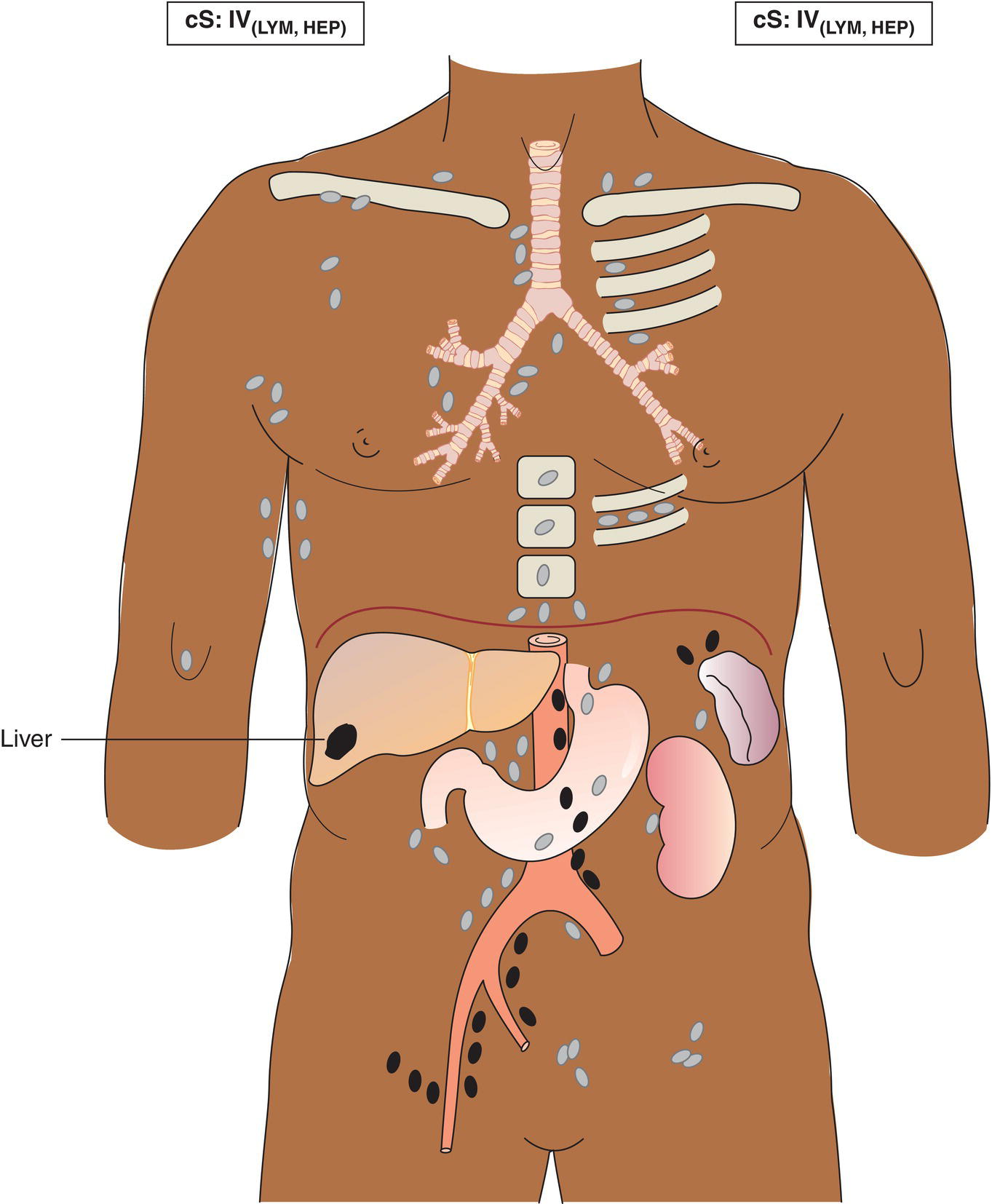
The current staging classification for Hodgkin Lymphoma is a modification of the Ann Arbor classification first adopted in 1971. Over the past 50 years the practice has changed, making the previously used staging laparotomy and the resulting pathological staging classification obsolete. The consensus conference that took place in 2012 in Lugano suggested an even more simplified system, putting together Stages I and II as Limited Stage and Stages III and IV as Advanced Stage lymphoma. The Lugano Classification, a modification of the Ann Arbor classification, has been published and accepted by the UICC.1 This is determined by history, clinical examination, imaging, blood analysis and the initial biopsy report. Bone marrow biopsy must be taken from a clinically or radiologically non‐involved area of bone. Clinical evidence of liver involvement must include either enlargement of the liver and at least an abnormal serum alkaline phosphatase level and two different liver function test abnormalities, or an abnormal liver demonstrated by imaging and one abnormal liver function test. Clinical evidence of spleen involvement is accepted if there is palpable enlargement of the spleen confirmed by imaging. The lymphatic