Lymphedema is caused when protein-rich fluid accumulates in the interstitium due to impaired function of lymphatic tissues. Proteins and other macromolecular wastes with water constitute lymphatic functional loads; these wastes rely upon specially structured absorptive and transport structures in regional anatomy for their return to the central circulation. When lymph stasis prevails, inflammatory processes and fibrosis (lymphostatic fibrosis) trigger changes in tissue density, further entrapping superficial vessels and accelerating the mechanical insufficiency.1
A brief description of primary lymphedema highlights the genetic variability of lymphatic constitution and may explain why seemingly similar patients receiving the same surgical protocol will have different risks for lymphedema over time (Table 104-1). In primary lymphatic diseases and syndromes (eg, Turner syndrome, Noonan syndrome), which include lymphatic dysplasias, these structural malformations involve the mechanisms for uptake, transport, and filtration of lymph. Inherited traits are found in Nonne–Milroy disease, Meige disease, and Distichiasis syndrome.2 The majority of these developmental insufficiencies (87%)1,3 manifest before the age of 35 and cause hypoplasia of vessels and nodes. Syndromes involving hyperplasia, node fibrosis, or aplasia also occur with much less frequency (Fig. 104-1).2 As a result, dysplasia predisposes regions of drainage to inadequate lymph collection, resulting in edema and secondary tissue changes such as chronic inflammation and reactive fibrosis. Since there is no method currently available to image lymphatic vessels safely, it remains a mystery how robust the constitution of lymphatic anatomy is in each patient. Of interest are variations in upper limb drainage that circumvent the axillary nodes to empty at supraclavicular sites. Anatomists theorize that these particular anomalies create advantageous collateral pathways to prevent lymphedema in those fortunate enough to possess these variations.4
The focus of this chapter’s discussion is secondary lymphedema, the type caused by a known, significant insult to lymphatic tissues (Table 104-2). It is well documented that lymphedema occurs with great frequency when direct trauma is rendered to regional nodes and/or vessel structures. Degradation of lymphatic health is also noted when adjacent tissues such as superficial and deep veins become diseased, when cellulitis occurs, or when accumulations of adipose or radiation fibrosis mechanically disrupt drainage of skin lymphatics.1,3,5 The following discussion is aimed at assisting with differential diagnosis, since all edemas do not occur in pure form.
Lymphedema always involves a compromised mechanical function within the lymphatic tissues leading to inadequate uptake of tissue loads and protein-rich fluid accumulation. The unique function of lymphatic vessels as compared to blood vessels involves peristaltic pulsation (intrinsic contractions) of lymphangion segments, resulting in continual transport of fluid regardless of activity level or limb position.4,6 At the level of the lymph capillary plexus, inelastic collagen-anchoring filaments tethered to overlapping inter-endothelial junctions (“swinging tips”)4 passively respond to increases in interstitial fluid pressure by opening under tension, allowing fluid to be pulled into the lumen to form lymph. Once this heightened interstitial pressure has been alleviated, the junction closes as the anchoring filament relaxes.4,7 Because lymph uptake relies upon continual, uninterrupted emptying of proximal vessels and nodes, disruption of afferent vessels at the node results in lymph vessel hypertension, valvular incompetence with reflux, vessel fatigue/failure, and lack of new lymph formation at the capillary level.4 Further mechanical compromises occur when surrounding tissues form lymphostatic fibrosis, which acts to entrap vessels and capillary plexuses, limiting free function.
During lymph stasis, activated macrophages respond to accumulations of lipoperoxides caused by free radicals not absorbed by compromised lymphatics. In the interstitium these macrophages release interleukin 1, causing fibroblast migration and fibrocyte or adipocyte accumulations.4 These inflammatory processes and cells responding to protein accumulation populate the interstitium, creating additional lymphatic loads (LLs) on already mechanically insufficient lymphatic tissues.4,6,8 Furthermore, hypertensive lymph collectors themselves become obstructed by clot formations, and vessel walls sclerose (mural insufficiency) if treatment is not administered to interrupt and reverse disease progression.4,9 Additionally, although lymph collectors and capillaries are not as radiation sensitive as the nodes, microvascular retreat within radiation tissues further accelerates fibrotic tissue formation, leading to regional vessel entrapment. Interestingly, as undifferentiated cells, fibroblast morphology seems to depend upon host body type, resulting in a hardened (fibrosclerosis) tissue formation in thin individuals or “spongy” swelling of the subcutis in heavier patients.
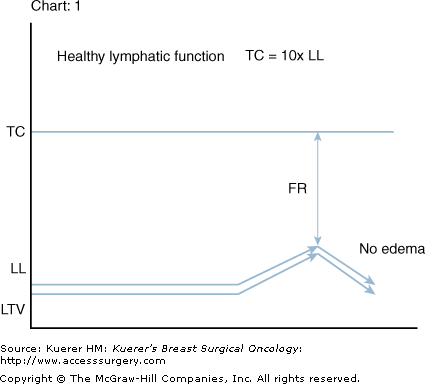
Transport capacity (TC) is generally estimated to be 10 times the level of daily-required LL.3,4,9 Lymph time volume (LTV) directly reflects the current LL by adjusting with an increase in contractile amplitude and frequency in tissues local to the newly formed fluids. Systemically LL is approximately 2 L/d; if required LTV adjusts (lymphatic safety factor)4 when necessary to a level of approximately 20 L/d without failure for extended periods. Therefore, TC equals maximum LTV (TC = MLTV) and functional reserve (FR) represents the difference between TC and LL at any moment in time (Fig. 104-2).4
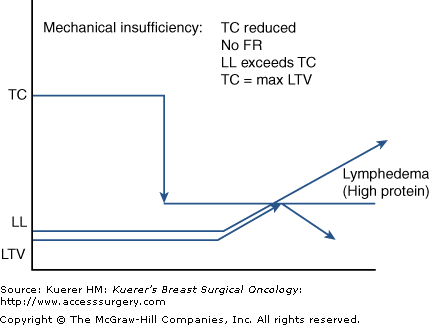
In secondary lymphedema, mechanical insufficiency results when TC is reduced from an assumed robust and normal level due to external trauma. FR is therefore decreased and LL approaches or exceeds the diminished TC, resulting in accumulations of macromolecular debris.4,5,9,10 Because FR is determined by ill-defined, inherited lymphatic constitution minus the relative amount of lymphatic trauma suffered, a timeline to the expression of lymphedema symptoms remains an unpredictable and highly variable phenomenon (Fig. 104-3).
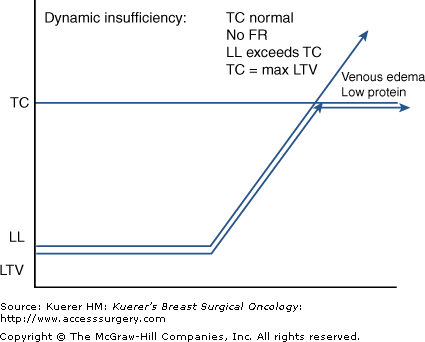
With its adjustable LTV (lymphatic safety factor) and considerable FR, the lymphatic system both regionally and systemically has an impressive capacity to absorb and transport fluid loads. When the neighboring venous system is patent, the net ultrafiltrate (UF) at the level of the microcirculation is approximately 10%. This net UF represents a LL, with the remaining 90% of tissue fluids returning directly through the venous limb of the capillary loop.4,5,9,10 When venous health is degraded, hypertension and resultant passive hyperemia cause an increased blood capillary pressure (BCP), resulting in high levels of UF from the venous limb, cancelling venous resorptive capacity.5 These excessively high LLs represent a potential pathologic demand, taxing the lymphatic system if not therapeutically countered. Such “dynamic” forces may consume much of the FRs, but if not exceeding maximum LTV, tissues will not show clinically evident edema. In this scenario, once the venous water loads exceed the TC of the still healthy lymphatic system, excesses will substantiate a low-protein, clinically apparent venous edema.3,4,9 It is important to note that this physiologic imbalance leads to secondary lymphedema as the lymphatic system’s adaptive function fatigues and then fails (Table 104-3, Fig. 104-4).
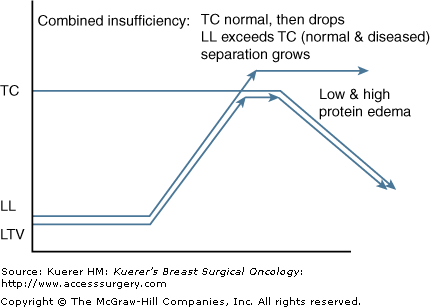
Based upon the former described pathologies it is possible to encounter a combination of mechanical and dynamic insufficiencies. With a lower than normal TC and a higher than normal LL, the gap between demand and capacity yields more of a burden on the lymphatic and venous systems. Postmastectomy-related lymphedema with concomitant congestive heart failure, DVT, or local tumor recurrence sets the stage for extremely high water loads with greatly limited uptake from surrounding veins and lymphatics (Fig. 104-5).
Lymphedema progression can be best described by clinical impression rather by calculation of limb volume or girth change. Lymphedema progression is most accurately described by clinical impression rather than limb volume calculation or girth measurement. As such, the clinical findings of limb enlargement represent both fluid retention as well as tissue metaplasia accompanied by palpable changes in density and texture (induration) at the epidermal and subcutaneous tissue layers.3,4,9 Since mechanically insufficient lymphatic response leads to increased population of interstitial proteins, extravascular oncotic pressure draws water away from the microcirculation, feeding the swelling. The resulting lymphedema does not resolve with limb positioning or elevation, as this oncotic pressure holds fluid firmly against the forces of gravity (Table 104-5).4
| Stage 0: Latency stage | Reduced transport capacity and functional reserve No visible, palpable edema, subjective complaints |
| Stage 1: Reversible | Reduces with elevation, pitting when present, no fibrosis |
| Stage 2: Spontaneously irreversible | No resolution, may fluctuate, pitting more difficult, fibrosis present |
| Stage 3: Lymphostatic Elephantiasis | Dermal hardening, non-pitting, papillomas, hyperkeratosis, extreme girth in some |
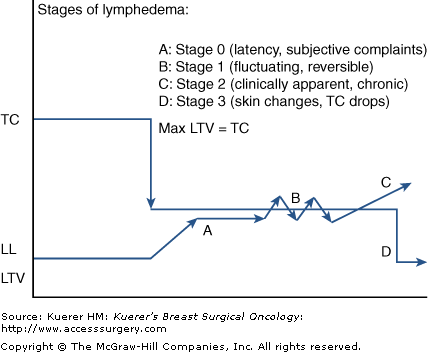
The stages of lymphedema progression involve secondary connective tissue disease combined with disturbed uptake and transport of fluid and reflect these attributes in a universal and classic clinical picture. Four distinct stages exist.
Much evidence exists to support the relevance of stage 0 regarding disease progression. Clinical expression is not yet evident unless highly sensitive measurement devices are employed. In this stage patients verbalize a classic list of complaints since there is sound cause to validate the imbalance within the regional lymphatic system. Typical descriptors, such as limb heaviness, achiness, pain, “waterlogged,” or tightness, are universal and quite common.11-15 Until recently, such subjective utterances were dismissed as inconsequential or baseless; however, when not addressed with therapeutic suggestions, stage 0 lymphedema always progresses to stage 1.
As lymph load approaches TC, patient sensitivity to these subtle changes can be reflected in limb volume changes of between 3% and 5% above baseline.14 While clinical examination is not sensitive enough to detect such subtle imbalances, lymphedema specialists have learned to regard these complaints as highly relevant (Table 104-4).
In the natural progression of ever-increasing macromolecular waste, the diminished TC is more easily reached or exceeded, creating a transient swelling state. During stage 1, limb elevation, decreased activity level, or self-administered manual therapy resolves the condition as the lymphatic system catches up with the workload placed upon it. Swelling is too recent to have triggered an inflammatory response, so the term “reversible” has been coined to describe its fully remitting characteristics.1,3,4
In this stage the swelling is ever-present, although it may appear minimal. The fact that a clinically measureable, and visibly apparent swelling is unremitting indicates excessive LLs that cannot be handled even when the limb is elevated or when activity levels are lessened (and so cannot spontaneously reverse).3,4 In stage 2 the first indications of fibrosis are apparent, as pitting is subtly more difficult to achieve and skin texture is palpably harder when compressed. Stage 2 is generally characterized as being free of papilloma formations and hyperkeratosis, which are only associated with stage 3.1,3,4,16 If neglected, stage 2 can advance, becoming massively swollen and pitting resistant even in the absence of such extreme external skin changes. As such, stage 2 may be a lifelong stage for many patients with upper extremity lymphedema.
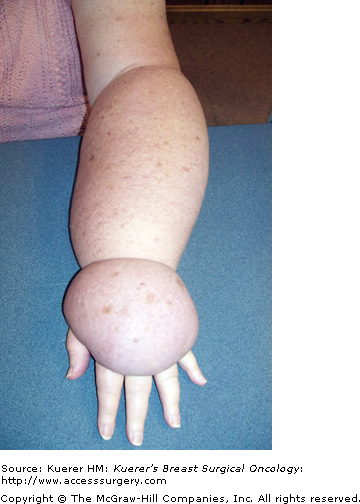
This stage indicates the presence of extreme lymphostatic fibrosis and is usually associated with limb contour distortions and massive girth (Fig. 104-6). Although subcutaneous fibrosis impregnates the tissues throughout the natural course of progression, an additional rough, warty keratin layer (hyperkeratosis) appears with or without papilloma formation (papillomatosis). These severe tissue changes characterize the term elephantiasis, and are unique to lymphedema as a disease process.1,3,4,9,16 Stage 3 is not uncommon in lower-extremity cases due to neglect, gravity, dependency, and masking or hiding the problem under clothing. Additionally, legs experience a higher incidence of primary lymphedema, which follows a more protracted timeline without an appropriate, accurate diagnosis. Fortunately in the breast cancer population it is uncommon to see prolonged lymphostasis leading to such severe disease expression. This is likely attributable to the vast educational resources available to breast cancer patients, which prompt recognition and earlier diagnosis and treatment. Additionally, patient self-consciousness and the inability to hide a hand swelling attract more attention, prompting therapeutic interventions earlier in the disease progression (Fig. 104-7).
Lymphedema results in regional immune suppression and leads to an increase in opportunistic infections such as cellulitis. As skin integrity suffers, scaling and dryness allow resident skin pathogens (strep and staph) to gain access through the skin barrier. Protein-rich interstitial fluid creates a favorable medium for bacterial colonization. Lymphocyte migration is reduced, and dissected or irradiated node sites are slow to detect invaders. Furthermore, stagnant lymph fosters a further delay on immune response.3,4,9,17
Pain is not directly associated with lymphedema in the majority of cases, since the skin accommodates a gradual and insidious accumulation of fluids.3,4,9,18 Secondary discomfort at the shoulder, elbow, or wrist may be explained by the increased weight of the limb,4,18 deconditioning, or decreased range of motion. Since the progression is strikingly slow in most cases, gravity and centrifugal forces pull fluids towards distal limb areas, causing an entrenched and stubborn pitting quality. As lymph vessels congest, valvular incompetence feeds the distal worsening to include fingers, toes, and dorsal hand/foot regions. Prominent upper extremity structures such as the styloids, epicondyles, and olecranon become progressively less distinct, creating a columnar limb appearance. As distinct lymphatic failure is not taxing to the venous system, skin color remains normal and blood supply patent, warding off the development of secondary ulcerations (Table 104-6).3,4
| Slow, gradual progression |
| Pitting in early stages |
| Distal to proximal advancement (may spare hand) |
| Loss of bony contours (columnar) |
| Dorsal “buffalo hump” if hand involved |
| Normal skin color (accept stage 3) |
| History of infection common |
| Ulcerations rare (pure lymphedema) |
| Rarely painful (discomfort common) |
| Asymmetric if bilateral (lower extremities) |
In upper extremity presentations the most notable departures from the norm involve hand-sparing and proximally pronounced lymphedema. When encountered, these presentations suggest a proximal traumatic disturbance, which has not yet resulted in distal vessel fatigue. In time forearm and hand regions will likely show signs of fluid accumulations as tendonous features and superficial veins become immersed. Rarely, fluid tends to pool in one limb region (hand, mid-arm, forearm only) without progressing to involve more tissue. Another atypical characteristic involves a markedly accelerated progression through the stages of severity leading to extreme fibrotic tissue changes with or without massive limb girth expressions.
The severity of breast cancer related lymphedema can be directly correlated to factors such as timeline since initial expression and extent of cancer therapy (number of nodes resected, number of positive nodes, and use of radiotherapy).11,19-21 Additionally, common exacerbators such as the number of episodes of infection,4,9,18 weight gain, injury,12,22 diuretics,18,22 disuse, pneumatic compression therapy4,23 and ill-fitting compression sleeves/gloves, contribute to worsening in most cases. The single most important contributor to increasing severity is lack of patient education leading to lack of treatment and/or improper attempts at therapy, which cause further harm.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree