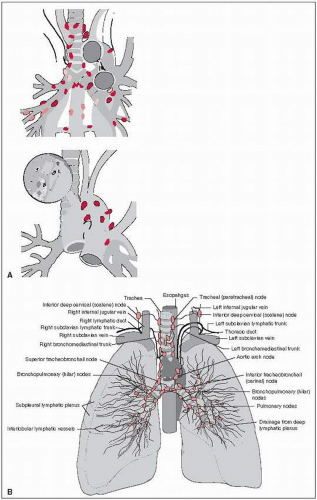
The right lung is composed of the upper, middle, and lower lobes, which are separated by the oblique (or major) and horizontal (or minor) fissures.
The left lung is composed of two lobes separated by a single fissure.
The lingular portion of the left upper lobe corresponds to the middle lobe on the right.
The trachea enters the superior mediastinum and bifurcates approximately at the level of the fifth thoracic vertebra.
The hila contain the bronchi, pulmonary arteries and veins, various branches from the pulmonary plexus, bronchial arteries and veins, and lymphatics.
The lung has a rich network of lymphatic vessels that ultimately drain into various lymph node stations: the intrapulmonary lymph nodes, along the secondary bronchi or in the bifurcation of branches of the pulmonary artery; the bronchopulmonary lymph nodes, situated either alongside the lower portions of the main bronchi (hilar lymph nodes) or at the bifurcations of the main bronchi into lobar bronchi (interlobar nodes) (8); and the mediastinal lymph nodes. The mediastinal lymph nodes are divided into the superior nodes, which are above the bifurcation of the trachea (carina) and include the upper (paratracheal, pretracheal, retrotracheal) nodes, the lower (paratracheal) nodes (azygos nodes), and a group of nodes located in the aortic window; and the inferior nodes, which are situated in the subcarinal region and inferior mediastinum and include the subcarinal, paraesophageal, and pulmonary ligament nodes (Fig. 22-1) (25).
Lymph from the right upper lobe flows to the hilar and tracheobronchial lymph nodes. Lymph from the left upper lobe flows to the venous angle of the same side and to the right superior mediastinum.
The right and left lower lobe lymphatics drain into the inferior mediastinal and the subcarinal nodes and from there to the right superior mediastinum (the left lower lobe also may drain into the left superior mediastinum) (74).
The pattern of spread may be local (intrathoracic), regional (lymphatic), or distant (hematogenous).
Small-cell carcinomas have a higher incidence of distant metastasis than non-small-cell cancers; of the latter, adenocarcinoma has the highest potential for distant metastasis.
The incidence of nodal involvement is lowest in lobectomy series (37%) and highest in necropsy series (94%).
Hilar lymph node metastases from lung cancer occur in approximately 60% of right upper lobe and middle lobe lesions and 75% of lower lobe tumors.
Mediastinal nodal involvement, which has been studied in both surgical (early cases) and autopsy series, occurs in 40% to 50% of operative specimens (8).
The incidence of scalene (supraclavicular) nodal involvement is 2% to 15%, predominantly from ipsilateral upper lobes or in patients with superior mediastinal metastases.
Hematogenous spread with multiple-organ involvement is frequent. The most common metastatic sites are the lung, liver, brain, and bone.
Adrenal metastasis has been reported in 27% of patients with epidermoid (squamous cell) carcinoma, 35% to 40% of patients with small- or large-cell undifferentiated carcinoma, and 43% of patients with adenocarcinoma (25).
Abdominal lymph nodes are involved in over 50% of patients with small-cell undifferentiated carcinoma.
Cough is a major symptom in 75% of patients and is severe in 40%.
Hemoptysis occurs in 57% of patients.
Dyspnea and chest pain also are common symptoms, resulting from involvement of the pleura, chest wall, or mediastinal structures.
Nonspecific, initial symptoms such as weight loss, weakness, anorexia, and malaise occur in 10% to 15% of patients. Febrile respiratory episodes are less common (18, 70).
Tumors located in the apex of the lungs may involve the cervical and thoracic nerves, resulting in Pancoast’s or superior sulcus tumor syndrome (shoulder pain, brachial plexopathy, or Horner’s syndrome) (77).
