Chapter 20 Lopinavir
Lopinavir (formerly known as ABT-378) was the first of the ‘second-generation’ protease inhibitors (PIs) to be approved for clinical use. Principles of rational drug design and structure-based design were used to select a compound that is active against ritonavir-resistant isolates of human immunodeficiency virus type 1 (HIV-1). In addition, lopinavir is the only approved antiretroviral agent specifically co-formulated with ritonavir, a PI that inhibits lopinavir metabolism by the cytochrome P450 3A4 enzyme. The combination of lopinavir and ritonavir is marketed under the trade name Kaletra. The initial capsule formulation contained 133.3 mg of lopinavir and 33.3 mg of ritonavir. This was replaced in 2005 with a tablet formulation (often referred to as the Meltrex formulation) containing 200 mg of lopinavir and 50 mg of ritonavir. An oral solution, containing 80 mg of lopinavir and 20 mg of ritonavir per milliliter, is also available. In the absence of other medications that produce pharmacokinetic interactions, the standard dose of lopinavir/ritonavir in adults is 400/100 mg twice daily or 800/200 mg once daily. The use of lopinavir/ritonavir in combination with other antiretroviral agents has produced excellent clinical results in both antiretroviral-naive and antiretroviral-experienced patients.
CHEMICAL STRUCTURE
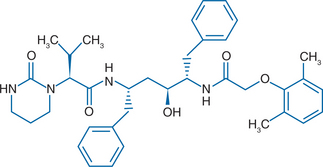
Lopinavir is a C2 symmetrical peptidomimetic inhibitor of HIV-1 protease. Chemically, the drug is designated {1S-[1R*, (R*), 3R*, 4R*]}-N-(4-{[(2,6 dimethylphenoxy)acetyl]amino}-3-hydroxy-5-phenyl-1-(phenylmethyl)pentyl)tetrahydro-a-(1-methylethyl)-2-oxo-1 (2H)-pyrimidineacetamide (Fig. 20-1). It has a molecular weight of 628.80 Da. Development of lopinavir was based on X-ray crystallographic studies of the interaction between the first-generation inhibitor ritonavir and HIV-1 protease.1 Binding of ritonavir to protease is strongly enhanced by interactions between the isopropylthiazolyl side chain of the P3 peripheral heterocyclic group of the molecule and the substrate-binding pocket of protease. Mutations in HIV-1 protease that lead to substitution of alanine, phenylalanine, or tyrosine for the wild-type valine at amino acid 82 disrupt these interactions, leading to a lower affinity for drug binding. To minimize the effect of resistance mutations on PI binding, modifications were introduced to the ritonavir molecule that reduced the importance of hydrophobic interactions with the side chain of Val82. Replacement of the isopropylthiazolyl P3 group with a cyclic urea, and replacement of the P2′ (thiazolyl) methoxycarbonyl moiety with a dimethylphenoxyactyl group produced lopinavir.
IN VITRO ACTIVITY
Lopinavir is a potent inhibitor of HIV-1. The drug inhibits 93% of wild-type protease activity at a concentration of 0.5 nM and binds with a K1 of 1.3 pM.1 Inhibition of HIV-1 protease by lopinavir is highly specific compared to inhibition of mammalian aspartyl proteases such as renin, cathepsin D, and cathepsin E. Lopinavir is ∼10 times more potent than ritonavir against wild-type virus. In the absence of human serum, the mean 50% effective concentration (EC50) of lopinavir ranges from 10 to 27 nM (0.006–0.017 μg/mL) against HIV-1 laboratory strains and from 4 to 11 nM (0.003–0.007 μg/mL) against several clinical strains. The presence of 50% human serum increases the mean EC50 of lopinavir against HIV-1 laboratory strains by seven to 11-fold, resulting in values that range from 65 to 289 nM (0.04–0.18 μg/mL).2
PHARMACOKINETICS
When administered by itself, lopinavir achieves relatively modest plasma concentrations due to rapid metabolism by the cytochrome P450 3A4 enzyme (CYP3A4). The area under the curve (AUC) for lopinavir is greatly increased by concomitant administration of ritonavir, which inhibits the metabolism of lopinavir by CYP3A4 with a KI of 0.013 μM.3 In healthy volunteers, simultaneous administration of a 400-mg dose of lopinavir together with 50 mg of ritonavir increases the lopinavir AUC 77-fold over that achieved with lopinavir alone.1 The AUC of lopinavir varies with the dose of the drug and the dose of concomitant ritonavir. In HIV-infected subjects, a 34% increase in AUC is achieved when lopinavir 400 mg bid is admin-istered with ritonavir 100 mg bid as compared to lopinavir/ritonavir doses of 200/100 mg bid.4
A multiple-dose pharmacokinetic study in HIV-seropositive subjects using the 400/100 mg dose of lopinavir/ritonavir determined that at steady state the peak plasma concentration (Cmax) of lopinavir is 9.8 ± 3.7 μg/mL, and the time to peak concentration (Tmax) is ∼4 h. The minimum steady-state concentration (Cmin) of lopinavir in patients receiving the 400/100 mg dose twice daily is 5.5 ± 2.7 μg/mL, which exceeds the protein-binding-adjusted IC50 for wild-type isolates of HIV-1 by more than 30-fold.2,4 Plasma concentrations of lopinavir and ritonavir after administration of two 200/50 mg lopinavir tablets are similar to three 133.3/33 mg lopinavir/ritonavir capsules taken after food, with less pharmacokinetic variability.2 Once-daily dosing of 800/200 mg of lopinavir/ritonavir produces a mean lopinavir Cmax of 11.8 + 3.7 μg/mL with a Tmax of ∼6 h. The Cmin of lopinavir in patients receiving the 800/200 mg dose once daily is 1.7 ± 1.6 μg/mL. The 100 mg twice-daily or 200 mg once-daily dose of ritonavir achieves drug levels that are less than 7% of those achieved with the standard dose of ritonavir (600 mg bid). Therefore the observed in vivo activity of the lopinavir/ritonavir co-formulation is attributable almost exclusively to the lopinavir component.
Lopinavir is 98-99% bound to plasma proteins, primarily to α1-acid glycoprotein and albumin. The extent of protein binding, which is constant throughout the therapeutic range, is similar in seronegative volunteers and HIV-infected subjects. The drug is extensively metabolized by CYP3A4. Although ritonavir is a potent inhibitor of CYP3A4, it also induces other P450 isozymes, including those responsible for its own metabolism. As a result, lopinavir trough concentrations decline somewhat over time, reaching a steady state within 10–16 days. Following a 400/100 mg dose of lopinavir/ritonavir, ∼10% of administered lopinavir can be recovered from urine and 83% from feces; ∼22% of the drug is excreted unchanged.2 After multiple doses less than 3% of lopinavir is excreted unchanged in the urine. The drug exposure following a standard dose of lopinavir/ritonavir in patients on hemodialysis is similar to patients with normal renal function indicating that dosage adjustments are not necessary in the setting of diminished renal function.5 In patients with HIV, hepatitis-C virus infection, and mild to moderate hepatic impairment, a 30% increase in lopinavir AUC and a 20% increase in Cmax were noted when compared to HIV-infected patients with normal hepatic function.2
The pharmacokinetics of lopinavir/ritonavir have been studied in children aged 6 months to 12 years. A lopinavir dose of 230 mg/m2 combined with a ritonavir dose of 57.5 mg/m2 (using the oral solution) provides lopinavir plasma concentrations similar to those observed in adults receiving the 400/100 mg bid dose of lopinavir/ritonavir, resulting in a mean steady-state AUC of 72.6 ± 31.1 μg h−1 mL−1, a mean Cmax of 8.2 ± 2.9 μg/mL, a mean Ctrough of 4.7 ± 2.9 μg/mL, and a mean Cmin of 3.4 ± 2.1 μg/mL.2,6
For both adults and children, lopinavir/ritonavir drug levels can be altered by co-administration of other drugs that affect hepatic metabolism. For example, if co-administered with nevirapine or efavirenz, inducers of CYP3A4, lopinavir concentrations are reduced. In adults, increasing the dose of lopinavir/ritonavir to 600/150 mg (three tablets) twice daily or 533/133 mg (using the solution) twice daily compensates for this effect. In children when co-administered with either nevirapine or efavirenz, a lopinavir/ritonavir dose of 300/75 mg/m2 provides lopinavir plasma concentrations similar to those observed in adults receiving the 400/100 mg dose without nevirapine or efavirenz.6,7
In 2005, a new tablet formulation of lopinavir/ritonavir containing 200 mg of lopinavir and 50 mg of ritonavir was approved by the FDA. This was accomplished with a novel melt extrusion technology which allows a poorly soluble compound like lopinavir/ritonavir to be dissolved in a polymer in a solvent-free environment. Studies of the new tablet formulation have documented less variability in absorption among study volunteers and also between the fed and fasted state.8 Consequently, the new tablet can be administered with or without food. Other advantages of the new formulation include fewer pills (the standard daily dosing with four tablets is equivalent to six capsules) and the absence of a requirement for refrigeration, the latter an important limitation with the capsules.
EFFICACY
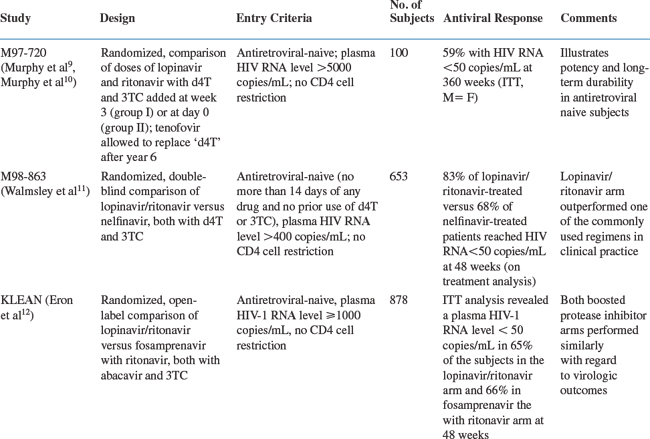
The efficacy of lopinavir/ritonavir has been examined in clinical trials in both antiretroviral-naive and antiretroviral-experienced patients. The results of the most important major clinical trials are reviewed below and summarized in Table 20-1.
The antiviral activity of several dosage levels of lopinavir/ritonavir in combination with stavudine (d4T) and lamivudine (3TC) was tested in a study of 100 antiretroviral-naive adults (study M97-720).9 Subjects initially received (1) one of two dosages of lopinavir/ritonavir (400/100 mg or 200/100 mg twice daily) alone followed by the addition of d4T and 3TC at week 3 (group I) or (2) one of two dosages of lopinavir/ritonavir (400/100 mg or 400/200 mg twice daily) together with d4T and 3TC beginning at day 0 (group II). Baseline characteristics of the enrolled patients included a median baseline CD4+ T-lymphocyte count of 398 and 310 cells/mm3 in groups I and II, respectively, and a mean plasma HIV-1 RNA level of 4.9 log10 copies/mL in both groups. According to an intention-to-treat (ITT) analysis, which counted missing patients as treatment failures, a plasma HIV-1 RNA level of less than 50 copies/mL was achieved in 75% and 79% of patients in groups I and II, respectively, at 48 weeks. The CD4+ T-lymphocyte count increased by ∼200–250 cells/mm3 over this same time period. After 48 weeks, all patients in this study received lopinavir 400 mg twice daily plus ritonavir 100 mg twice daily, along with d4T and 3TC at standard doses and the study was continued to examine the long-term efficacy of this regimen. A modification at year six allowed 37 patients to replace stavudine with tenofovir. At 360 weeks (7 years), 59% of patients have maintained an HIV-1 plasma RNA level <50 copies/mL using an ITT analysis where noncompleters are counted as failures. Using an on-treatment analysis, 95% of patients have an HIV-1 plasma RNA level <50 copies/mL. Over the 7-year study, the mean CD4+ T-lymphocyte count increased by 501 cells/mm3.10
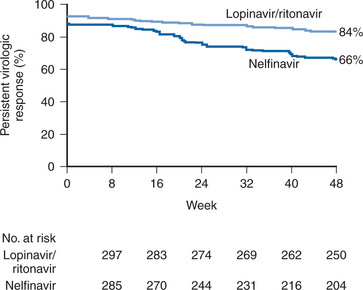
A randomized, double-blind phase III study compared the efficacy of lopinavir/ritonavir with that of nelfinavir in treatment-naive patients (study M98-863).11 Both PIs were used in combination with d4T and 3TC. Lopinavir/ritonavir was dosed at 400/100 mg twice daily; nelfinavir was dosed at 750 mg three times a day, but subjects were allowed to change to 1250 mg twice daily at approximately week 24, when data on twice-daily dosing of nelfinavir became available. At base-line the mean plasma HIV-1 RNA level and CD4+ T-lymphocyte count among the 653 participants were 4.9 log10 copies/mL and 259 cells/mm3, respectively. At 48 weeks, the ITT analysis showed that a plasma HIV-1 RNA level of less than 50 copies/mL was achieved in 67% of subjects on the lopinavir/ritonavir arm versus 52% in the nelfinavir arm. An on-treatment analysis at 48 weeks confirmed the virologic superiority of the lopinavir/ritonavir arm in this study, with 83% of subjects on the lopinavir/ritonavir arm achieving a plasma HIV-1 RNA level of less than 50 copies/mL versus 68% in the nelfinavir arm. This study illustrates the potency of a lopinavir/ritonavir-containing regimen in antiretroviral-naive patients when compared to one of the unboosted PI treatment regimens commonly used in clinical practice at that time (Fig. 20-2).
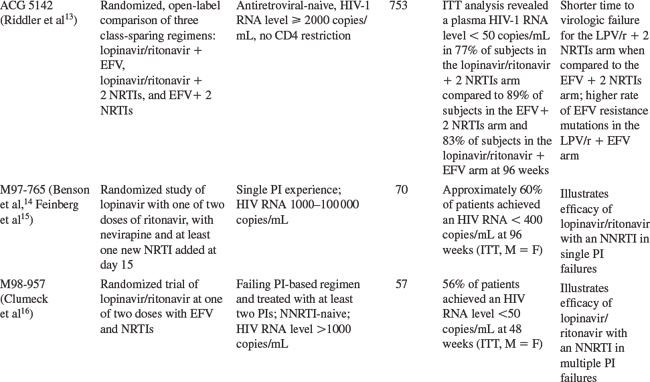
More recently, lopinavir/ritonavir was compared to another boosted PI regimen, fosamprenavir with ritonavir, each with abacavir/lamivudine, in a prospective, randomized, open-label trial in antiretroviral-naive patients. Lopinavir/ritonavir was dosed 400/100 mg twice daily while fosamprenavir and ritonavir were dosed 700 mg twice daily and 100 mg twice daily, respectively. Baseline data for the ITT population of 878 patients included a median plasma HIV-1 RNA level of 5.07 log10 copies/mL and a CD4+ T-lymphocyte count of 192 cells/mm3. At 48 weeks, an ITT analysis revealed a plasma HIV-1 RNA level <50 copies/mL in 65% of the subjects in the lopinavir/ritonavir arm and 66% in the fosamprenavir with ritonavir arm. A prospective, randomized three-arm trial (AIDS Clinical Trials Group 5142) compared lopinavir/ritonavir with two nucleoside reverse transcriptase inhibitors (NRTIs) versus efavirenz with two NRTIs versus lopinavir/ritonavir and efavirenz without NRTIs. Baseline data for the 753 subjects included a median plasma HIV-1 RNA level of 5 log10 copies/mL and a CD4+ T-lymphocyte count of 182 cells/mm3. At 96 weeks, an ITT analysis revealed a plasma HIV-1 RNA level <50 copies/mL in 77% of subjects in the lopinavir/ritonavir with two NRTIs arm compared to 89% of subjects in the efavirenz with two NRTIs arm and 83% of subjects in the lopinavir/ritonavir with efavirenz arm (P = 0.003 for the comparison of the efavirenz with two NRTIs arm versus the lopinavir/ritonavir with two NRTIs arm). The time to virologic failure was significantly shorter for the lopinavir/ritonavir with two NRTIs arm when compared to the efavirenz with two NRTIs arm (P = 0.006). In addition, there was a trend for more efavirenz resistance mutations in the lopinavir/ritonavir with efavirenz arm when compared to the efavirenz with two NRTIs arm.13
The activity of lopinavir/ritonavir in patients failing antiretroviral therapy that included a PI with two NRTIs was evaluated in study M97-765.14,15 Patients failing their first PI-based regimen with plasma HIV-1 RNA levels between 1000 and 100 000 copies/mL were eligible for study if they were naive to NNRTIs and to at least one NRTI. Patients received lopinavir 400 mg twice daily plus ritonavir at a dose of either 100 or 200 mg twice daily for 15 days, after which nevirapine along with at least one new NRTI were added. Seventy patients enrolled in this study. The median baseline CD4+ T-lymphocyte count and plasma HIV-1 RNA level were 349 cells/mm3 and 4.0 log10 copies/mL, respectively. Prior PI use included indinavir (44%), nelfinavir (36%), saquinavir (13%), ritonavir (6%), and amprenavir (1%). Phenotypic resistance testing at study entry revealed that viruses from 32% of patients had a fourfold or more loss in susceptibility to three or more PIs. No significant difference in treatment response was observed between the two groups. At week 2, prior to the addition of nevirapine, 80% of patients had either a more than 1 log10 copies/mL decrease in plasma HIV-1 RNA level or achieved a plasma HIV-1 RNA level of less than 400 copies/mL. The ITT analysis at 