structures are as follows: The lymph nodes are grouped into regions and one or more (2, 3, etc.) may be involved. The spleen is designated S and extralymphatic organs or sites E. Lung involvement limited to one lobe, or perihilar extension associated with ipsilateral lymphadenopathy, or unilateral pleural effusion with or without lung involvement but with hilar lymphadenopathy is considered as localized extralymphatic disease. Liver involvement is always considered as diffuse extralymphatic disease. Involvement of a single lymph node region (I) (Figs. 550, 551, 552, 553) or localized involvement of a single extralymphatic organ or site (IE) (Fig. 554). Involvement of two or more lymph node regions on the same side of the diaphragm (II) (Fig. 555), or localized involvement of a single extralymphatic organ or site and its regional lymph node(s) with or without involvement of other lymph node regions on the same side of the diaphragm (IIE) (Fig. 556). Stage II disease with a single nodal mass greater than 10 cm in maximum dimension or greater than a third of the thoracic diameter as assessed on CT (Figs. 557, 558, 559). Involvement of lymph node regions on both sides of the diaphragm (III) (Fig. 560), which may also be accompanied by localized involvement of an associated extralymphatic organ or site (IIIE) (Fig. 561), or by involvement of the spleen (IlIS), or both (IIIE + S) (Fig. 562). Disseminated (multifocal) involvement of one or more extralymphatic organs, with or without associated lymph node involvement (Figs. 563, 564); or non‐contiguous extralymphatic organ involvement with involvement of lymph node regions on the same or both sides of the diaphragm (Fig. 565). Note The site of Stage IV disease is identified further by specifying sites according to the notations listed above. Each stage should be divided into A and B according to the absence or presence of defined general symptoms. These are: Note Pruritus alone does not qualify for B classification, nor does a short, febrile illness associated with a known infection.
HODGKIN LYMPHOMA
Introductory Notes
Clinical Staging (cS)
Liver Involvement
Spleen Involvement
Lymphatic and Extralymphatic Disease
Lung Involvement
Liver Involvement
Clinical Stages (cS)
Limited Stage
Stage I
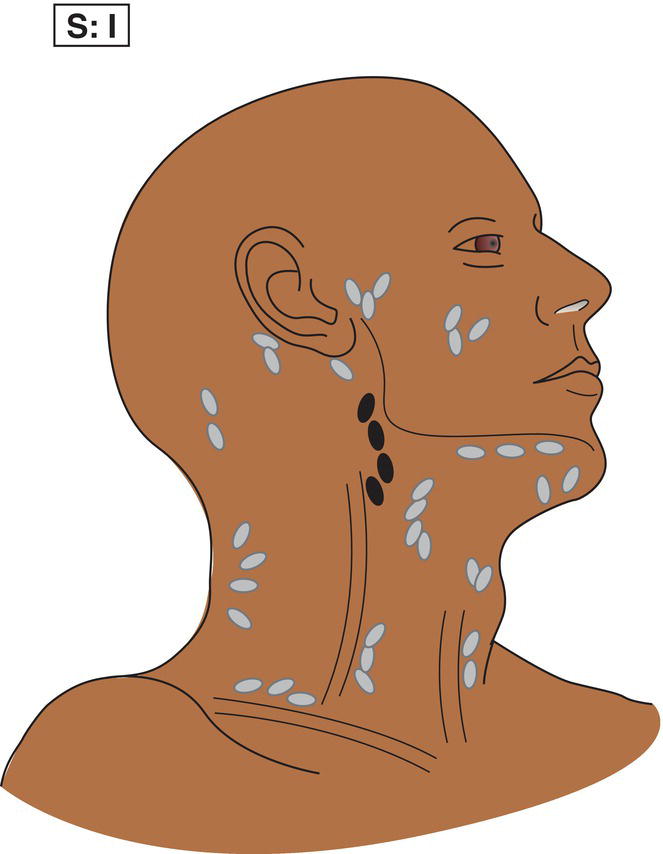
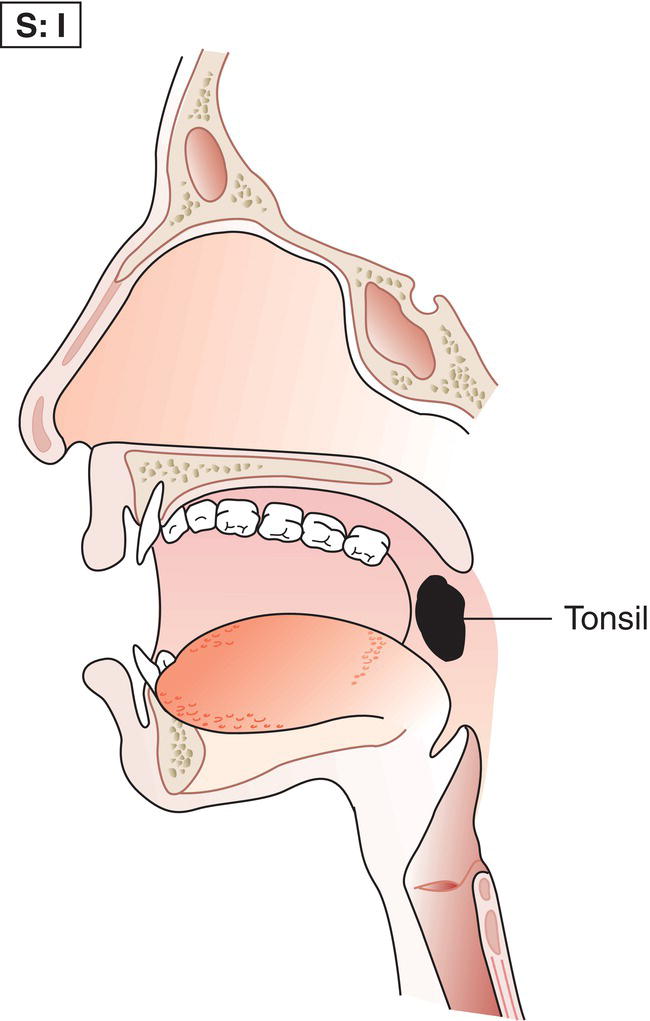
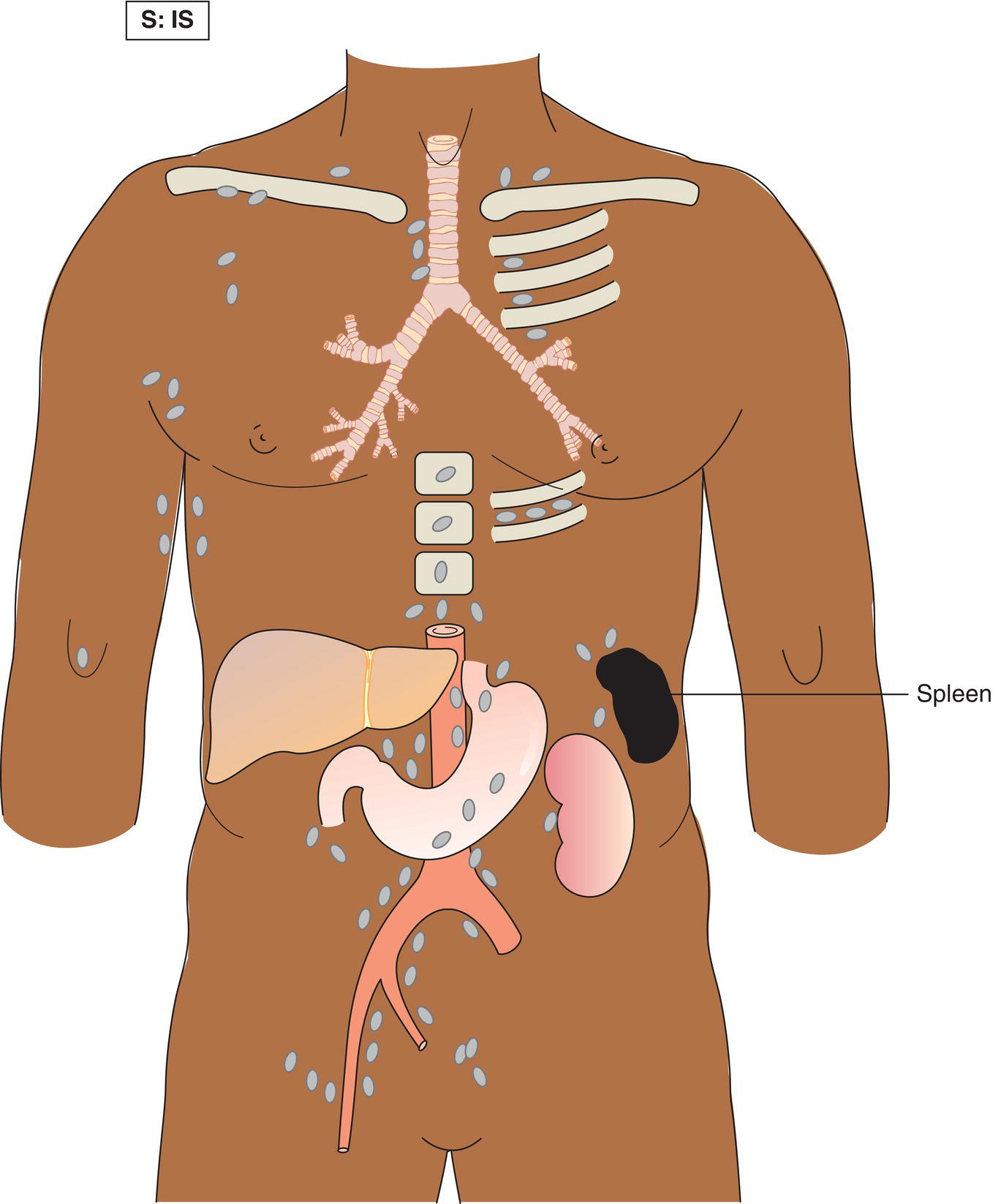
Stage II
Bulky Stage II
Advanced Stage
Stage III
Stage IV
A and B Classification (Symptoms)
Summary
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree