Fig. 15.1
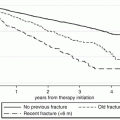
Decrease in absolute risk of new vertebral fractures in patients treated with risedronate in two different clinical trials (left panel) and zoledronate in the same clinical trial (right panel). (A) VERT-NA; (B) VERT-MN; (C) HORIZON, patients with no prevalent vertebral fractures; (D) HORIZON, patients with ≥2 prevalent vertebral fractures; RRR relative risk reduction, NNT number needed to treat; note the difference in NNT for the same compound in two different clinical trials or in one clinical trial depending on baseline fracture risk. From: Appelman-Dijkstra NM, Papapoulos SE. Prevention of incident fractures in patients with prevalent fragility fractures: Current and future approaches. Best Practice and Research Clinical Rheumatology 2013; 27:805–820
The evidence of the antifracture efficacy of pharmacological interventions varies among approved agents, and for treatment decisions, the highest level of available evidence should be selected. Properly designed and performed RCTs and meta-analyses of RCTs are at the top of the hierarchy of evidence. RCTs and meta-analyses provide different perspectives. RCTs address a specific question in a given population, whereas the primary purpose of meta-analysis is to synthesize information from prior studies and provide an estimate of the magnitude of the effect of treatment .
Benefits and Risks of Treatments
An evidence-based approach to treatment of the individual patient with osteoporosis involves the use of the best data available from clinical studies combined with clinical judgment and the patient’s preferences and values. The final decision, however, strongly depends on the balance between the benefit and the harm of a given intervention. The fact that any medication, even OTC preparations such as acetaminophen or NSAIDs, may have very serious adverse consequences is frequently overlooked. The best way to achieve zero risk would be never to take a drug, a decision that should be weighed against the price of ignoring the benefit [5]. The benefit of antiosteoporotic treatments is the reduction of the risk of fractures at all skeletal sites including the hip which has the most devastating clinical consequences. In addition, potential extra-skeletal beneficial effects should be considered, but this rarely occurs in clinical practice. It should be appreciated that the true risk of any treatment should be calculated as a fraction, the numerator of which is the number of patients with a given adverse effect and the denominator is the total number of patients who used the medication over the same period of time. Consequently, adverse events are classified as common (1–10 %), uncommon (0.1–1 %), rare (0.01–0.1 %), and very rare (less than 0.01 % or less than 1:10,000). This transparent expression of the risk of a treatment differs from the fear generated by reports in the media of very rare events that may affect the willingness of patients to take or continue a medication but also of physicians to prescribe the medication. Such fear has contributed to the gradual fall of sales of antiosteoporotic agents, particularly bisphosphonates, in recent years [6] despite their efficacy and the generally recognized low uptake of treatments by patients with increased risk of fractures [7, 8]. For example, in the USA, the use of antiosteoporotic medications within one year after a hip fracture fell from 40.2 % in 2002 to 20.5 % in 2011 [9].
There are several examples of the importance of the estimation of the benefits and harms for the approval and use of agents for the management of osteoporosis. Tibolone , an agent with estrogenic, progestogenic, and androgenic effects, has been used for many years for the control of menopausal symptoms and prevention of osteoporosis. In an RCT of 4538 women with postmenopausal osteoporosis, tibolone reduced the risk of vertebral and non-vertebral fractures by 45 % and 26 %, respectively, after a median period of 34 months [10]. In addition, compared with placebo, tibolone reduced the rate of invasive breast cancer by 68 % and that of colon cancer by 69 %, without an increase in the incidence of thromboembolism or coronary heart disease. Women who received tibolone were more likely to report vaginal bleeding, endometrial thickness, weight increase, and increases in liver enzymes. However, because of a significantly increased risk of stroke in women treated with tibolone compared with those treated with placebo [HR 2.19 (1.14–4.23)], the study was discontinued. This example, illustrates the importance of the assessment of the risk–benefit balance for the approval of medications for the treatment of osteoporosis. The same also applies to already approved medications for the treatment of osteoporosis, such as intranasal calcitonin which was withdrawn from the market in Europe due to an unfavorable risk–benefit profile.
The risk–benefit balance has also been used to better position approved medications in the treatment of osteoporosis. Hormonal treatment, the dominant intervention for prevention and treatment of osteoporosis in the past, is not any more considered first line of therapy based mainly on the results of the Women’s Health Initiative study [11]. In this study, the beneficial effect of hormonal therapy on fractures, including those of the hip, and colon cancer was thought not to clearly exceed the risks of treatment (Fig. 15.2). Another example is the restriction of the use of strontium ranelate , an agent shown to reduce the risk of vertebral and non-vertebral fractures in women with postmenopausal osteoporosis by 24 and 15 % after 5 years [12]. In 2014, the Committee for Medicinal Products for Human Use (CHMP) in Europe recommended that strontium ranelate should only be used to treat severe osteoporosis in postmenopausal women and men at risk of fractures, for which treatment with other medicinal products approved for the treatment of osteoporosis is not possible due to, for example, contraindication or intolerance. Strontium ranelate should further not be used in patients with established, current, or past history of ischemic heart disease, peripheral arterial disease, and/or cerebrovascular disease or uncontrolled hypertension. Decisions should be made on the assessment of the individual patient’s risk [13]. This recommendation was based on the assessment of the risk for myocardial infarction and venous, thrombotic, and embolic events in postmenopausal women with osteoporosis treated with this agent.
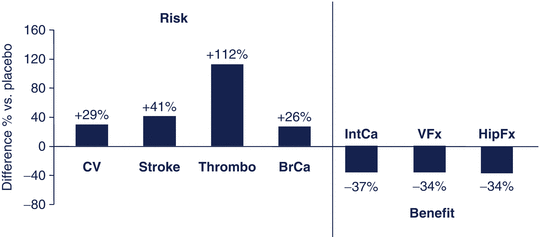

Fig. 15.2
Benefits and risks of HRT relative to placebo in postmenopausal women aged 50–79 years in the Women’s Health Initiative Study. CV cardiovascular events, thrombo thromboembolism, BrCa breast cancer, IntCa intestinal cancer, VFx vertebral fractures, HipFx hip fractures. Data from JAMA 2002;288: 321–333
There are currently four agents that have been shown to reduce the risk of all osteoporotic fractures with variable efficacy; the bisphosphonates alendronate, risedronate, and zoledronate and the RANKL inhibitor denosumab. All these are inhibitors of bone resorption and turnover. Long-term studies are available for these agents and help to better formulate therapeutic decisions. In the absence of head-to-head studies with fracture endpoints, these data together with the safety profiles of these agents should be considered in tailoring therapeutic choices to individuals at risk. Long-term data are also available for other agents [14, 15], but due to either restrictions in their use (Strontium Ranelate) or lack of efficacy in reducing the risk of non-vertebral and hip fractures (SERMs), these as well as PTH peptides, the use of which is restricted to 18–24 months, will not be further discussed.
Rationale for the Use of Inhibitors of Bone Turnover
The definition of osteoporosis, as formulated more than 20 years ago, recognized that low bone mass is not the only determinant of bone fragility and that the strength of the skeleton depends also on other properties of the bone tissue, collectively termed bone quality. As with other materials, the structure and material composition of bone together with its mass will determine its ability to resist structural failure. Bone is, however, different from other materials due to its ability to be continuously renewed throughout life by the process of bone remodeling. The generally higher rates of bone remodeling in osteoporosis combined with the negative balance between bone formation and bone resorption at the basic multicellular unit (BMU) lead to loss of bone mass, an increased number and depth of resorption cavities, perforation of trabecular plates, and loss of trabecular elements of cancellous bone and thinning and porosity of cortical bone [16–19]. They also reduce the degree of mineralization and of the amount of collagen of the bone matrix and may also impair the maturation and cross-linking of collagen fibers [19]. Thus, mass, structure, and material composition of bone can all be affected in patients with osteoporosis compromising bone strength and increasing bone fragility. Currently available inhibitors of bone turnover reduce the rate of bone resorption to different degrees depending on the potency and mechanism of action of the different classes of agents. The decrease of bone resorption is invariably followed by a decrease in the rate of bone formation leading to an overall lower rate of bone turnover. These actions have beneficial effects on bone strength by reducing the remodeling space, maintaining or sometimes improving trabecular or cortical architecture, correcting the hypomineralization of bone tissue, and increasing bone mineral density. The clinically relevant outcome is the reduction in the risk of fractures.
Bisphosphonates
The Benefit
Bisphosphonates reduce the rate of bone resorption and turnover leading after 3–6 months to a new steady state of lower rate of bone turnover that is maintained for at least 10 years of continuous treatment [20]. This response illustrates, in addition, that the accumulation of bisphosphonate in the skeleton is not associated with cumulative effects on bone remodeling. The importance of the reduction of bone resorption and turnover for the antifracture efficacy of bisphosphonates has been suggested by meta-analysis of results of clinical trial [21] and was demonstrated for alendronate by analysis of individual patient data from the Fracture Intervention Trial (FIT) [22]. Bisphosphonates have also been suggested to prolong the life span of osteocytes by reducing their rate of apoptosis by a mechanism different from that of their action in osteoclasts [23].
Antifracture Efficacy of Oral Bisphosphonates
Vertebral fractures, the most representative osteoporotic fractures, are a key outcome in studies of antiosteoporotic treatments. They are less influenced by extrinsic factors (e.g., falls), they increase the risk of new clinical fractures, and they result in more frequent hospitalizations and are associated with increased mortality. The efficacy of alendronate in reducing the risk of vertebral fractures was examined in the FIT study. In the vertebral fracture arm of this trial (FIT 1), postmenopausal women with femoral BMD T-score < −1.6 and at least one prevalent vertebral fracture were assigned to placebo (n = 1005) or alendronate (n = 1022) for 3 years [24]. The clinical fracture arm (FIT2) included women with femoral neck BMD T-score < −1.6 but without vertebral fractures at baseline of whom 2218 received placebo and 2214 received alendronate for 4 years [25]. The dose of alendronate was initially 5 mg/day and was increased to 10 mg/day after 2 years because other studies suggested that this dose had greater effects than 5 mg on BMD and bone markers with similar tolerability. Spine radiographs were obtained after 2 and 3 years in FIT 1 and after 4 years in FIT2. Alendronate reduced the incidence of new vertebral fractures by 47 % and 44 % in FIT1 and FIT2, respectively.
The VERT study was the pivotal trial that examined the efficacy of risedronate in reducing the risk of vertebral fractures. In VERT North America (NA), women with two or more prevalent vertebral fractures or one prevalent vertebral fracture and low lumbar spine BMD were assigned to placebo (n = 820) or risedronate 5 mg/day (n = 821); in a third group that received risedronate 2.5 mg/day, treatment was discontinued after the first year because data from other trials indicated that this dose was less effective than the 5 mg/day dose [26]. Spine radiographs were obtained after 1, 2, and 3 years. Compared with placebo, risedronate reduced the incidence of vertebral fractures by 41 % after 3 years. VERT International (VERT-MN) included women at higher risk (2 or more prevalent vertebral fractures) who were assigned to placebo (n = 407) or risedronate 5 mg/day (n = 407); a third group that received risedronate 2.5 mg/day was discontinued after 2 years [27]. In this study, risedronate reduced the risk of vertebral fractures by 49 % after 3 years.
The consistency of the efficacy of alendronate and risedronate in reducing the risk of vertebral fractures has been demonstrated by meta-analyses of RCTs [28, 29].
The impact of clinical fractures on morbidity, hospitalization, mortality, and health-care costs is immediately obvious. Furthermore, the time of occurrence is easily determined as all these fractures require medical attention and radiographs are made. A treatment effect on the risk of non-vertebral fractures is, however, more difficult to demonstrate, and in no study of oral bisphosphonates was the incidence of non-vertebral fractures a primary efficacy point. Clinical fractures, that included non-vertebral and vertebral fractures, were a primary efficacy outcome in FIT2. A large study with risedronate (HIP) was the only one to assess the efficacy of an oral bisphosphonate on the risk of hip fractures as primary endpoint of the trial [30]. It should be noted that non-vertebral fractures are strongly influenced by extra-skeletal factors, such as trauma, and their definition in clinical trials varies among studied agents which may influence the outcome.
Alendronate reduced the risk of non-vertebral fractures by 20 % and 12 % in FIT1 and FIT2, respectively, both nonsignificant risk reductions. However, in FIT1, alendronate reduced significantly the risk of hip fractures by 51 % and those of the wrist by 48 %, and in post hoc analysis of FIT2, it decreased the risk of any clinical fracture by 34 % and of hip fractures by 56 % in women with osteoporosis (T-score < −2.5). In a preplanned pooled analysis of women with osteoporosis (prevalent vertebral fracture or BMD T-score < −2.5) of the combined FIT cohorts, alendronate reduced significantly the incidence of non-vertebral fractures by 27 %, of non-vertebral osteoporotic fractures by 36 %, and of hip fractures by 53 % [31].
In the VERT-NA study, risedronate 5 mg/day reduced significantly the cumulative incidence of non-vertebral osteoporotic fractures by 39 % and in the VERT-MN by 33 %, a nonsignificant reduction. The HIP study was designed to assess the efficacy of risedronate on the risk of hip fractures and included 9331 patients, 5445 women 70–79 years old with osteoporosis (femoral neck BMD T-score < −4.0 or <−3.0 plus a nonskeletal risk factor for hip fracture, e.g., poor gait or propensity to fall) and 3886 women ≥80 years old with at least one nonskeletal risk factor for hip fracture or low BMD; the latter group comprised only 16 % of this cohort [30]. The women were randomly assigned to receive treatment with risedronate 2.5 or 5.0 mg/day or placebo for 3 years. In this study, the combined effect of the two risedronate doses was estimated. Compared with placebo, risedronate reduced significantly the incidence of non-vertebral fractures by 20 % due to the effect of the bisphosphonate in the women selected on the basis of osteoporosis. In a post hoc analysis of women with osteoporosis who had, in addition, prevalent vertebral fractures, the risk of non-vertebral osteoporotic fractures was significantly reduced by 30 %. In the whole population of the study, risedronate decreased significantly the incidence of hip fractures by 30 %, 40 % in the women with osteoporosis (p = 0.009), and 20 % in the older women with risk factors (p = 0.35).
Post hoc analyses, pooled analyses, and meta-analyses of the incidence of non-vertebral and hip fractures during treatment with alendronate and risedronate have been performed [32–37]. Overall, results confirmed the significant effect of these two bisphosphonates in reducing the risk of non-vertebral and hip fractures. For example, a meta-analysis of the Cochrane Collaboration of the efficacy of alendronate and risedronate to reduce the incidence of non-vertebral and hip fractures in women with osteoporosis reported significant reductions of 23 % (RR 0.77; 95 % CI 0.74–0.94) and 53 % (0.47; 95 % CI 0.46–0.85), respectively, for alendronate and 20 % (RR 0.80; 95 % CI 0.72–0.90) and 26 % (0.74; 95 % CI 0.59–0.94), respectively, for risedronate.
Daily administration of bisphosphonates, though highly efficacious, is inconvenient because of strict dosing instructions and may also be associated with gastrointestinal adverse effects. These reduce adherence to treatment and can diminish the therapeutic potential of bisphosphonates [38]. To overcome these problems, more convenient once-weekly formulations, the sum of seven daily doses, have been developed for alendronate and risedronate and shown to be pharmacologically equivalent to daily formulations and to significantly improve patient adherence to treatment [39–42]. For risedronate, other preparations are also available; a once-monthly preparation of 150 mg (or 75 mg given on two consecutive days once a month) and a 35 mg once-weekly preparation that can be taken after breakfast [43–45].
Antifracture Efficacy of Intravenous Zoledronate
The efficacy of zoledronate in reducing the incidence of osteoporotic fractures was examined in the HORIZON-PFT trial [46]. Postmenopausal women with osteoporosis (femoral neck BMD T-score ≤ −2.5 with or without prevalent vertebral fractures or T-score ≤ −1.5 with at least one moderate or two mild vertebral fractures) were randomized to receive a single 15-min infusion of zoledronate 5 mg (n = 3889) or placebo (n = 3876) at baseline, at 12 months, and at 24 months. New vertebral fractures (in patients not taking concomitant osteoporotic medications) and hip fractures (in all patients) were primary endpoints, while non-vertebral fractures were a secondary efficacy endpoint. Spine radiographs were taken annually. Compared with placebo, zoledronate reduced the incidence of vertebral fractures by 70 % and that of hip fractures by 41 % after 3 years. The risk of non-vertebral fractures was also significantly reduced by 25 %.
In the HORIZON-RFT [47], men and women with a hip fracture were randomized to receive yearly intravenous zoledronate 5 mg (n = 1065) or placebo (n = 1062) within 90 days after surgical repair of the fracture. Patients received also a loading dose of vitamin D (50,000–125,000 IU) 14 days before the first infusion of the bisphosphonate if serum 25-OHD was ≤15 ng/ml or if the level was not available. Thereafter, they received vitamin D 800–1200 IU/day and calcium 1000–1500 mg/day. After a median follow-up of 1.9 years, zoledronate decreased the risk of any clinical fracture by 35 % (HR 0.65; 95 % CI 0.50–0.84), of vertebral fractures by 46 % (HR 0.54; 0.32–0.92), of non-vertebral fractures by 27 % (HR 0.73; 95 % CI 0.55–0.98), and of hip fractures by 30 % (HR 0.70; 0.41–1.19). This is a unique study because patients were not selected by the level of BMD or the presence/absence of vertebral fractures; only 41 % of patients had a BMD T-score < −2.5 and 35.3 % had osteopenia, while in 11.4 % BMD was normal and in 12.1 % of patients BMD data were not available. The results of this prospective study demonstrated that not only patients with prevalent vertebral fractures but also patients with hip fractures should receive treatment independently of the level of BMD.
Extra-skeletal Effects of Bisphosphonates
Extra-skeletal effects of bisphosphonates include improvement of aspects of the quality of life, reduction of the incidence of certain cancers, and, more importantly, reduction of the risk of dying. These benefits of bisphosphonate treatment are rarely considered in the choice of a treatment in clinical practice. Alendronate was shown in the FIT trial to reduce the number of days of bed disability and days of limited activity caused by back pain [48]. Similarly, in the HORIZON-PFT trial, zoledronate reduced significantly the number of days that patients reported back pain and limited activity and bed rest due to a fracture [49]. In the HORIZON-RTF trial, compared with placebo, zoledronate decreased significantly all-cause mortality by 28 %. This is a remarkable result, the underlying mechanism of which is unclear at present, but it was not related to the reduction in the incidence of fractures. It appears that the efficacy of bisphosphonates in reducing mortality is not restricted to zoledronate. Reduction of mortality has also been reported in different cohorts, but not in RCTs, of patients treated with other bisphosphonates [50–52]. In addition, reduction of the incidence of colon cancer and mortality rate, once colon cancer is diagnosed, was reported in alendronate-treated patients [53]. The occurrence of heart failure was investigated in a cohort study of 102,342 bisphosphonate users compared with 307,026 age- and gender-matched controls from the general population [54]. Alendronate users in this cohort showed a dose-dependent, significant reduction in the risk of heart failure. Moreover, in a post hoc analysis of a prospective cohort study of 19281 patients with rheumatoid arthritis in the USA, the adjusted risk of myocardial infarction was 0.72 (0.54–0.96, p = 0.02) in bisphosphonate users compared with nonusers [55]. A reduction in the risk of myocardial infarction and strokes was also reported in patients with fractures treated with bisphosphonates [56, 57].
Long-Term Effects on Bone Fragility
Skeletal fragility on long-term bisphosphonate therapy has been examined in extensions of four clinical trials (VERT-MN with risedronate, Phase III and FIT with alendronate, and HORIZON with zoledronate) (Fig. 15.3). None of these extension studies were specifically designed to assess antifracture efficacy, but rather safety and efficacy on surrogate endpoints as well as consistency of the effect of bisphosphonates over longer periods were evaluated. Fractures were, however, collected in all studies (Table 15.1).
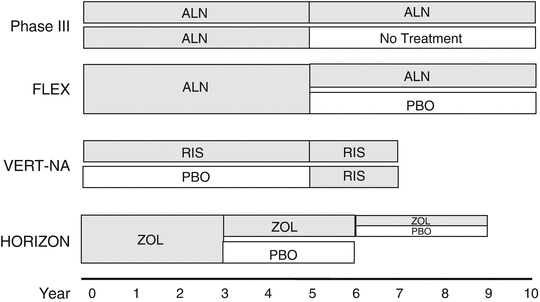

Fig. 15.3
Schematic presentation of the design of long-term controlled studies of bisphosphonates in osteoporosis. ALN alendronate, RIS risedronate, ZOL zoledronate, PBOplacebo
Table 15.1
Incidence of fractures in long-term studies of bisphosphonates
Study | Patients (nr) | Treatment | Years BP | VFx | NVFx (%) | HFx (%) |
|---|---|---|---|---|---|---|
Phase III | 286 | ALN 10 mg/day | 10 | 6.6 % | 8.1a | n.a. |
ALN | 5 | 5.0 % | 12a | n.a. | ||
FLEX | 1099 | ALN | 10 | 2 %b | 19 | 3 |
ALN/PBO | 5 | 5 %b | 20 | 3 | ||
VERT-NA | 136 | RIS | 7 | 3.8 %/year | 6c | n.a. |
PBO/RIS | 2 | 3.8 %/year | 7.4c | n.a. | ||
HORIZON | 1283 | ZOL | 6 | 3.0 %d | 8.2 | 1.3 |
ZOL/PBO | 3 | 6.2 %d | 7.6 | 1.4 |
The first study consisted of two 2-year extensions of the VERT-MN trial [58, 59]. During the first 5 years of the study, two groups of osteoporotic women received either placebo or risedronate 5 mg/day, while in the following 2 years, all patients received active treatment. The annualized incidence of new vertebral fractures during years 6 and 7 was similar in patients who received placebo previously and those who received risedronate continuously (3.8 %/year); the incidence of vertebral fractures in the risedronate group was similar to that observed in years 0–3 (4.7 %/year) and years 4–5 (5.2 %/year). Moreover, the percentage of women with non-vertebral fractures was not significantly different between the two groups during years 6–7 (7.4 vs. 6.0 %). These results indicate consistency of the effect of risedronate on the incidence of fractures with time. Apart from the lack of a placebo group during the whole observation period, an additional limitation of this, as well as of other extension studies, is the substantial decrease in the number of participants with time. For example, 814 patients were initially randomized of whom 473 completed the first 3 years; of these, 260 entered the first extension (4–5 years), 164 the second extension (6–7 years), and 136 completed the study. While loss of patients in such extension studies is expected by the length of the study and the aging of the participants, results should be interpreted with caution.
The second study was an extension of the Phase III clinical trial originally reported by Liberman et al. with alendronate [20]. Patients received alendronate either 5 or 10 mg/day continuously for 10 years or 20 mg/day for 2 years, followed by 5 mg/day for 3 years (providing a total dose equivalent to 10 mg/day for 5 years). The rate of non-vertebral fractures in the pooled alendronate group during years 0–3 was 8.5 %; during years 6–10, this was 11.5 % in patients on 5 mg/day and 8.1 % in those on 10 mg/day, similar, thus, to the initial rates and lower than the estimated rates of the original placebo group adjusted for the effect of aging on the risk. In this study, 247 women of 482 originally assigned to alendronate treatment participated in all three extensions. These results supported the consistency of the long-term effect of alendronate on bone fragility.
In the extension of the FIT trial (FLEX), 1099 patients who participated in the FIT and received on average alendronate for 5 years were randomized to placebo, alendronate 5 mg/day, or alendronate 10 mg/day and were followed for another 5 years [60]. At the end of the 10-year observation period, the incidence of non-vertebral and hip fractures in the ALN/PBO group was similar to that in the ALN/ALN groups (20 % vs. 19 % and 3 % vs. 3 %, respectively), but the incidence of clinical vertebral fractures was significantly reduced in the ALN/ALN groups compared with the ALN/PBO group (2 % vs. 5 %). A post hoc analysis of the FLEX study reported a significant relationship between BMD values at the start of the 5-year extension and incidence of non-vertebral fractures at the end of the study in women with no prevalent vertebral fractures [61]. Compared with placebo, patients on alendronate who entered the extension with BMD T-score ≤ −2.5 showed a significant 50 % decrease in the incidence of non-vertebral fractures (RH 0.50; 95 % CI 0.26–0.96), while those who entered the extension with BMD T-score >2.0 showed a 41 % nonsignificant increase. Except BMD and age, no other clinical or biochemical characteristic could identify patients who would benefit from continuation of treatment with alendronate beyond 5 years [62]. In a further analysis, Black et al. reported that patients with vertebral fractures and femoral neck BMD <−2.0 at discontinuation had a lower incidence of clinical vertebral fracture if they continued treatment for another 5 years [63].
The last long-term study was a 3-year extension of the HORIZON-PFT [64]. In this clinical trial, 1233 women who received zoledronate during the first 3 years of the study were randomized to continue yearly infusions of zoledronate or placebo for another 3 years. Compared with women who received 3 years of treatment followed by placebo, those who were treated for 6 years had a significantly lower incident of new morphometric vertebral fractures (OR 0.51; 95 % CI 0.26–0.95). There were no significant differences in the incidence of hip or all clinical fractures between the two groups [HR 0.9 (0.33–2.49) and 1.04 (0.71–1.54), respectively]. In a post hoc analysis, Cosman et al. [65] showed that predictors of fracture in the discontinuation group were an osteoporotic hip BMD at the start of the extension and the presence of incident morphometric vertebral fractures during the initial treatment period. On the other hand, women with total hip BMD >−2.5, no recent incident fractures, and no more than one risk factor for fractures have a low risk for a subsequent fracture if treatment was discontinued. These results are generally similar to those obtained with long-term alendronate treatment .
Taken together, the findings of the long-term extension studies of bisphosphonates are reassuring and indicate that prolonged exposure of bone tissue to bisphosphonate maintains the effect of treatment and is not associated with adverse effects on bone fragility. Whether continuation of treatment offers additional antifracture benefit is not entirely clear, but, within the limitations of the studies, the data strongly suggest that patients with increased fracture risk can benefit from continuation of treatment for up to 6 or 10 years with zoledronate and alendronate, respectively. For clinical decisions, analysis of changes of surrogate endpoints such as BMD and biochemical markers of bone turnover can be of additional value.
Resolution of the Effect of Treatment
Bisphosphonates have the unique properties to be selectively taken up by bone, preferentially at sites of increased bone remodeling, to be embedded in bone for long after completing their action on the surface, and to be slowly released from bone. The capacity of the skeleton to retain bisphosphonate, which is biologically inert, is large, and saturation of binding sites in bone with the doses used in the treatment of osteoporosis is unlikely even if these are given for a very long time [66]. These characteristics differentiate the pharmacodynamics of bisphosphonates from those of all other agents used in the treatment ofosteoporosis and play an important role in the interpretation of their long-term results on bone tissue and their implementation in clinical practice.
Pharmacodynamic responses following cessation of bisphosphonate therapy given for prevention of bone loss were adequately investigated in the Early Postmenopausal Intervention Cohort (EPIC) study [67, 68]. Early postmenopausal women were given alendronate for 2, 4, or 6 years, or placebo, and were followed for 6 years. Cessation of treatment after 2 or 4 years was associated with progressive increases in biochemical markers of bone resorption toward the levels of women treated with placebo, and BMD decreased at a rate similar to that of placebo-treated women . There was, thus, no rapid increase in the rate of bone resorption and no “catch-up” bone loss as observed in a parallel group that received hormone replacement therapy for 4 years.
Bagger and colleagues analyzed the results of studies of 203 women given different daily doses of alendronate or placebo for varying periods for the prevention of postmenopausal bone loss and were followed for up to 9 years [69]. Seven years after withdrawal of treatment, women who received alendronate (2.5–10 mg/day) for 2 years had a 3.8 % higher BMD than those who received placebo. The residual effect was proportionally larger in women who received treatment for 4 or 6 years (5.9 % and 8.6 %, respectively), but the largest residual effect was observed in women who received alendronate 20 mg/day for 2 years (9.7 %). Similar to the EPIC study, bone turnover markers tended to revert back to placebo levels, and the rate of bone loss following cessation of alendronate treatment was comparable to the bone loss observed in the placebo group. This study provides information additional to that obtained in EPIC. It shows that alendronate has a residual effect on bone metabolism that is proportional to the length of treatment with doses between 2.5 and 10 mg/day. The highest residual effect was obtained with the dose of 20 mg/day, although this was given for only 2 years, corresponding to 4 years of treatment with 10 mg/day or 8 years of treatment with 5 mg/day.
About 20 years ago, in exploratory studies of women and men with osteoporosis treated with daily oral pamidronate for 6.5 years, we reported that cessation of treatment was not associated with decreases of bone mineral density of the spine and the femoral neck and that the rate of vertebral fractures remained stable during 2 years of follow-up without bisphosphonate [70]. We hypothesized that resumption of bone remodeling after stopping treatment led to release of bisphosphonate previously embedded in bone. The concentration of the released bisphosphonate was probably sufficient to correct the imbalance between bone resorption and bone formation and to protect skeletal integrity, but insufficient to maintain the decrease of bone resorption to the same level and to further increase BMD. In a later study, we showed that pamidronate can be released in the circulation of humans for at least 8.0 years after stopping treatment [71]. The long-term responses of women with osteoporosis treated with other nitrogen-containing bisphosphonates are in agreement with these early observations. For example, cessation of alendronate after 5 years of treatment was followed by modest increases in biochemical markers of bone turnover to levels lower than those before any treatment was given [20]. The lack of a control group receiving placebo during the whole period of observation precludes any conclusions about the precise magnitude of this response. Spine BMD remained stable during 5 years off treatment, while it increased further on continuing treatment. Importantly, BMD of hip sites showed some decrease, but not back to baseline. In the FLEX study, patients who received placebo after 5 years of alendronate therapy showed a gradual increase in biochemical markers of bone resorption that remained within premenopausal values during the following 5 years without bisphosphonate [60]. Changes in BMD were similar to those in the extension of the Phase III study, with the exception of total hip BMD, which reached pretreatment values after 5 years off treatment. McNabb et al. performed a detailed analysis of the BMD changes of patients who received placebo following alendronate in the FLEX and found that in these untreated elderly women with osteoporosis, total hip and femoral neck BMD decreased by only 3.6 % and 1.7 %, respectively, after 5 years [62]. However, in 29 % of the women, total hip BMD decreased by more than 5 % after 5 years. In an attempt to identify prognostic markers for this BMD loss, the authors examined a number of risk factors for bone loss and fractures including bone turnover markers but failed to identify women at risk of higher rates of bone loss. They concluded that risk factors are currently of limited utility for predicting bone loss following discontinuation of alendronate treatment after 5 years of continuous administration of the bisphosphonate.
Resolution of the effect of long-term risedronate treatment on biochemical bone markers and BMD was examined in 61 patients with postmenopausal osteoporosis who participated in the three extension studies of the VERT-NA [72]. In patients who received PBO/RIS or RIS/RIS, the bone resorption marker NTx/Cr increased to the same extent by about 20 % after one year remaining clearly below baseline values. The resolution of the effect of risedronate was not different between the two groups with different exposures to therapy (2 vs. 7 years). This finding may be related to the lower affinity of risedronate for bone mineral. These changes were associated with decreases in total hip BMD , but lumbar spine and femoral neck BMD were maintained or increased off treatment. Results of bone markers and BMD longer than 1 year after treatment arrest of risedronate are not available.
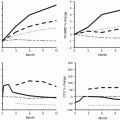
In the extension of the HORIZON study, levels of markers of bone turnover changed slightly; serum CTX was 0.16 ng/ml in the ZOL/ZOL group and 0.18 ng/ml in the ZOL/PBO group (p = 0.45), and serum P1NP was 28.6 ng/ml and 25.8 ng/ml (p = 0.0001), after 6 years, respectively [64]. Compared with the ZOL/ZOL group, BMD of the total hip and the femoral neck decreased significantly in the ZOL/PBO group after 3 years but did not reach pretreatment values (Fig. 15.4).
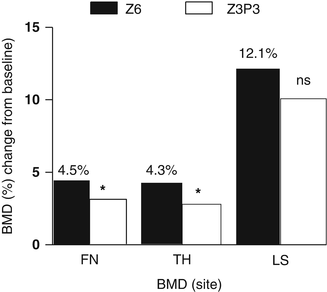

Fig. 15.4
Bone mineral density (BMD) changes after 6 years treatment with yearly infusions of zoledronate 5 mg (Z6) or 3 years treatment with zoledronate followed by 3 years with placebo (Z3P3); FN femoral neck, TH total hip, LS lumbar spine; ns nonsignificant, * p < 0.05. Data from Journal of Bone and Mineral Research 2012; 27: 243–54
The combined observations of the long-term studies of bisphosphonates allow some treatment recommendations based on the risk of fracture of the individual patient. In patients with low fracture risk, zoledronate or alendronate treatment may be stopped after 3 or 5 years, respectively; such approach can also have economic implications. Patients should be followed regularly, but reported data do not allow a precise definition of the length of follow-up intervals, and decisions should be based on clinical judgment; yearly clinical assessments with measurements of BMD and markers of bone turnover can be recommended. In patients at high risk of fracture, treatment should be continued beyond 3 and 5 years for zoledronate and alendronate, respectively, as this may offer additional therapeutic benefits. Alendronate treatment may be continued at a dose of 70 mg every 2 weeks as in the FLEX study 5 mg/day was sufficient to fully maintain the responses of biochemical markers of bone turnover and BMD. This suggestion is based, however, on theoretical considerations as no data supporting a similar efficacy of 5 mg/day and 70 mg every 2 weeks are available. Decisions about long-term therapy should not only be based on these efficacy data but also on potential harm associated with prolonged administration of bisphosphonates.
The Risk
Considering the different molecules, doses, routes of administration, and multiple indications for their use, bisphosphonates are generally safe compounds (reviewed in [73, 74]).
General Toxicity
Short-term adverse effects of bisphosphonate treatment are discussed in detail in Chap. 20. In brief, these include upper GI side effects with nitrogen-containing bisphosphonates that occur more frequently with daily oral dosing; ulcerative esophagitis that occurs rarely and is usually associated with improper use of the bisphosphonates; acute phase response, mainly with intravenous nitrogen-containing bisphosphonates after the first treatment; ophthalmic reactions (e.g., conjunctivitis, uveitis, scleritis, and keratitis) that occur infrequently and are usually attributed to the acute phase response; renal toxicity associated mainly with intravenous administration of bisphosphonate to patients with impaired renal function [75]; hypocalcemia especially in patients with increased rates of bone turnover, concurrent vitamin deficiency, and/or impairment of renal function treated with intravenous bisphosphonate; defective mineralization of bone tissue, an earlier concern of treatment with etidronate, has not been observed with nitrogen-containing bisphosphonates.
The abovementioned adverse events were identified and thoroughly investigated because of the known pharmacology and targets of bisphosphonate action and metabolism. During the course of clinical trials and long-term pharmacovigilance, however, unexpected adverse effects potentially associated with bisphosphonate use were identified and extensively studied. These are atrial fibrillation (discussed in Chap. 20), osteonecrosis of the jaw (ONJ), and atypical femoral fractures (AFF).
Osteonecrosis of the Jaw
In 2003, a report brought attention to an unexpected and previously unrecognized potential adverse effect of bisphosphonate treatment in the jaws of patients suffering mainly from malignant diseases [76]. This was initially termed “avascular necrosis of the jaws” and later “osteonecrosis of the jaws” (ONJ) [77] in analogy with the condition osteoradionecrosis of the jaw resulting from radiotherapy of patients with head and neck cancers. The original publications were followed by a large number of case reports and case series describing an association between bisphosphonate treatment and ONJ (discussed in detail in Chaps. 12–14). A causal relationship between bisphosphonate treatment and ONJ has not been established [78–81], there is no generally accepted pathogenetic mechanism of ONJ in bisphosphonate-treated patients, and an appropriate animal model of ONJ is not available [82]. An International Classification of Diseases code (ICD ) and a working definition for ONJ were introduced for the first time in 2006–2007.
The American Association of Oral and Maxillofacial Surgeons (AAOMS) and the American Society of Bone and Mineral Research (ASBMR) proposed in 2007 the following definition of ONJ:
1.
Current or previous treatment with bisphosphonates
2.
Exposed necrotic bone in the maxillofacial region, which has been present for at least 8 weeks
3.
No history of radiation therapy to the jaws
This definition was widely accepted and formed the basis of studies that examined the frequency and pathogenesis of ONJ. The definition was revised in 2014 by AAOMS as follows:
1.
Current or previous treatment with antiresorptive or antiangiogenic agents
2.
Exposed bone or bone that can be probed through an intraoral or extraoral fistula(e) in the maxillofascial region that has persisted for more than 8 weeks
3.
No history of radiation therapy to the jaws or obvious metastatic disease to the jaw
The main difference of this from the earlier definition of ONJ is the recognition that the disorder does not occur only in bisphosphonate-treated patients, as was widely believed. Patients at risk or with established medication-related ONJ can also present with other common clinical conditions not to be confused with medication-related ONJ. Commonly misdiagnosed conditions include alveolar osteitis, gingivitis/periodontitis, carries, fibro-osseous lesions, and chronic sclerosing osteomyelitis [81]. Importantly, ONJ occurs also in patients not exposed to antiresorptive or antiangiogenic agents . The pathophysiology of ONJ has not been fully elucidated, and various hypotheses have been proposed to explain the unique localization in the jaws including altered bone remodeling, inhibition of angiogenesis, constant microtrauma, vitamin D deficiency, soft tissue bisphosphonate toxicity, and inflammation or infection.
Initial surveys, using different ways to define ONJ, revealed that the condition was much more frequent in patients treated with bisphosphonates for malignant diseases, particularly multiple myeloma and breast cancer . The incidence of ONJ in patients with malignant diseases treated with intravenous zoledronate in clinical trials is approximately 1.0 % [83]. It should be noted that the dose of zoledronate given to patients with malignant diseases for prevention of skeletal-related events is 4 mg every 3–4 weeks accounting for a total yearly dose of about 50 mg; this dose is tenfold higher than the dose of zoledronate used for the treatment of osteoporosis. The estimated prevalence of ONJ in patients with osteoporosis treated with bisphosphonates range between 10 cases per 10,000 and <1 case per 100,000 patients exposed [84]. In clinical trials of oral bisphosphonates in osteoporosis, no cases of ONJ were identified in more than 60,000 patient-years, while in the HORIZON-PFT , the only study of bisphosphonates in osteoporosis in which cases of ONJ were prospectively collected and adjudicated, two documented cases were reported: one in the placebo-treated group and one in the zoledronate-treated group; another case was documented in the zoledronate group of the extension study [46, 64]. Overall, the risk of ONJ among patients treated with either zoledronate or alendronate for osteoporosis approximates the risk of ONJ in patients treated with placebo [81]. The risk appears to increase with time on treatment and is 100 times lower than in cancer. The studies of ONJ in patients treated with bisphosphonates have identified other risk factors that contribute to the development of the condition, the most common being a dental procedure and the use of glucocorticoids . In contrast to malignant diseases, there are no specific recommendations for dental procedures in patients with osteoporosis on treatment with bisphosphonates. A dental procedure should not be deferred because the risk of ONJ is extremely low. An advice to temporarily stop treatment, for example, 2–3 months, before and after the procedure is more for reassurance of the patient and the dentist and is not based on scientific evidence. Various treatment strategies are used by dental surgeons depending on the stage of ONJ (see Chap. 14). PTH treatment has been reported to have a quick, favorable effect in some cases. In conclusion, the risk of ONJ in patients with osteoporosis treated with bisphosphonate is very low and does not affect the favorable benefit-to-risk balance of bisphosphonate treatment of osteoporosis.
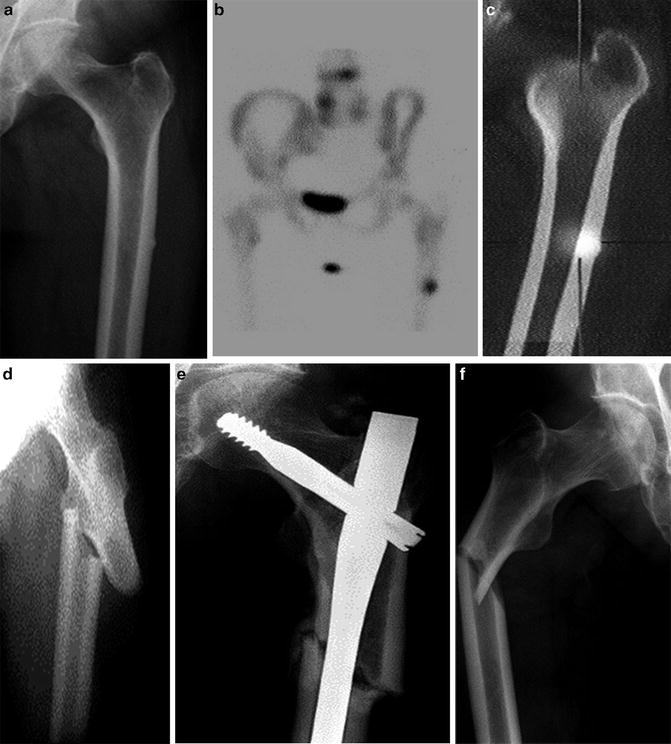
Atypical Fractures of the Femur (AFF)
In recent years, there has also been concern about the potential relation between fractures of the femur below the lesser trochanter deemed to be unusual for patients with osteoporosis on the basis of their localization and radiographic characteristics and long-term use of bisphosphonate. These fractures were termed AFF and were reported to occur as a result of no or minimal trauma, and they may be complete, extending across the entire femoral shaft, or incomplete affecting only the lateral cortex of the femur. They are morphologically transverse or short oblique, often in areas of thickened femur cortices, and they are not comminuted (Fig. 15.5). They are often preceded by prodromal pain and can be bilateral, and healing may be delayed. The first report of AFF in patients treated with oral bisphosphonate [84] for osteoporosis was followed by case reports and case series of patients with AFF while on treatment with oral bisphosphonates (reviewed in [85–87]). In 2010, a Task Force of the ASBMR proposed criteria for the identification and diagnosis of AFF which were revised in 2014 as shown in Table 15.2 [88]. It is now generally accepted that AFF are stress fractures that may proceed to complete fractures.