96 weeks for the two groups combined showed that ∼60% of patients achieved a plasma HIV-1 RNA of less than 400 copies/mL.15 These results demonstrate the efficacy of lopinavir/ritonavir in antiretroviral-experienced patients. However, patients in this study had not received more than one other PI, and other approved PIs have also been effective in this setting. Moreover, activity of the treatment regimen was bolstered by adding two other effective drugs (nevirapine and a previously unused NRTI).
The use of lopinavir/ritonavir in patients with a greater degree of PI experience was assessed in another study (M98-957).16 Patients with plasma HIV-1 RNA levels of more than 1000 copies/mL on a failing PI regimen who had been treated with at least two PIs were eligible for study if they were naive to NNRTIs. At baseline, the median plasma HIV-1 RNA level was 4.5 log10 copies/mL. Altogether, 68% of baseline viral isolates demonstrated a fourfold or more loss in susceptibility to three or more other PIs. Patients were randomized to receive lopinavir/ritonavir at a dose of 400/100 mg twice daily (n = 29) or 400/100 mg twice daily for 14 days, after which the dose was increased to 533/133 mg twice daily (n = 28). All patients also received efavirenz (600 mg daily) beginning at day 0. Twenty-five percent of patients also received at least one new NRTI during the first 8 weeks of the study. After week 24 the dose of lopinavir/ritonavir was changed to 533/133 mg twice daily in all patients. This change in dosing was prompted by the observation that lopinavir trough levels and the lopinavir AUC were reduced in patients who received lopinavir/ritonavir together with efavirenz, which induces lopinavir/ritonavir metabolism. The ITT analysis (missing = failure) at 48 weeks demonstrated that 56% of patients achieved a plasma HIV-1 RNA level of less than 50 copies/mL. Successful suppression of plasma HIV-1 RNA was achieved in most patients even though the mean susceptibility of lopinavir among baseline viral isolates was 16-fold higher than that of the wild-type virus. Results of this study support the concept that resistance to PIs can be overcome in part by increasing drug exposure through ritonavir-mediated inhibition of CYP3A4. Lopinavir/ritonavir was compared to atazanavir with ritonavir and atazanavir with saquinavir in a randomized, open-label 48 week trial in patients who had failed at least two prior antiretroviral regimens.17 In this study, 358 patients with a baseline HIV RNA level >1000 copies/mL and a CD4+ T-lymphocyte count >50 cells/mm3 were randomized 1:1:1 to atazanavir 300 mg once daily with ritonavir 100 mg once daily, atazanavir 400 mg once daily with saquinavir 1200 mg once daily, or lopinavir/ritonavir 400/100 mg twice daily, each with tenofovir and one NRTI. At week 48, the primary efficacy endpoint (plasma HIV-1 RNA reduction assessed by time-averaged difference) was similar in the lopinavir/ritonavir and atazanavir with ritonavir arms; the atazanavir with saquinavir arm was inferior. Mean reductions from baseline in HIV-1 RNA levels at 48 weeks were 1.93 log10 copies/mL in the atazanavir with ritonavir arm, 1.55 log10 copies/mL in the atazanavir with saquinavir arm, and 1.87 log10 copies/mL in the lopinavir/ritonavir arm.
A three-arm study compared lopinavir/ritonavir 400/100 mg twice-daily to two different dosing regimens of fosamprenavir and ritonavir, each with two NRTIs, in PI-experienced patients. Fosamprenavir was dosed at 700 mg twice daily with ritonavir 100 mg twice daily in one arm and 1400 mg once daily with ritonavir 200 mg once daily in the other arm. At 48 weeks, an HIV-1 RNA level <50 copies/mL was achieved in 50% of subjects in the lopinavir/ritonavir arm compared to 46% of subjects in the twice-daily fosamprenavir with ritonavir arm and only 37% of subjects in the once-daily fosamprenavir with ritonavir arm.2,18
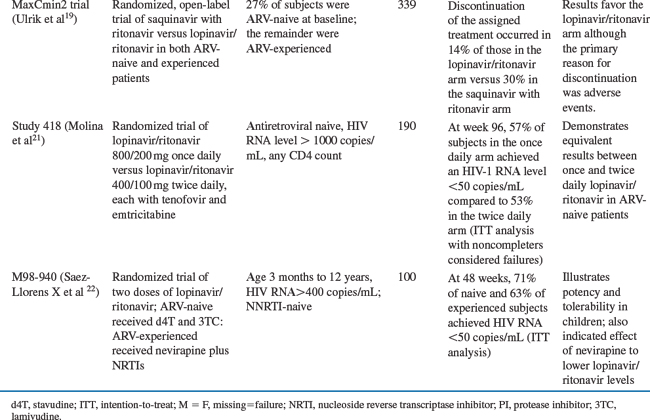
A randomized, open-label study compared lopinavir/ritonavir to another ritonavir-boosted PI, saquinavir with ritonavir, each administered with two or more NRTIs, in both antiretroviral-naive and antiretroviral-experienced subjects (the MaxCmin2 trial). Lopinavir/ritonavir was dosed at the standard dose of 400/100 mg twice daily whereas saquinavir was dosed at 1000 mg twice daily with 100 mg of ritonavir twice daily. The 48-week analysis, by ITT, revealed treatment failure in 29/163 (18%) subjects in the lopinavir/ritonavir arm versus 53/161 (33%) in the saquinavir with ritonavir arm. The primary reason for treatment discontinuation in both arms was adverse events, which occurred more commonly in the saquinavir with ritonavir arm.19
When lopinavir/ritonavir was initially approved by the FDA, it was administered twice daily. A small randomized, open-label trial involving 38 antiretroviral-naive patients found comparable virologic results between 800/200 mg once-daily dosing and 400/100 mg twice-daily dosing.20 These data led to a larger randomized, open-label trial comparing lopinavir/ritonavir 800/200 mg once daily compared with 400/100 mg twice daily, each with tenofovir and emtricitabine, in antiretroviral-naive subjects with a baseline HIV-1 RNA level subjects with a baseline HIV-1 RNA level >1000 copies/mL and any CD4+ T-lymphocyte count.21 At baseline the median HIV-1 RNA levels were 4.8 and 4.6 log10 copies/mL in the once-daily and twice-daily arms, respectively. The median baseline CD4+ T-lymphocyte counts were 214 and 232 cells/mm3 in the once-daily and twice-daily arms, respectively. Patients were randomized 3:2 with 115 patients receiving once-daily therapy and 75 patients receiving twice-daily therapy. At week 96, 57% of subjects achieved an HIV-1 RNA level <50 copies/mL in the once-daily arm compared to 53% in the twice-daily arm (ITT analysis with noncompleters considered failures). Diarrhea was more common in the once-daily arm. This study led to the approval of lopinavir/ritonavir for once-daily dosing in antiretroviral-naive patients. Two dosage levels of lopinavir/ritonavir have been studied in treatment-naive and treatment-experienced HIV-1-infected children (study M98-940).22 Lopinavir/ritonavir was administered every 12 h at a dose of 230/57.5 mg/m2 or 300/75 mg/m2 in combination with d4T and 3TC (for antiretroviral-naive children) or together with nevirapine and one or two NRTIs (for antiretroviral-experienced patients). After 12 weeks, all patients received the 300/75 mg/m2 dose. A total of 100 patients were enrolled in this study. At entry, the median plasma HIV-1 RNA level was 4.9 log10 copies/mL in the antiretroviral-naive group (n = 44) and 4.5 log10 copies/mL in the antiretroviral-experienced group (n = 56). An ITT analysis at 48 weeks showed that 84% of antiretroviral-naive subjects and 75% of antiretroviral-experienced subjects achieved a plasma HIV-1 RNA levels of less than 400 copies/mL; 71% and 63%, respectively, had plasma HIV-1 RNA levels of less than 50 copies/mL.
Novel Treatment Strategies
The use of lopinavir/ritonavir has been studied in several novel treatment strategies including monotherapy (either as initial therapy or following simplification from a combination regimen) and as part of nucleoside analog-sparing regimens. In one small study, 30 patients with a mean baseline HIV-1 RNA of 262 000 copies/mL and a CD4+ T-lymphocyte count of 169 cells/mm3 began monotherapy with lopinavir/ritonavir dosed at either 400/100 mg or 533/133 mg twice daily. Of the 19 who completed the 48-week study, all 19 achieved an HIV-1 RNA level <400 copies/mL; 16/19 achieved an HIV-1 RNA level of <50 copies/mL (54% by an ITT analysis where noncompleters are considered failures).23 The MONARK study randomized antiretroviral-naive subjects to either lopinavir/ritonavir monotherapy (n = 83) or lopinavir/ritonavir with zidovudine and lamivudine (n = 53). This study favored combination therapy; an on-treatment analysis at 48 weeks revealed a plasma HIV-1 RNA level of less than 50 copies/mL in 84% of subjects in the lopinavir/ritonavir monotherapy arm compared to 98% of subjects in the combination therapy arm.24 In a study evaluating treatment simplification to boosted PI monotherapy, 42 patients without prior PI treatment failure, on lopinavir/ritonavir with two NRTIs, and with a plasma HIV-1 RNA level less than 50 copies/mL for at least 6 months, were randomized to either simplification to monotherapy with lopinavir/ritonavir 400/100 mg twice daily or continuation of the three-drug regimen. At 48 weeks after the switch, 17/21 (81%) of patients in the monotherapy arm maintained an HIV-1 RNA level <50 copies/mL.25 A number of other studies have evaluated this approach.26–28 However, PI monotherapy as initial therapy or as a simplification strategy should still be viewed as investigational.
Combination of PIs with NNRTIs (a nucleoside-sparing approach) is a possible treatment strategy that has been investigated in several recent trials.13,29 In the recent ACTG trial 5142, reviewed above, an ITT analysis at 96 weeks revealed a plasma HIV-1 RNA level <50 copies/mL in 83% of subjects in the lopinavir/ritonavir and efavirenz arm compared to 77% of subjects in the lopinavir and two NRTIs arm and to 89% of subjects in the efavirenz and two NRTIs arm. There was a trend towards higher rates of efavirenz resistance in the nucleoside-sparing (lopinavir/ritonavir with efavirenz) arm when compared to the efavirenz with two NRTIs arm.13 In another trial involving 31 antiretroviral-experienced patients with prolonged viral suppression, the combination of lopinavir/ritonavir with nevirapine was compared to lopinavir/ritonavir with two NRTIs. At 48 weeks, all patients had maintained their virologic suppression.29
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree