Summary of Key Points
- •
The main oncologic emergencies most germane and specific to lung cancer are central airway obstruction, massive hemoptysis, and massive pleural effusion.
- •
Central airway obstruction due to lung cancer is traditionally classified as exophytic (intraluminal tumor), extrinsic (compression of the airway by tumor within or external to the wall of the airway), or mixed.
- •
Treatment of central airway obstruction generally correlates with the type of lesion: ablative modalities (e.g., laser, cryotherapy, argon plasma coagulation) for exophytic lesions; airway stenting for extrinsic compression; and combined therapies for mixed lesions.
- •
While treatment of central airway obstruction can improve survival, the major aim is the relief of symptoms.
- •
Massive hemoptysis may present in a dramatic fashion and with a rapidly fatal outcome. Rapid, efficient, and expert management and treatment are therefore of paramount importance.
- •
Patients with massive hemoptysis generally die of asphyxiation, not exsanguination, and although not all patients with massive hemoptysis require endotracheal intubation and mechanical ventilation, airway management with vigilant ongoing attention to airway assessment and management is a primary concern.
- •
Endovascular embolization (bronchial artery in 90% of cases) is the mainstay treatment of massive hemoptysis.
- •
Malignant pleural effusion is the most common cause of a massive pleural effusion.
- •
Point-of-care ultrasound is an important part of the evaluation and management of massive pleural effusions.
- •
Indwelling pleural catheters are an effective and safe method to help in the management of symptoms for patients with malignant pleural effusions.
The oncologic emergencies encountered by patients with lung cancer are not exclusive to lung cancer and, for the most part, may affect all cancer patients. Nevertheless, some circumstances and aspects of lung cancer emergencies are unique. Worldwide, lung cancer ranks first in cancer incidence and mortality, but in the United States, it ranks third in incidence behind prostate and breast cancer. Yet, among patients who present to the emergency department, more than three times as many have lung cancer as the next most common type, colorectal cancer. Not surprisingly, respiratory problems are the most common chief complaint for patients with lung cancer on presentation to the emergency department. These patients also appear to be more acutely ill; more than five times as many patients with lung cancer die in the emergency department as compared with patients with colorectal cancer. Although most lung cancer emergencies, such as pneumonia, respiratory failure, neutropenic sepsis, shock, intracranial catastrophe, spinal cord compression, and pathologic fracture, also occur among patients with other cancers, three problems stand out as being most specific and germane to lung cancer, including central airway obstruction, massive hemoptysis, and massive pleural effusions. This chapter offers practical insights and perspectives into the etiology, evaluation, and management of these three complex, controversial, and dreaded complications of lung cancer.
Central Airway Obstruction
For the purposes of this chapter, central airway obstruction is defined as obstruction of the trachea and main bronchi as well as of the lobar and segmental bronchi. Central airway obstruction is a complex and frequent sign of progressive disease in patients with lung cancer and in patients with malignancies that metastasize to the lungs and airways. Lung cancer-related central airway obstruction often requires emergent evaluation and treatment to prevent hospitalization and admission to the intensive care unit, to control progression of disease, to palliate and treat other life-threatening diseases, and to avoid immediate death.
Central airway obstruction is associated with many presenting signs and symptoms, a handful of diagnostic modalities used in its evaluation, a multitude of available interventional therapies, and, most importantly, a number of patient-related issues relevant to the diagnostic and therapeutic management of this emergency. In this chapter, we briefly address some of these issues as they relate to the oncologist, radiologist, cytopathologist, interventional pulmonologist, critical care specialist, thoracic surgeon, medical ethicist, and radiation oncologist operating as members of a multidisciplinary team for lung cancer management.
Types of Central Airway Obstruction Presenting as Emergencies
Traditionally, central airway obstruction is classified as exophytic (intraluminal), extrinsic (i.e., compression of the airway from tumor beyond or involving the airway wall), or mixed ( Fig. 57.1 ). This classification is enhanced by specifying the location and extent of the airway abnormality; describing whether the obstruction is focal, multifocal, or diffuse; and indicating whether associated abnormalities are present, such as edema, bronchitis, airway necrosis, purulent secretions, obvious infection (which may be primary or secondary), bleeding, perforation or fistula, dehiscence, or airway distortion. It is also helpful to ascertain whether the abnormality is a primary or secondary disorder. For example, central airway obstruction may be a result of new, progressive, or recurrent disease, or it may be an iatrogenic complication after a procedure, such as airway intubation, mechanical ventilation, stent insertion, brachytherapy or laser resection, other bronchoscopic airway manipulation, external-beam radiotherapy, or thoracic surgical intervention.
Additional features that may be relevant in management decisions include whether the obstructing lesion is dynamic (alters the size of the airway during inspiration and expiration) or fixed (airway diameter remains unchanged during respiratory cycles), whether the lesion has associated malacia (softening of airway cartilage) or excessive dynamic airway collapse (exaggerated invagination of the posterior membrane), and the extent to which symptoms adversely affect the patient’s functional status and quality of life. When the airway obstruction has caused an emergency, one must determine whether the emergency is immediately life-threatening. This last point has important implications for diagnosis, treatment, and ethical aspects of care.
Symptoms of Lung Cancer-Related Central Airway Obstruction
The symptoms of central airway obstruction associated with lung cancer are similar to those found in other instances of central airway obstruction and include dyspnea, cough, hemoptysis, hoarseness, and respiratory failure. These symptoms may be progressive or of sudden onset, and can easily be considered to be consistent with the patient’s preexisting symptoms of the lung cancer itself. They can be signs of progressive although manageable disease or represent an immediate precursor or cause of death. Central airway obstruction should be suspected in all cases of new onset or increasing symptoms in any patient with known or suspected lung cancer and in patients who have recently undergone palliative or curative therapeutic interventions for their lung cancer. Of course, a patient’s comorbidities may be contributing to or causing these nonspecific symptoms. The medical evaluation, therefore, must ascertain the presence, severity, and contributing roles of possible heart failure; esophageal extension; pleural disease; other malignancies extending to the lung, mediastinum, and airways; emphysema and chronic bronchitis; pneumonia; radiation-induced lung or airway injury; clinical depression; malnutrition; and failure to thrive.
Central airway obstruction can be suspected during an outpatient clinic visit, prompt the patient to make an emergency room visit, or be responsible for sudden deterioration requiring intubation, hospitalization, or both. Central airway obstruction often requires admission to the intensive care unit. On some occasions, the obstruction is discovered only after a patient has emergency intubation and is placed on mechanical ventilation. In other cases, symptoms of significant obstruction may warrant intubation, raising issues about life-sustaining treatment, appropriate use of medical resources, costs, and roles of palliative care and procedures. A third scenario may involve a patient with dyspnea or other complications who is denied admission to the intensive care unit and further diagnostic evaluation, either because the diagnosis of central airway obstruction is not considered or because the condition is diagnosed but may be considered irreversible. This last scenario raises issues of professionalism, competency, and resource allocation because levels and quality of care depend, in part, on physician expertise, team experience, institutional biases, finances, and societal philosophies regarding extent of care for patients with life-threatening illnesses. Good communication with the patient and with other health-care providers involved in the patient’s care is essential, and a properly executed informed consent is a prerequisite to a thorough understanding of available therapeutic alternatives, including the potential consequences of choosing to accept or refuse minimally invasive surgical interventions.
The setting of emergency central airway obstruction is often complex and stressful for health-care providers, patients, and their families. Patients with malignant central airway obstruction may have a median survival as short as 3 months. One-year survival may be only 15%. In general, the survival rate beyond 90 days in nonsurgical patients with lung cancer requiring admission to the intensive care unit is only 37%, and in the case of associated acute respiratory failure, the prognosis is very poor. Usually, respiratory failure in patients with lung cancer is caused by pneumonia, acute lung injury or acute respiratory distress syndrome, diffuse alveolar hemorrhage, airway bleeding, or venous thromboembolism, as well as central airway obstruction. Mortality increases with the number of failed organs, severity of comorbidities, and presence of airway obstruction. In one study, the hospital mortality rate was 83% for patients with lung cancer and central airway obstruction who were receiving mechanical ventilation, compared with 62% in patients without an obstructed airway. In other studies, when respiratory failure was caused by airway obstruction, only 25% of patients were successfully weaned from mechanical ventilation, although some patients with malignant central airway obstruction benefited from interventional bronchoscopic procedures aimed at restoring airway patency.
Diagnosis of Central Airway Obstruction
The diagnosis of central airway obstruction is made with a combination of clinical findings, radiographic imaging, and bronchoscopic techniques. Because they are noninvasive, chest radiography or computed tomography (CT) is usually performed first. In some life-threatening situations, flexible bronchoscopic inspection is performed to provide immediate information to assist in establishing indications for or against therapeutic interventions to restore airway patency, alleviate dyspnea, postpone or prevent the onset of respiratory failure requiring intubation, or palliate other symptoms (such as hemoptysis).
Clinical Findings
Findings related to central airway obstruction may include decreased breath sounds on chest auscultation, prolonged expiration, and unilateral wheezing. Patients may lose the ability to phonate in cases where an airway stent has migrated proximally to impinge on the vocal cords from below. Vocal cord paralysis may be suggested by cough, hoarseness, change in voice, or episodes of recurrent aspiration and may be related to a primary lung mass or enlarged mediastinal lymph node impinging on the recurrent laryngeal nerve. Hemoptysis may suggest central airway obstruction in patients with known lung cancer or cancers that are known to metastasize or otherwise spread into the airways (such as colon cancer, malignant melanoma, renal cell carcinoma, thyroid cancer, esophageal cancer, adenoid cystic carcinomas, sarcomas, and some lymphomas).
Chest Radiographs Often Aid in Diagnosis
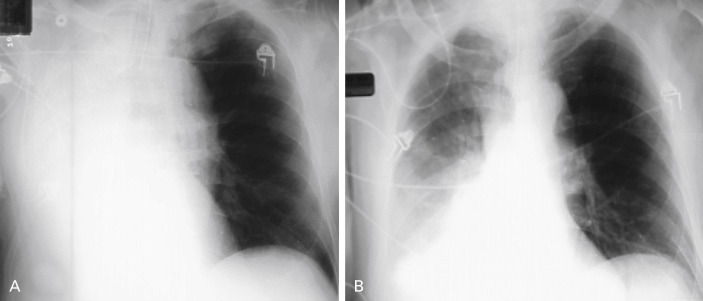
Chest radiographs may show atelectasis, ipsilateral mediastinal shift, lobar consolidation, stent migration, or a mass impinging on the central airway ( Fig. 57.2 ). CT is used to confirm the diagnosis and obtain more detailed information about the cause, extent, type, and morphology of the obstructing lesion. Associated findings include, among others, mediastinal widening, other lung or airway lesions, pleural disease, volume loss, atelectasis, and consolidation. Patients with a history of radiotherapy may have signs of fibrosis or radiation pneumonitis. CT may also provide information regarding the extent of peribronchial involvement and airway distortion, which may be underestimated by bronchoscopy alone. In some instances, ventilation–perfusion scans can be performed to ascertain whether functional lung exists beyond the level of the obstruction, but the results are not always precise and negative findings do not necessarily preclude successful reestablishment of airway patency and restored ventilation.
Flexible Bronchoscopic Examination
Flexible bronchoscopy provides information about the morphology, extent, etiology, and severity of the airway obstruction. It also provides information pertaining to associated airway abnormalities that may affect management decisions and indications for or against palliative or curative interventions to restore airway patency. In experienced hands, airway inspection is performed very quickly with minimal risk to the patient. Depending on the setting, bronchoscopy can be performed through the nares or the mouth (using a bite block), from behind the head of the patient or standing in front of and to the side of the patient, always in conjunction with supplemental oxygen and with or without sedation. For example, in a patient with impending respiratory failure, bronchoscopy can be performed with the patient receiving high-flow oxygen and/or through a continuous positive airway pressure mask, without sedation (to avoid risks of iatrogenic respiratory suppression), and with the patient in the seated position (to avoid aspiration or respiratory suppression related to the supine position). Such a procedure done in the emergency department, hospital ward, or procedure suite may prompt an immediate referral to the operating room for a therapeutic bronchoscopic intervention. If absolutely necessary, the patient can be intubated temporarily with an endotracheal tube over the flexible bronchoscope, or, after appropriate sedation and airway management, intubation can be performed via laryngoscopy before transport to the operating room or interventional bronchoscopy suite.
Evaluation of Patients
Patients with a diagnosis of lung cancer–related central airway obstruction require a careful evaluation to obtain information that will guide management. Many decision points must be considered, some of which are disease related, whereas others are lesion related, patient related (e.g., clinical status and treatment preferences), and health-care team related ( Table 57.1 ). To be certain that information is obtained to address each of these aspects of care, a four-step approach can be used that includes an initial evaluation, a review of procedural strategies, procedural techniques and expected/known results, and a long-term management plan ( Table 57.2 ). Many examples for dealing with cancer-related central airway obstruction can be found in the textbook Bronchoscopy and Central Airway Disorders: A Patient-Centered Approach .
| Category | Description |
|---|---|
| Disease related | Severity and extent of comorbid conditions Extent of disease (organ failure, metastases) Prognosis without further systemic therapy Prognosis with additional systemic therapy |
| Lesion related | Extent of abnormalities Duration of central airway obstruction and symptoms of respiratory insufficiency Amenable to bronchoscopic removal or palliation Amenable to stent insertion Potential response to radiotherapy |
| Patient status and preference related | Functional status Expected survival in the absence of central airway obstruction Do-not-resuscitate status Response to informed consent Family support system Risk tolerance Desire to live: goals, expectations |
| Health-care team related | Team experience with bronchoscopic techniques Physician competence and experience Multidisciplinary team management Palliative care and medical ethics consultation availability Resource availability (intensive care unit beds, equipment, instruments) Proactive versus reactive behavioral profiles Realistic, nihilistic, or unrealistic desires and expectations Cost, health-care insurance, societal philosophies regarding resource allocation |
| Initial Evaluation | Procedural Strategies |
|---|---|
|
|
| Procedural Techniques and Results | Long-Term Management Plan |
|---|---|
|
|
Treatment Modalities for Emergency Management of Lung Cancer–Related Central Airway Obstruction
The goals of therapy are to restore airway patency, improve symptoms, enhance quality of life, improve patients’ functional status so they may undergo additional systemic or local–regional therapies, transfer hospitalized patients to a lower level of care (from the intensive care unit to the wards or from the wards to home), and increase survival. In recent years, a commonly used therapeutic palliative approach is the combination of endobronchial debulking (using thermal, nonthermal, or mechanical techniques) with or without stent insertion, followed by external-beam radiotherapy and/or systemic therapy, if indicated or possible.
Bronchoscopic Laser Resection
Bronchoscopic laser resection, usually using the neodymium:yttrium aluminum garnet (Nd:YAG) laser, is a mainstay of emergency bronchoscopic intervention for patients with central airway obstruction. Laser can be used in conjunction with mechanical debulking and stent insertion, but requires that the health-care team have experience with laser technologies and, most importantly, knowledge of various laser–tissue interactions related to the use of low- and high-power densities. According to numerous studies, laser resection is an effective palliative procedure for central airway obstruction. Complications are uncommon in well-trained hands, but physicians should always consider the possibility of failure to control the airway, airway fires (especially with indwelling airway stents or endotracheal tubes), failure to control bleeding, and airway necrosis. In general, the depth of penetration of the Nd:YAG laser allows excellent coagulation of vessels that are several millimeters in diameter because this thermal ablative technique achieves substantial vasoconstriction and vaporization of tissues.
Airway Stenting
Silicone airway stents are extremely valuable for emergency treatment of central airway obstruction. Not only can these stents be deployed, if necessary, without prior thermal ablative techniques, but they also ensure airway patency and give health-care providers time to address other issues relevant to a patient’s care. Data from numerous studies confirm the efficacy of silicone stents to restore and maintain airway patency, although complications such as stent migration, kinking, obstruction by tumor or mucus, and even infection have been reported. Silicone stents, however, require rigid bronchoscopy and general anesthesia. Models are available in all shapes and sizes, including stents that fit onto the carina or secondary bifurcations. By improving functional status, stent insertion allows the medical team to proceed with palliative chemotherapy or radiotherapy if indicated ( Fig. 57.3 ).
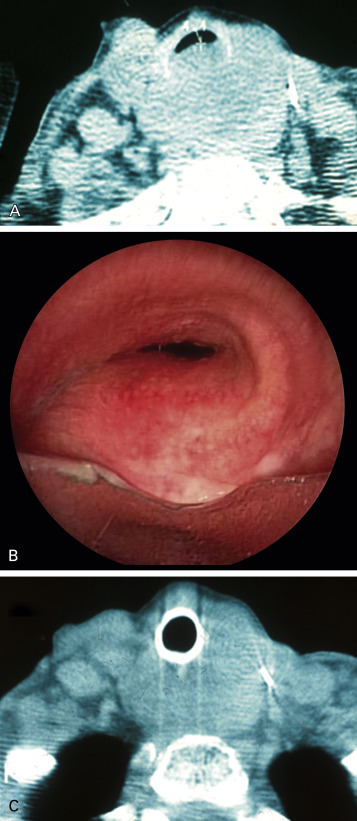
Stent insertion becomes necessary when symptomatic airway stenosis is mixed, when stenosis is caused by extrinsic compression, or when a patient has had repeated removal of an intraluminal lesion at short intervals because of a fast-growing tumor. Stent selection has traditionally been based on an operator’s previous experience with a particular stent and the local availability of various stents and other equipment. Stent retrievability is important among patients with malignancy for whom a temporary stent placement is expected; these include patients with malignant central airway obstruction who will undergo further surgical or systemic chemotherapy and/or radiotherapy (e.g., patients with thyroid cancer, primary lung cancer, and esophageal cancer impinging on or involving the airways).
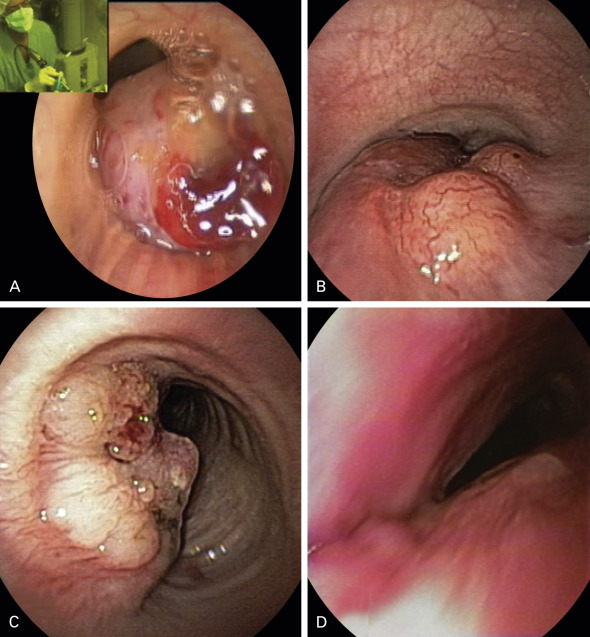
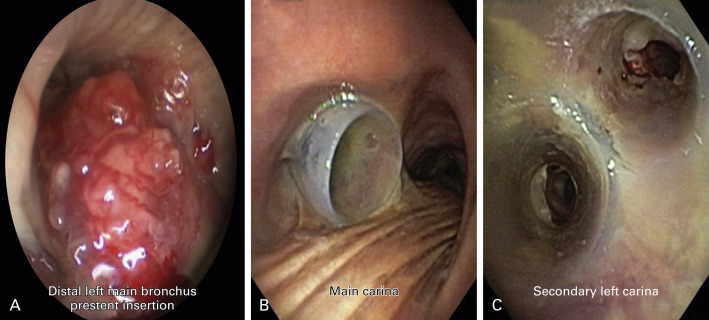
In addition to the morphology and consistency of the tumor, the mechanical properties of the stent should be considered in selecting the appropriate stent. The expansile force (strength) and ability to withhold angulation (also known as buckling) vary among different types of stents. The studded-silicone-type stent has a high expansile force. In a distorted, curved airway, angulation properties are important because they determine whether a stent can conform to the acutely angulated airway and remain patent ( Fig. 57.4 ). Patients with indwelling airway stents benefit from having a stent medical-alert card, which informs emergency room physicians about stent type, size, location, and construction and provides instructions about emergency procedures in case of stent-related complications.
External-Beam Radiotherapy
External-beam radiotherapy is a feasible, noninvasive therapeutic alternative, but when the patient has associated severe airway obstruction resulting in atelectasis, the response rate is 20% to 50% in studies involving more than 50 patients. Smaller studies showed that bronchial obstruction can be relieved in up to 74% of patients, resulting in complete or partial reexpansion of the collapsed lung. The time to initiation of treatment matters, because 71% of patients who had radiotherapy within 2 weeks after radiographic evidence of atelectasis had complete reexpansion of their lungs, compared with only 23% among patients having radiotherapy after 2 weeks. The main limitation of external-beam radiotherapy is unwanted exposure of the normal lung parenchyma, heart, spine, and esophagus. Improvements in imaging and treatment planning using three-dimensional conformational radiation and respiratory gating can precisely target radiation delivery and, by decreasing normal tissue margins included to account for uncertainties in position, can diminish the risk of clinically significant pneumonitis and esophagitis. The restoration of airway patency usually improves a patient’s functional status and performance score, accelerates the initiation of systemic therapy if indicated, and can improve survival.
Endobronchial Brachytherapy
Endobronchial brachytherapy has proven efficacy in patients with endoluminal tumor even when a substantial extrabronchial component is present. This treatment is based on the principle of the inverse square law, which states that dose rate decreases as a function of the inverse square of the distance to the source center, making it possible to achieve a high radiation dose in the center of the radiation source with a fast decrease toward the periphery. Endobronchial brachytherapy offers palliation, with rates of recanalization ranging from 60% to 90%. Symptomatic improvement is seen in 70% to 80% of well-selected patients. The variability in reported results is explained by patient selection, different treatment schemes, and the use of additional treatments. For palliation of symptoms of nonsmall cell lung cancer (NSCLC), however, a Cochrane meta-analysis concluded that endobronchial brachytherapy alone was less effective than external-beam radiotherapy. Endobronchial brachytherapy is usually performed via flexible bronchoscopy. Treatment effects are delayed and complications include hemoptysis, which can be fatal in up to 21% of patients. Other complications include fistula formation, radiation bronchitis (10%), and bronchial stenosis. Squamous cell histology and, most importantly, treatment in the upper lobes are associated with the highest incidence of hemoptysis, probably because of the proximity of the great vessels. Radiation delivered anywhere in the vicinity of major vessels, however, probably increases bleeding risk, as does combined therapy such as laser resection plus endobronchial brachytherapy, which together may augment the likelihood of tissue necrosis. Patients with poor performance status may also be at higher risk for periprocedural complications such as cough, bronchospasm, and pneumothorax caused by placement of the brachytherapy catheter.
Photodynamic Therapy
Photodynamic therapy can also be performed via flexible bronchoscopy and is approved for local–regional palliation for advanced NSCLC. This modality is most effective when the patient has more than 50% narrowing from mucosal disease. The outcome of photodynamic therapy seems to be the best, however, when patients have a relatively good performance status. In addition, this therapy is less than ideal in the emergency setting because the therapeutic effect is delayed for at least 48 hours and the associated risk of phototoxicity is approximately 20% at 4 weeks after the intervention. Photodynamic therapy may actually worsen airway obstruction during the initial posttreatment period because of sloughing of airway mucosa and retained tumor debris. Sloughed tissue can occlude the airway and cause complete obstruction, resulting in worsening symptoms and postobstructive pneumonia. Similar to endobronchial brachytherapy, photodynamic therapy is contraindicated among patients at high risk for fatal massive hemoptysis. Hemorrhage has been reported for 0% to 2.3% of patients, but the risk may be higher when the disease involves major blood vessels.
Cryotherapy
Cryotherapy causes thrombosis and necrosis of tumor tissues. Although cryotherapy poses no risk of airway fire or perforation, it can cause cold-induced bronchospasm. Cryotherapy has been shown to be most effective when performed in combination with external-beam radiotherapy. Similar to photodynamic therapy and endobronchial brachytherapy, cryotherapy has a delayed effect and initially may worsen airway obstruction, causing postobstructive pneumonia from sloughed necrotic tissue. Among patients with lung cancer and endoluminal obstruction, cryotherapy is reportedly effective in up to 75% of cases, but it is not a therapy of choice in the emergency setting or when extrinsic compression is present.
Argon Plasma Coagulation
Argon plasma coagulation and electrocautery allow removal of exophytic disease and provide superficial cauterization (3 mm to 6 mm), which may not be sufficient to stop large airway bleeding. Depth of penetration and distribution of heat-induced necrosis within tissues are not as predictable as they are with lasers because electrical current follows the path of least electrical resistance within different tissue types. Argon gas is heavy, inert, and much less soluble in the body than carbon dioxide. As gas is forced into the airway wall, perforation may occur, or gas may collect in a blood vessel and pass into the systemic circulation, causing life-threatening gas embolism. Erosion from tumor also presents a risk to major vessels. Risks may be increased when treating highly vascularized lesions and in the proximity of large blood vessels.
Covered Self-Expanding Metal Stents
Self-expanding metal stents, also called hybrid stents, have been used to relieve airway obstruction and seal off fistulae to avoid symptoms of aspiration. Among patients receiving mechanical ventilation, self-expanding metal stents can be inserted via rigid bronchoscopy under general anesthesia or via flexible bronchoscopy under fluoroscopic guidance. Some patients, however, are not suitable candidates for rigid bronchoscopy with a general anesthetic because of the severity of their illness and comorbidities or their unwillingness to have an operation. Fluoroscopy requires special facilities that may not be available in every intensive care unit. Self-expanding metal stents are more costly than silicone stents and can be difficult to remove.
Bronchoscopic Balloon Dilatation
Balloon dilatation is usually performed for airway strictures and is probably not ideal for cases of mixed obstruction from malignant disease. A balloon can be used to expand the airway lumen to facilitate the atraumatic passage of a rigid bronchoscope or endotracheal tube in cases where the operator has limited procedural experience. Mechanical resection of exophytic disease is possible using a specially designed resector balloon.
Expected Outcomes of Emergency Bronchoscopic Management of Central Airway Obstruction
It is not the purview of this chapter to cite the results of bronchoscopic treatment of central airway obstruction. It is common sense, and many studies show, that restoring airway patency improves symptoms, quality of life, exercise capacity, and survival. Therefore, bronchoscopic treatments have universally become the standard of care and should be considered for all patients with a diagnosis of central airway obstruction related to lung cancer.
In some settings, referral to the appropriate specialist is necessary. When in doubt, it is better to ask the specialist to evaluate the patient than to assume that intervention is not technically possible, that the patient cannot tolerate or survive the procedure because of a poor functional status, or that the intervention will not improve quality of life or survival. To enhance the care of all patients with lung cancer, including patients who require emergency treatment for central airway obstruction, the benefits of consulting a multidisciplinary team cannot be overemphasized. Such a team can help make lung cancer management decisions, has ready access to airway specialists with experience and knowledge of minimally invasive interventional techniques, and is well trained in one or more therapeutic bronchoscopy procedures.
Because this section is dedicated to airway emergencies in patients with central airway obstruction, it is reasonable to comment on the subject of patients with respiratory failure due to central airway obstruction. In these cases, interventional bronchoscopic procedures such as mechanical debulking, thermal ablation, and stent insertion have more immediate results than external-beam radiotherapy, help obviate the need for continued mechanical ventilation, provide time to initiate additional therapies, and prolong survival and quality of life. In many instances, patients who seem close to death are able to live productive, comfortable lives after airway patency is restored.
The Society of Critical Care Medicine recommends admission to the intensive care unit for all patients with advanced cancer who have a reversible condition such as pulmonary embolism, cardiac tamponade, or airway obstruction. Accompanying this recommendation is a prioritization scale identifying patients who will benefit most from admission to the intensive care unit (priority 1) and patients who will not benefit at all (priority 4). Patients with cancer complicated by airway obstruction are assigned priority 3, defined as unstable patients who are critically ill but have a reduced likelihood of recovery because of underlying disease or the nature of their acute illness. It is well recognized that emergency bronchoscopic intervention can be beneficial in many critically ill patients. In 2012, we reported the results of emergent therapeutic bronchoscopic intervention in 12 patients with NSCLC who had intubation and mechanical ventilation in the intensive care unit during a 6-year study period. Airway patency was restored in 11 (92%) of 12 patients. Bronchoscopic intervention resulted in immediate extubation and discontinuation of mechanical ventilation in 9 (75%) of 12 patients. The overall median survival was 228 days (range, 6 days to 927 days). For 9 individuals who were extubated within 24 hours after intervention, the median survival was 313 days (range, 6 days to 927 days).
In another study, Jeon et al. reported the results for 36 patients with respiratory failure and malignant central airway obstruction from various tumors. They noted that patients who had systemic therapy or radiotherapy in addition to bronchoscopic intervention survived longer than patients who had bronchoscopic palliation of central airway obstruction alone (median survival, 38.2 months vs. 6.2 months). In a retrospective study from Holland, 14 patients with advanced disease from esophageal cancer (5 patients) or NSCLC (9 patients) had immediate symptomatic relief after bronchoscopic intervention. As a result, Dutch general practitioners responsible for terminal home care remarked that bronchoscopic intervention and airway stent insertion are worthwhile elements of a patient-focused treatment plan.
Massive Hemoptysis
Hemoptysis is not a rare event, and it will occur at some point in approximately 20% of patients with lung cancer. Massive hemoptysis is far less common, but 3% of patients will have massive hemoptysis as their terminal event. When hemoptysis is trivial in amount, it is usually straightforward to diagnose and treat; yet it may also foreshadow a more critical or fatal event. At times, hemoptysis has such a sudden onset and large volume that it prompts emergent hospitalization and treatment, yet is not considered massive by all definitions in the literature. At other times, it may arise or advance in such dramatic volume and fashion as to be truly shocking to both patient and clinician. With this unmistakable presentation, it is universally acknowledged as massive and is clearly recognizable as a critical life-threatening condition. However, no universally accepted term is used (major, massive, catastrophic, life-threatening, and severe have all been used) to describe these different scenarios, and even more elusive is a precise volume or clinically relevant definition. Perhaps most important is the physiologic effect of hemoptysis; for example, although 500 mL of blood loss is insufficient to cause exsanguination, it can lead to rapid asphyxiation and death. Rapid and efficient evaluation and treatment of hemoptysis are therefore of paramount importance. Mobilizing the resources to accomplish this task is a challenge, particularly in centers that may have limited experience and resources. Therefore, not surprisingly, the reported mortality rate is highly variable, ranging from 0% to 78%, depending on the definition, etiology, era, treatment center, study design, and, potentially, the treatment approach.
For the purposes of this review, the term massive hemoptysis is used to encompass the various terms and definitions encountered in the medical literature, but the reader is encouraged to consider the broader context of potentially life-threatening hemoptysis. Data regarding massive hemoptysis specifically in the context of lung cancer are limited. Therefore much of the following discussion is derived from data regarding hemoptysis in general, but, wherever possible, the discussion is focused on lung cancer. Accumulating, albeit imperfect, evidence suggests the benefit of a multidisciplinary approach to the diagnosis and management of massive hemoptysis for patients with lung cancer.
Definition
The volume of expectorated blood that has been used to define massive hemoptysis varies from more than 100 mL to more than 1000 mL in 24 hours, but is often considered to be greater than 600 mL. The volume of expectorated blood has long been recognized to correlate with disease severity and outcome, including mortality. In a retrospective, single-institution review of 887 patients with hemoptysis of greater than 200 mL in 24 hours, Corey and Hla found that the mortality rate was 58% for patients with hemoptysis greater than 1000 mL in 24 hours, whereas the rate was 9% for patients with less than 1000 mL in 24 hours. However, the volume of expectorated blood is difficult for patients to quantify and is somewhat subjective. Furthermore, the expectorated volume may vastly underestimate the amount of blood remaining in the alveolar spaces and airways. For this reason, a chest x-ray may reflect more accurately the clinical significance of the bleeding. Perhaps more important than the precise volume of expectorated blood is its physiologic effect. It has been estimated that 400 mL of blood in the alveolar spaces is sufficient to impair oxygen transfer. The same volume of blood can cause significant obstruction of the large airways, asphyxiation, and death. In addition, the rate of bleeding, underlying morbidity, and the patient’s ability to maintain a patent airway all affect severity, independent of the absolute volume. Alternative definitions based more on the physiologic effects of airway obstruction and hemodynamic instability have been proposed.
In a large series published in 2012, Fartoukh et al. shed light on predicting the in-hospital mortality and severity of hemoptysis and hence clarified how we may consider, define, characterize, and manage this disorder. In their study, the volume of expectorated blood was an apparent predictor of mortality in a univariate analysis, but no longer remained an independent predictor of death after adjustment for other factors. Using data from a retrospective review of 1087 patients with hemoptysis admitted to the intensive care unit or step-down unit over 14 years at a single institution, they developed and validated a multiregression model for predicting in-hospital mortality. They devised a simple scoring system assigning points for chronic alcoholism (1 point), cancer (2 points), aspergillosis (2 points), pulmonary artery involvement (1 point), two or more chest x-ray quadrants (1 point), and initial mechanical ventilation (2 points). In-hospital mortality increased with increasing score, as follows: 0 = 1%, 1 = 2%, 2 = 6%, 3 = 16%, 4 = 34%, 5 = 58%, 6 = 79%, and 7 = 91%. These results suggest that rather than using a cutoff of expectorated volume for defining massive hemoptysis, a scoring system might better define hemoptysis and stratify patients according to the level of risk. Only with this type of objective and standardized definition can we begin to study how best to triage, manage, and treat these patients.
Etiology of Hemoptysis
Regarding massive hemoptysis in general, infectious etiologies (tuberculosis, bronchiectasis, mycetomas, and necrotizing pneumonia) predominate worldwide. Lung cancer is the cause in 3% to 10% of the cases that are severe enough to require bronchial artery embolization and in 17% of patients admitted to the intensive care unit. Tuberculosis is the most common cause in specific areas of the world, yet is a rare cause in other areas. Lung cancers, like most other cancers, are highly vascular and, particularly when endobronchial, may lead to massive hemoptysis. Patients with lung cancer may have chemotherapy-induced thrombocytopenia, comorbidities such as renal disease and/or liver disease, vascular disease requiring antiplatelet therapy, or thrombotic disorders requiring anticoagulation, and these factors compound the problem. Some of these conditions and medication effects can be mitigated, corrected, or reversed. Of special note are the antiangiogenesis factors and tyrosine kinase inhibitors (some of which also have significant angiogenesis inhibition) that can lead to massive hemoptysis. The biologic effect of these medications cannot be reversed and lasts for weeks.
Massive hemoptysis in a patient with lung cancer should not be automatically assumed to be coming directly from the lung cancer lesion. As already discussed, patients often have comorbidities that may predispose them to various other etiologies of hemoptysis, including cancer-related hypercoagulability leading to pulmonary emboli, coagulopathy leading to alveolar hemorrhage, and immunosuppression leading to necrotizing pneumonia. The clinician must therefore thoroughly evaluate hemoptysis in patients with lung cancer without assuming that the lung cancer is the source of the hemoptysis. This evaluation is important in treatment considerations; for example, patients with diffuse alveolar hemorrhage and/or coagulopathy respond to specific treatments and are not likely to derive benefit from invasive therapeutic procedures such as endovascular embolization.
Vascular Source of Bleeding
In approximately 90% of cases of hemoptysis, the bronchial artery circulation is the source of bleeding. This circulation is of relatively low flow, representing only a small percentage of cardiac output. Consequently, the bleeding may be self-limited and minimal in quantity. However, the bleeding is at the same time driven by high systemic pressures that may flow directly into the airways, where no counter-pressure exists to provide tamponade against the bleeding. This so-called high-pressure–low-flow source of bleeding can rapidly overwhelm the patient’s ability to keep the airways clear and avoid suffocation. Malignancy and chronic lung inflammation promote neovascularization, recruitment, hypertrophy, and proliferation of bronchial arteries. Chronic pleural inflammation promotes abnormalities in the circulation, which may originate from the mammary, subclavian, intercostal, thoracic, pericardial, phrenic, and thyrocervical arteries. These vessels may enter the lung through the pulmonary ligament or parietal or diaphragmatic pleura and represent another high-pressure–low-flow source, which has been reported in 3% to 25% of cases of hemoptysis. Conversely, although the pulmonary artery circulation is driven by relatively low right ventricular pressures, a pulmonary artery bleed may represent a significant percentage of the cardiac output. This relatively low-pressure–high-flow circulation is responsible for only a minority of cases of massive hemoptysis, but may be just as dramatic in presentation, such as with a ruptured Rasmussen artery in patients with tuberculosis. Remy et al. detected a pulmonary vascular source of bleeding in 11 (6%) of 189 patients treated with transcatheter bronchial or pulmonary artery embolization for massive or repeated hemoptysis, and Wang et al. recognized a pulmonary artery source in 2 of 30 patients. The authors of other large series have failed to report the pulmonary artery circulation as a significant source of hemoptysis. However, failure to consider the pulmonary circulation as a potential source of hemoptysis may explain why angiography fails to identify a definitive source of hemoptysis in approximately 11% of cases. Of final note, the pulmonary venous circulation is also not well recognized as a potential source of massive hemoptysis. It has even lower pressures, equivalent to left atrial pressures, and represents a potential very-low-pressure–high-flow source of massive hemoptysis ( Table 57.3 ).
| Source | Approximate Incidence (%) |
|---|---|
| Bronchial circulation | 90 |
| Other systemic circulation | 3–25 |
| Pulmonary vascular circulation | 6 |
Bronchial artery anatomy can be quite variable, and a thorough understanding and evaluation are necessary to determine the precise vascular origin of hemoptysis. The bronchial arteries originate from the descending aorta between T5 and T6 in 70% of people. Another 20% of people have ectopic branching arising from the subclavian, internal thoracic, pericardiacophrenic, innominate, thyrocervical trunk, and inferior phrenic arteries or abdominal aorta. In 10%, the bronchial arteries arise from other regions of the descending thoracic aorta and aortic arch. Branching patterns of the bronchial arteries themselves are highly variable, with nine patterns described. Multidetector CT provides an accurate road map for embolization by identifying the etiology and origin of the hemoptysis. It can depict the precise anatomy and nature of the bronchial and nonbronchial arteries involved, their course and size, and their relationship to the spinal artery.
Clinical Assessment, Initial Resuscitation, and Stabilization
Although assessment, resuscitation, and stabilization will be addressed separately from the approach to diagnosis, in reality all of these tasks are performed in parallel and are to a great degree integrated. As in most critical care situations, the initial clinical assessment, resuscitation, and stabilization of massive hemoptysis generally take precedence over complex or comprehensive diagnostic testing. Given that most patients die of asphyxiation rather than exsanguination, airway assessment and management are the priority. Initial hospital management should take place in the emergency room or the intensive care unit, with the most experienced personnel available rendering care. Whenever possible, these patients should be cared for at centers with the expertise and resources to optimally manage them. Transfer to tertiary centers may be advised.
Airway assessment is similar to that for any emergency patient, and the decision to perform endotracheal intubation and initiate mechanical ventilation rests on sound clinical judgment. If the patient has evidence of respiratory distress, one should not hesitate to secure an airway. However, not all patients with massive hemoptysis require endotracheal intubation. The aims of endotracheal intubation are to establish a secure airway, achieve adequate ventilation and oxygenation, and maintain airway clearance, which, in the nonintubated patient, are dependent on several factors, including the flow, volume, and duration of hemoptysis; cough and airway clearance mechanics; and cardiopulmonary reserve. Prophylactic intubation is not usually warranted for a patient with massive hemoptysis who is not in distress. Nevertheless, one must pay careful attention to signs that a patient is failing to maintain airway clearance, such as tachycardia, tachypnea, hypertension, hypotension, and hypoxemia. A chest x-ray with two or more quadrants involved indicates a large volume of aspirated and incompletely cleared blood, suggesting that these patients are at an increased risk of death. Early intubation may be warranted for these and other selected patients. Again, clinical judgment must be exercised, taking into consideration such factors as the patient’s ability to tolerate transport, supine positioning, and sedation for angiography or other procedures.
Patients should be confined to bed rest and should be in a decubitus position with the bleeding lung down. When intubation is deemed necessary, a large-bore tube, size 8 or larger (to facilitate bronchoscopy and suctioning), should be inserted by the most experienced operator available. Bronchoscopic intubation is often preferred because it not only facilitates intubation, but may also be diagnostic and therapeutic. Furthermore, it allows selective intubation of the nonbleeding right or left lung when it is deemed necessary. Selective intubation of the left main bronchus can rapidly establish a secure airway as well as isolate and protect a nonbleeding left lung. Selective intubation of the right main bronchus is more problematic given the very proximal right upper-lobe takeoff. Placement of a balloon in the bleeding bronchus will cause tamponade and terminate hemoptysis, as well as further protect the nonbleeding lung. A double-lumen endotracheal tube can accomplish the same goals. Although placement of a double-lumen endotracheal tube has been advocated to isolate and protect the nonbleeding lung, its use is not without problems: misplacement occurs in about 50% of cases, life-threatening trauma to the airway has been reported, and the small diameters of the lumen make bronchoscopy and suctioning difficult.
Rigid, as opposed to flexible, bronchoscopy has been advocated as a means of securing an airway and simultaneously providing a platform to diagnose and control massive hemoptysis. However, it is not readily available at all centers, and two large series on the management of massive hemoptysis suggested that flexible bronchoscopy (preferred as first-line therapy over rigid bronchoscopy) can be used safely, with a mortality rate of 0% to 4%. Without head-to-head comparisons, the choice of rigid or flexible bronchoscopy remains largely based on individual experience, availability, and institutional practice.
Two large-bore intravenous catheters should be placed, and a central venous catheter should be considered. Appropriate aggressive volume resuscitation including blood and, when necessary, intravenous vasoactive medications should be administered. Chest x-ray, laboratory testing, and type and screening for blood should be performed. Disorders of coagulation should be corrected whenever possible.
As discussed earlier, the in-hospital mortality rate is 34% for patients with a score of 4 or more on the scoring system by Fartoukh et al., and admission to the intensive care unit should be strongly considered for these patients. Death from massive hemoptysis is difficult to predict, and even patients with a score of 3 or less may benefit from admission to the intensive care unit; clinical judgment must be exercised.
A thorough history and physical examination should be performed, with attention paid to the following: (1) the quantity, duration, and quality of hemoptysis; (2) the patient’s lung cancer history including type, status, radiotherapy, surgical procedures, and antineoplastic drug therapy; (3) medication history; (4) underlying liver, kidney, and cardiopulmonary diseases; (5) signs and symptoms of respiratory distress; (6) signs and symptoms of other sites of bleeding; and (7) history of alcohol use.
Approach to Diagnosis
The major diagnostic studies for massive hemoptysis are laboratory tests to evaluate for coagulopathy and other potential causes of hemoptysis (e.g., vasculitis and alveolar hemorrhage), chest x-ray, CT of the chest, and bronchoscopy. Laboratory testing is aimed at identifying correctible or treatable causes of hemoptysis, whereas the remaining diagnostic studies are aimed at rapid and efficient triage of patients for definitive invasive interventional treatment. Although a chest x-ray should be performed as an important prognostic indicator and may guide triage decisions, chest x-rays are not helpful in approximately 50% of cases.
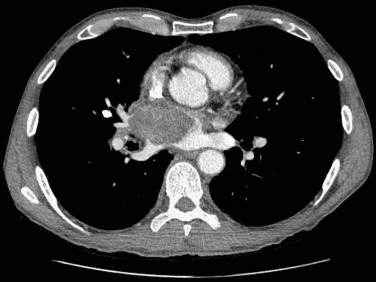
In the critical setting of massive hemoptysis, localization of bleeding to a specific site or at least the relevant side is crucial in allowing the interventional radiologist to narrow his or her focus and to perform endovascular embolization in an efficient manner. CT images provide information on the site and potential causes of bleeding and may indicate the precise vascular origin and source ( Fig. 57.5 ). Bronchoscopy more accurately identifies endobronchial lesions and provides a potentially temporizing and, on occasion, a definitive therapeutic option. A practical approach is to perform CT if the bleeding has been stabilized and to perform a diagnostic and potentially therapeutic bronchoscopic procedure on patients who are not stable enough for transport to CT because of uncontrolled bleeding. When necessary, these procedures can be performed sequentially.