Sympathetic nerve involvement results in Horner’s syndrome (enophthalmos, ptosis, meiosis, and ipsilateral loss of sweating).
Involvement of the recurrent laryngeal nerve may lead to hoarseness; this is more common with tumors of the left lung and with aorticopulmonary window lymphadenopathy.
Involvement of the phrenic nerve can result in dyspnea and paralysis of the hemidiaphragm.
Dysphagia may result from compression of the tumor on the esophagus.
Primary tumors located in the right lung or metastatic tumors in the right mediastinal lymph nodes may cause superior vena cava syndrome.
Secondary tumor effects (paraneoplastic syndromes) are sometimes seen (18).
Routine chest x-ray is the most common radiologic examination.
Computed tomography (CT) of the chest with upper abdomen (to assess for liver, adrenal involvement) is the most valuable radiologic study for evaluation, staging, and therapeutic planning of lung cancer, but it cannot differentiate inflammatory disease from neoplasia.
Mediastinal nodes less than 1 cm in diameter are considered unlikely to contain metastatic disease. Nodes 1 to 2 cm are intermediate, and those larger than 2 cm in a patient with bronchogenic carcinoma almost certainly are metastatic (44).
Positron emission tomography (PET) scanning is routinely used to determine the malignant nature of suspicious lesions, to more accurately define tumor extent, including lymph node involvement (27, 58, 64, 109), and to aid in three-dimensional conformal radiation therapy (3-D CRT) treatment planning (71).
Studies have uniformly shown improvements in sensitivity and specificity for staging of individual patients ranging from approximately 60% to more than 85%, particularly if the PET scans were read in conjunction with the CT scans (108).
Kalif et al. (48) prospectively studied 105 patients with non-small-cell lung cancer to assess the impact of 18F-fluorodeoxyglucose PET on clinical management. PET influenced the
radiation delivery in 22 (65%) of 34 patients receiving definitive radiotherapy. Twelve patients considered probably inoperable on conventional imaging studies were downstaged by PET and underwent potentially curative surgery. CT and PET understaged 3 of 20 surgical patients, and PET missed one small intrapulmonary metastasis apparent on CT. No pathological N2 disease was missed on PET.
A brain CT or magnetic resonance imaging (MRI) scan is frequently used in the workup of small-cell carcinoma and advanced non-small-cell carcinoma.
Pulmonary function tests (which yield parameters such as FEV1, DLCO) are important predictors of the patient’s ability to undergo surgical resection or withstand irradiation.
Sputum cytology can diagnose malignancy in 65% to 75% of patients, but with lower yield for peripheral tumors.
Bronchoscopic examination provides important data, even in the presence of preoperative cytologic proof of cancer; endobronchial ultrasonography permits visualization of suspicious lymph nodes for needle sampling.
In patients with suspicious, undiagnosed peripheral lung lesions seen on x-ray or CT imaging, radiographically guided percutaneous biopsy can be performed.
Other procedures used in establishing the diagnosis/staging are mediastinoscopy (to assess level 2, 4, 7 nodes), thoracoscopy, anterior mediastinotomy/Chamberlain procedure (to assess level 5, 6 nodes), thoracotomy, and biopsy of scalene/supraclavicular lymph nodes or distant metastatic sites.
For the seventh edition of its staging system, the American Joint Committee on Cancer has adopted the tumor-node-metastasis system proposed by the International Association for the Study of Lung Cancer (Table 22-1) (36).
Small-cell lung carcinomas are generally staged more simply as limited (confined to one hemithorax/encompassed by a single radiation portal) or extensive.
Primary lung carcinoma is divided into non-small-cell carcinoma (including squamous cell/epi-dermoid, large cell undifferentiated, adenocarcinoma, and carcinoid) and small-cell carcinoma.
Bronchoalveolar carcinoma is a subtype of adenocarcinoma that commonly presents in nonsmokers.
Tumor size, stage, histologic type, performance status (Karnofsky score), and weight loss are the most important prognostic factors related to survival.
Genetic prognostic factors include mutations in the K-ras oncogene, deletion of tumor suppressor genes (e.g.,p53 gene), presence of N-cam expression, and elevated serum levels of neuron-specific enolase.
The first management step for non-small-cell lung cancer is to decide whether the treatment aim is definitive or palliative and whether the tumor is resectable or unresectable.
TABLE 22-1 AJCC Staging System for Lung Cancer | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Non-small-cell carcinoma of the lung should be treated surgically, if resectable.
Lobectomy is preferred over pneumonectomy; wedge resection is associated with a high local failure rate (34).
Several collaborative studies failed to show significant improvement in survival with use of preoperative irradiation (105).
Preoperative chemotherapy has been associated with improved survival versus surgery alone for patients with N2 disease (86, 88).
Trimodality preoperative chemoirradiation for N2 patients proved to be an encouraging approach in the SWOG 8805 trial (1).
INT 0139/RTOG 9309 trial demonstrated that preoperative chemoirradiation to 45 Gy is associated with improved progression-free survival versus chemoradiation to 61 Gy (5-year: 22% versus 11%) and better 5-year overall survival; pN0 disease was associated with improved survival, indicating the prognostic importance of mediastinal sterilization by preoperative chemoirradiation; trimodality therapy may not be appropriate if pneumonectomy is required, due to a high rate of treatment-related deaths (2).
Postoperative irradiation has been advocated for patients with pN2 disease (approximately 50 Gy), positive surgical margins (approximately 54 to 60 Gy), or nodal extracapsular extension (approximately 54 to 60 Gy).
The PORT meta-analysis demonstrated lower survival in patients receiving postoperative irradiation; this was related to greater irradiation sequelae due likely in part to poor radiation technique in many included studies (e.g., high doses, large fraction sizes, use of lateral fields, use of spinal cord blocks, use of 60Co, lack of CT-based planning). Although there was an
absolute reduction in 2-year overall survival from 55% to 48%, subgroup analyses indicated that this adverse effect was greatest for patients with stage I or II, N0 to N1 disease, whereas for those with stage III, N2 disease there was no evidence of an adverse effect (68, 82).
Adjuvant postoperative chemotherapy—generally cisplatin based—has been associated with an absolute survival benefit of approximately 5% at 5 years versus surgery alone (4, 14, 23, 111).
Postoperative chemotherapy is generally reserved for patients with pathologic stage more advanced than pT1N0 (although there is debate if pT2N0 patients benefit from chemotherapy)
Postoperative concurrent chemoirradiation did not offer a survival gain versus postoperative irradiation alone in the ECOG 3590/INT 0115 trial (49).
Definitive radiation therapy (±chemotherapy) is indicated for non-small-cell lung cancer patients who are technically operable but medically inoperable, with 5-year overall survival of approximately 15% (97).
The standard of care was established by the Radiation Therapy Oncology Group (RTOG) 73-01 dose-escalation trial (79). The incidence of local failure, evaluated clinically, was lower in patients treated to 60 Gy (33%) versus 50 Gy (39%) versus 40 Gy (44% to 49%). Accordingly, patients receiving definitive radiotherapy are generally treated with doses of 60 Gy or higher.
Stereotactic body radiotherapy (SBRT) is an alternative for medically inoperable node-negative patients with primary lesions up to about 5 cm in size, offering 80% to 95% local control (104); however, centrally located lesions should only be treated with SBRT on clinical trials due to potentially high risk of grade 3 to 5 toxicity (103).
Combined chemotherapy and irradiation is the treatment of choice for locally advanced, inoperable non-small-cell lung cancer patients with good performance status and absence of significant weight loss or those without other medical contraindications to chemotherapy (47).
Sequential cisplatin/vinblastine chemotherapy for two cycles, followed by irradiation (60 Gy in 6 weeks) has shown 11% to 13% absolute improvement in 2-year overall survival compared with 60 Gy alone (22, 91).
Chemotherapy regimens used with radiation include cisplatin/vinblastine, cisplatin/etoposide, paclitaxel/carboplatin, cisplatin/gemcitabine, cisplatin/pemetrexed (for nonsquamous histology).
Cisplatin-based concurrent chemoirradiation has been associated with 11% to 14% absolute improvement in 3-year overall survival compared with irradiation alone (93).
A number of trials have demonstrated superior overall survival for concurrent chemoirradiation versus sequential chemoirradiation (21, 32). RTOG 94-10 demonstrated improved median overall survival with concurrent chemoirradiation (cisplatin/vinblastine/60 Gy thoracic RT) versus sequential chemoirradiation (cisplatin/vinblastine —> 60 Gy): 17.0 versus 14.6 months (21).
Small-cell lung carcinoma is sensitive to many chemotherapeutic agents; multiagent drug combinations are more effective than single agents (37).
Initial chemotherapy induces complete response in about 40% to 68% of patients with limited disease and 18% to 40% with extensive disease (37).
A meta-analysis of 13 prospective randomized trials showed that thoracic irradiation combined with chemotherapy resulted in a 5.4% absolute increase in 3-year overall survival compared with chemotherapy alone (80). Cisplatin/etoposide is a regimen commonly combined with radiotherapy for small-cell lung carcinoma.
There has been interest in the inclusion of surgery in the multidisciplinary management of limited-stage small-cell carcinoma; however, prospective studies have shown that few patients are candidates for thoracotomy before or after chemotherapy (38), although surgery may be a reasonable option for very early-stage patients (e.g., T1 — 2, N0), followed by adjuvant chemotherapy (± radiotherapy).
INT 0096 demonstrated superior survival for 45 Gy b.i.d. versus 45 Gy q.d. in limited-stage patients receiving cisplatin/etoposide concurrent with thoracic irradiation (5-year overall survival: 26% versus 16%) but with increased rate of grade 3 esophagitis (27% versus 11%) (106).
The incidence of brain metastasis in small-cell carcinoma of the lung is as high as 50%. The actuarial incidence has been projected to be as high as 80% in patients surviving for 5 years.
Elective whole-brain irradiation decreases the cumulative incidence of intracranial metastasis by about 50% and provides approximately a 5% absolute 3-year overall survival increase in patients in complete remission (5).
Prophylactic cranial irradiation is associated with an increase in 1-year overall survival from 13.3% to 27.1% in extensive-stage patients with a response to chemotherapy (98).
25 Gy in 10 fractions is an appropriate dose for prophylactic cranial irradiation; there does not appear to be a benefit to dose escalation (56).
The volume to be treated and the configuration of the irradiation portals are determined by size and location of the primary tumor/involved lymph nodes, areas of lymphatic drainage (if elective nodal irradiation is delivered), prescription dose, and equipment/beam energies available.
Treatment portals are generally designed with a 1.5- to 2.0-cm margin around the gross tumor volume (GTV) of primary tumor plus involved lymph nodes determined by CT size (short axis diameter greater than 1.0 to 1.5 cm), PET avidity, and/or pathologic sampling positivity.
A significant problem in defining GTV is distinguishing between actual tumor and postob-structive atelectasis or pneumonitis. Significant interclinician variability in contouring target volumes has been reported (95). Consultation with a diagnostic radiologist is invaluable. Imaging modalities such as PET scanning may further improve tumor definition (71).
In a study of lung cancer surgical specimens, Giraud et al. (35) concluded that margins of 6 mm for adenocarcinoma and 8 mm for squamous cell carcinoma around the gross tumor are necessary to include microscopic tumor in 95% patients.
Classic radiotherapy portals incorporating elective nodal irradiation. However, the merit of elective mediastinal (± supraclavicular) nodal irradiation is highly questionable given an elective nodal failure rate of only 5% to 10% in a number of non-small-cell lung cancer series omitting elective nodal irradiation in both early-stage inoperable and locally advanced patients (87, 98). Indeed, involvedfield radiotherapy may facilitate dose escalation to gross disease without unacceptable toxicity (Fig. 22-2). Accordingly, the trend has been to move away from elective nodal irradiation.
For postoperative radiotherapy, the target volume is generally the mediastinum and the ipsilateral hilum, with boost fields to areas of extracapsular nodal extension or positive margins.
3-D CRT allows for more precise targeting of tumor volumes while limiting dose to normal structures, when compared with 2-D techniques (3, 41). Intensity-modulated radiation therapy may further enhance the therapeutic ratio, especially for relatively large or irregularly shaped tumor volumes (101).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree