Locally Advanced Squamous Carcinoma of the Head and Neck
Approximately 50,000 patients are diagnosed annually with squamous cell head and neck cancer (HNC) in the United States. Worldwide, approximately 600,000 patients are afflicted. Nearly 60% of this population presents with locally advanced but nonmetastatic disease. Locoregional failure constitutes the predominant recurrence pattern, and most fatalities result from uncontrolled local and/or regional disease.
Radiotherapy (RT) alone was long the standard nonsurgical therapy for locally advanced disease. The state of the art regarding radiation dose fractionation has evolved from once-daily treatment to hyperfractionation and accelerated fractionation.1,2,3–4 These newer strategies lead to a 7% to 10% improvement in locoregional control relative to once-daily treatment schemes. A recent meta-analysis of randomized trials testing modified fractionation schemes against conventional once-daily fractionation demonstrated that hyperfractionation was the most effective strategy, leading to an 8% absolute improvement in 5-year survival.5 Nonetheless, even the most effective RT regimens result in local control rates of 50% to 70% and disease-free survivals (DFSs) of 30% to 40%.
This circumstance has stimulated the investigation of treatments combining RT and chemotherapy. Review articles describe in detail the different chemotherapeutic agents and RT schemes of these treatment programs.6,7 Most trials have used sequential or neoadjuvant (induction) chemotherapy followed by RT. Randomized trials of induction cisplatin and 5-fluorouracil (5-FU) chemotherapy followed by standard fractionation versus laryngectomy and postoperative RT in advanced larynx and hypopharynx cancer performed by the Veterans Administration Cooperative Group8 and the European Organization for the Research and Treatment of Cancer (EORTC), respectively, initially showed that larynx preservation could be achieved without compromising overall survival.
Most randomized clinical trials show the superiority of combined RT and chemotherapy to RT alone for the treatment of locally advanced, nonmetastatic HNC. A meta-analysis of individual patient data from >17,346 participants in 93 trials conducted from 1965 to 2000 (Meta-Analysis of Chemotherapy on Head and Neck Cancer [MACH-NC]) demonstrated that the use of radiotherapy and concurrent chemotherapy (CRT) resulted in a 19% reduction in the risk of death and an overall 6.5% improvement in 5-year survival compared to treatment with RT alone (p < .0001).9 This benefit was predominantly attributable to a 13.5% improvement in local regional control. The 2.9% reduction in the risk of distant metastases was not statistically significant (Fig. 40.1).
FIGURE 40.1. Data from the Meta-Analysis of Chemotherapy in Head and Neck Cancer (MACH-NC) illustrating that the major therapeutic benefit of platinum-based chemotherapy results from an improvement in local-regional disease control when the drugs are given concurrently with radiotherapy. No significant improvement occurs with induction chemotherapy followed by radiotherapy.
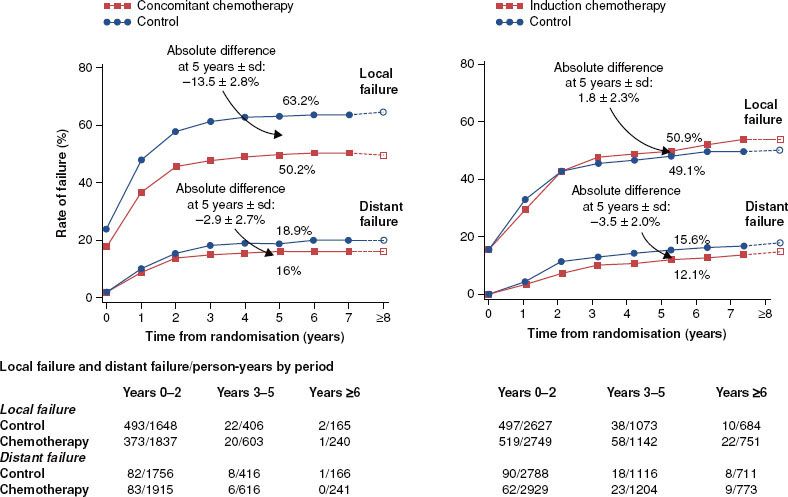
The MACH-NC also demonstrated a 2% improvement in 5-year survival from the use of induction chemotherapy followed by RT, which was not significant. A subset analysis of trials that used cisplatin and 5-FU as the induction regimen did show a 4% improvement in overall survival.10
Randomized comparisons of concurrent chemoradiation versus induction chemotherapy followed by radiotherapy alone are few but confirm that the former strategy is superior.11,12 The Radiation Therapy Oncology Group (RTOG) conducted a three-arm trial of radiation alone versus radiation and concurrent cisplatin versus induction cisplatin followed by irradiation in larynx carcinoma. Concurrent therapy constituted the most effective means of larynx preservation and provided the best disease control, albeit without a statistically significant survival benefit.12 Neoadjuvant chemotherapy followed by RT was no more efficacious than RT alone. Despite the lack of evidence supporting sequential chemoradiation strategies, this strategy was the most common method of integrating the two modalities in the community practice setting until recently.13 A more contemporary survey has demonstrated that concurrent RT and chemotherapy is now used more frequently.14
RT and concurrent chemotherapy represents the most commonly used strategy and is a more attractive approach because some chemotherapeutic agents may both radiosensitize cells and provide additive cytotoxicity. The superiority of concurrent CRT relative to RT alone has been demonstrated in randomized trials in squamous cell carcinoma of other anatomic sites including the esophagus and uterine cervix.15–17
Certain issues must be considered when evaluating randomized trials of CRT for advanced head and neck cancer. The first consideration relates to the effectiveness of the RT-alone control arm. Specifically, does it represent optimal single-modality treatment? If the CRT regimen is more effective than RT alone but the radiation is suboptimal, then it is difficult to accurately gauge whether or not the combined-modality regimen represents a true improvement in therapy. The second consideration concerns the toxicity of CRT itself. Typically, both acute and late toxicity from CRT are greater than from RT alone.18–20
Acute mucositis constitutes the most significant impediment to the timely delivery of concurrent therapy. Because prolongation of total treatment time adversely affects the success of RT in HNC,21,22–23 a major challenge has been the development of treatment schedules that integrate RT and chemotherapy and yet do not excessively increase total treatment time. A thorough understanding of toxicity is mandatory as avoidance of the functional morbidity associated with surgery in advanced head and neck cancer is one of the main reasons for the utilization of concurrent therapy in the first place.
 HISTORICAL DEVELOPMENT OF RADIATION AND CONCURRENT CHEMOTHERAPY
HISTORICAL DEVELOPMENT OF RADIATION AND CONCURRENT CHEMOTHERAPY
CRT may be administered in synchronous or alternating schemes. Synchronous administration results in the delivery of RT and chemotherapy (CT) on the same days. Typically, chemotherapy will be given for 1 or more days at the initiation of RT and then repeated in the same fashion several weeks later. Alternating regimens usually sandwich RT and CT around one another. Radiation and drugs are therefore not necessarily given on the same days. In such a scheme, CT would be given during the first week of treatment with RT following in subsequent week(s) before CT is given again.
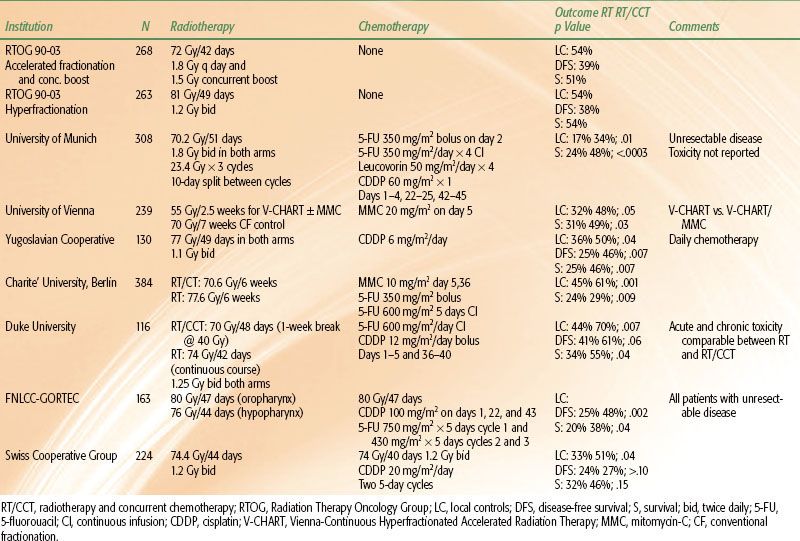
TABLE 40.1 RANDOMIZED TRIALS OF ONCE-DAILY IRRADIATION AND CONCURRENT CHEMOTHERAPY IN ADVANCED HEAD AND NECK CANCER
Synchronous Radiation and Single-Agent Chemotherapy
Synchronous treatment is completed more quickly than alternating treatment. It is therefore preferable from a theoretical standpoint in terms of addressing the issue of accelerated repopulation, albeit at the expense of increased acute side effects. Early randomized trials of conventionally fractionated RT and CT used single-agent chemotherapy. These studies are summarized in Table 40.1. The experimental arms of RTOG 90-03,1 EORTC 22791,3 and DAHANCA 6-74 are included to provide a basis for comparison with optimal regimens of RT alone.
Both the Northern California Oncology Group (NCOG) and the EORTC tested radiation and synchronous bleomycin against radiation therapy alone.24,25 Acute toxicity was worse in the combined-modality arm in both trials, but the outcomes were quite different with respect to efficacy. The EORTC trial showed no improvement in DFS or survival and the RT/bleomycin combination in the NCOG program led to a statistically significant doubling of locoregional control and DFS, as well as a near-significant improvement in overall survival from 24% to 43%.
Differences in study design and execution may explain the discrepancy between outcomes in the EORTC and NCOG trials. Fractionation was similar in the two studies, but patients in the EORTC trial received 15 mg of bleomycin twice weekly during the first 5 weeks of RT for a total dose of 150 mg, whereas the NCOG patients received 5 mg twice weekly for a total dose of 70 mg. Acute mucosal and skin toxicity was worse in the RT/bleomycin arm in both trials. Toxicity significantly prolonged the RT delivery time in 30% of the combined-modality patients in the EORTC trial but not in any of the RT-alone patients. This prolongation of treatment time and associated tumor repopulation in such a large proportion of patients may have negated any benefit accrued from the use of concurrent therapy. There were no differences in overall treatment time between the two treatment arms in the NCOG trial. An important lesson from these two studies is that the dose administration schedules of concurrent chemotherapy must be carefully designed so that toxicity does not adversely affect overall treatment compliance.
The Christie Hospital in Great Britain evaluated RT and 100 mg/m2 of single-agent methotrexate (MTX) given at the commencement of and after 2 weeks of a 3-week course of treatment.26 Most of the 313 patients in this protocol received 50 to 55 Gy in 15 or 16 fractions. Mucositis was significantly greater in the patients receiving MTX, but there was no difference in long-term toxicity. The addition of MTX increased local control from 50% to 70% (p = .02) and survival from 37% to 47% (p = .07). The greatest benefit was seen in patients with oropharyngeal primary tumors who constituted one-third of the study population. Local control with RT/MTX was 78% versus 38% with RT alone (p = .002) in this patient subset. Survival was 25% with RT alone and 50% with RT/MTX (p = .009). Unfortunately, the data from this trial are not generally applicable to current clinical practice because of the large radiation fraction sizes that were used.
5-FU has been used in conjunction with RT more frequently than any other chemotherapeutic agent. Lo et al.27 reported the first study to show a significant improvement in local control and survival with the addition of bolus 5-FU to RT in squamous carcinoma of the oral cavity. Browman et al.28 compared RT and continuous infusion 5-FU against RT alone in a placebo-controlled randomized trial sponsored by the National Cancer Institute of Canada. All 175 patients received 66 Gy in 2-Gy fractions. 5-FU was given at 1,200 mg/m2/day for the first 3 days of the first and third weeks of irradiation. Confluent mucositis was more frequent in the 5-FU arm than in the placebo arm (32% vs. 11%; p = .001), as was weight loss >15% from pretreatment baseline (41% vs. 11%; p < .0001). This increased acute toxicity did not prolong the delivery of RT in the RT/5-FU arm relative to the RT/placebo arm. Two-year DFS and survival were 30% and 50% for RT/placebo patients and 50% and 63% for RT/5-FU patients (p = .06 and .08), respectively.
The relative radioresistance of hypoxic cells in vitro is well understood.29 Clinically, the existence of hypoxia both in head and neck primary tumors and metastatic lymph nodes has been described, and its adverse impact on the prognosis of patients treated with RT has been demonstrated.30,31 Investigators from Yale University designed their treatment strategy around this principle. They treated 195 patients in two randomized trials with mitomycin-C (MMC). This agent is predominantly metabolized in and preferentially cytotoxic to hypoxic cells. The Yale treatment program consisted of 68 Gy ± MMC on days 1 and 43 of RT. Local control was improved with the addition of MMC from 54% to 76% (p = .003). Survival improved from 42% to 48%, but this was not statistically significant. The majority of patients in these trials received adjuvant postoperative or preoperative irradiation, however. Only 74 (38%) received definitive, primary RT, and the benefit from the addition of MMC in this subset is unclear.32
Synchronous Radiation and Multiagent Chemotherapy
Cisplatin (CDDP) is a radiosensitizer, too.33 The combination of CCDP and 5-FU is also one of the most active cytotoxic drug combinations against squamous cell HNC. Consequently, investigators have incorporated both of these drugs into a variety of concurrent treatment strategies. A randomized trial from the Cleveland Clinic assigned patients to receive 66 to 72 Gy ± two cycles of synchronous CDDP (20 mg/m2/day é 4) and infusional 5-FU (1,000 mg/m2/d é 4) during weeks 1 and 4 of RT.34 The main objective of this study was primary site organ preservation. Surgical salvage was allowed for patients with persistent disease. Acute toxicity was significantly greater in the combined-modality treatment arm, especially with respect to weight loss. Mucosal recovery usually required 8 to 12 weeks after completion of RT and chemotherapy. There were no differences in the total time required for RT delivery, however. Three-year DFS was significantly better for the patients receiving chemoradiotherapy (67% vs. 52%; p = 0.03). Three-year survival with primary site preservation was also higher in the combined-modality group (57% vs. 35%, p = .02), although there was no significant difference in overall survival.
MMC and 5-FU were used together in a trial of 209 patients conducted at the Princess Margaret Hospital.35 Patients were treated with continuous course RT alone at 2.5 Gy/day to 50 Gy in 28 days. Patients randomized to receive RT/chemotherapy received the same dose fractionation scheme as those receiving RT alone but over a total time of 56 days due to a planned 4-week treatment interruption after 25 Gy. Bolus MMC (10 mg/m2) was given on days 1 and 43. Two cycles of continuous infusion 5-FU (1,000 mg/m2/day) were given on days 1 to 4 and 43 to 46. The intent of the treatment break was to maintain comparable levels of acute toxicity in the two treatment arms. Acute toxicity was, in fact, equivalent in the two groups. Unfortunately, however, there was no difference in 4-year local control (~40%) or survival (~40%).
The Princess Margaret trial raises an important question: can one quantify the contribution provided by concurrent chemotherapy in terms of the delivery of an equivalent dose of irradiation? Approximately 0.6 Gy/day is necessary to compensate for the tumor repopulation that transpires with each day of prolongation of standard course RT.23 Thus, the total dose in the Princess Margaret Hospital RT/chemotherapy arm would have to have been about 67 Gy [(2.5 Gy é 20) + (0.6 Gy/day é 28 days)] in order to have been isoeffective with the 50-Gy regimen in the RT-alone arm. The equivalent efficacy of the two treatments in this trial therefore suggests that the chemotherapy compensated for the tumor repopulation that occurred during the treatment break. Thus, one could argue that the chemotherapy was equivalent to approximately 17 Gy of additional irradiation. There can be no doubt as to the inferiority of the split-course fractionation scheme in this trial had it been delivered without chemotherapy and compared head to head against the continuous course RT regimen. Conversely, if the combined-modality treatment had been given with continuous course RT, it would quite probably have been more efficacious than the RT-only regimen.
A Spanish three-arm randomized trial (N = 859) provides additional information that is pertinent to the estimation of the radiotherapeutic dose equivalent provided by the delivery of concurrent chemotherapy.36 Patients were assigned to receive one of the following regimens:
A. 2 Gy/day to 60 Gy/42 days,
B. 1.1 Gy twice daily to 70.4 Gy/44 days, or
C. 2 Gy/day to 60 Gy/42 days with concurrent bolus 5-FU 250 mg/m2 given every other day.
Progression-free survival and overall survival were significantly worse in arm A as compared with arms B and C, as one would expect. Arms B and C were equally efficacious. Not accounting for the different fractionation in arms B and C and the unconventional administration of chemotherapy, it is still clear that the addition of 5-FU was comparable to dose escalation of approximately 10 Gy. A recent modeling analysis of phase III trials comparing CRT with RT only suggests that concurrent chemotherapy provides the equivalent of a 10- to 12-Gy dose escalation.37
Neither the Princess Margaret nor the Spanish trial delivered maximally intensive radiotherapy in their respective control arms. The rationale for treatment intensification with the addition of concurrent chemotherapy as opposed to simple RT dose escalation is weak in such a context. The situation may be dramatically different, however, when the RT-alone arm is maximally intensive such as in RTOG 90-03 or EORTC 22791. Dose escalations of 10 to 12 Gy are not possible with accelerated regimens that already deliver 72 Gy during 6 weeks or with hyperfractionated regimens delivering 79 Gy in 7 weeks (see Radiation Fractionation Scheme section). Concurrent chemotherapy, however, can be added to modified fractionation regimens ≥70 Gy.
Alternating Radiotherapy and Chemotherapy
Alternating therapy produces less acute mucosal toxicity than synchronous therapy but may prolong the overall treatment time by several weeks. Although longer treatment times adversely affect efficacy in programs of standard RT alone due to tumor repopulation, the significance of overall treatment time (for RT) in a continuous course of alternating RT and chemotherapy is controversial. Some investigators have suggested that the usual time–dose relationships do not apply.38
The National Institute for Cancer Research in Italy conducted a phase III trial comparing RT with alternating RT and chemotherapy in 157 patients with unresectable head and neck cancer.39,40 The RT arm was designed to give 70 Gy/7 weeks via standard fractionation. The combined-therapy arm scheduled chemotherapy on weeks 1, 4, 7, and 10 and radiation (60 Gy) on weeks 2 to 3, 5 to 6, and 8 to 9. Each 2-week cycle of radiation consisted of 20 Gy/10 fractions. Each cycle of chemotherapy included 5 days of bolus CDDP (20 mg/m2/day) and bolus 5-FU (200 mg/m2/day). The incidence of grade 3/4 mucositis (18% to 19%) was the same in both treatment groups. However, RT treatment delays occurred more often in the RT-alone patients: 32% with a 1-week prolongation and 25% with a ≥2-week prolongation. Corresponding delays in the combined-modality treatment group were 11% and 15%, respectively. The median dose of RT delivered in the combined-modality-treatment group matched the planned dose of 60 Gy, but it was only 62 Gy in the RT-alone group. Five-year actuarial survival was significantly better in the combined-modality-treatment patients (24% vs. 10%; p = .01), as were DFS (21% vs. 9%; p = .008) and local control (64% vs. 32%; p = .04).
Given the similar levels of acute toxicity, it is unclear why treatment times were prolonged and total doses reduced so extensively in the RT-only patients. Better protocol compliance in the control arm might well have changed the outcome of this trial. There are no other randomized trials of RT and alternating chemotherapy. Further randomized trials of alternating therapy will be necessary to determine its true value because the deficiencies of the National Institute for Cancer Research study prevent definitive conclusions.
Despite its drawbacks, the Italian study, like the Princess Margaret Hospital trial, strongly reinforces the idea that in some settings, chemotherapy counteracts tumor repopulation during treatment. The total RT treatment time was prolonged in the combined-modality arms in both studies. The fundamental difference between these two trials is that patients received no treatment during the RT break in the Princess Margaret Hospital trial and the patients in the Italian trial received chemotherapy during each interruption of RT.
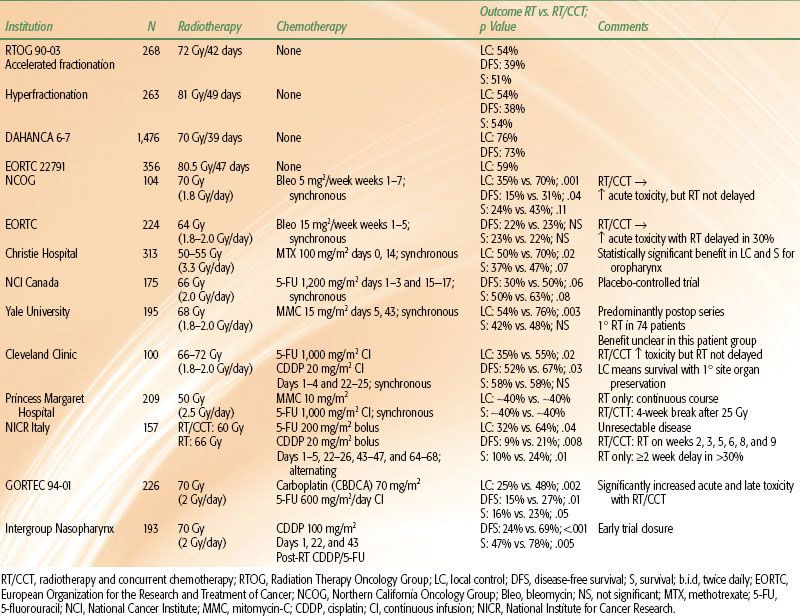
 CONTEMPORARY RANDOMIZED TRIALS OF RADIOTHERAPY AND CONCURRENT CHEMOTHERAPY
CONTEMPORARY RANDOMIZED TRIALS OF RADIOTHERAPY AND CONCURRENT CHEMOTHERAPY
Curative Intent Treatment
The French cooperative group trial, GORTEC 94-01, was performed in patients who had stage III/IV oropharyngeal carcinoma.19,41 Radiotherapy consisted of conventional 2 Gy, once-daily fractionation to 70 Gy. Patients on the CRT arm also received three cycles of concurrent carboplatin (70 mg/m2) and continuous infusion 5-FU (600 mg/m2/day é 4 days). Two hundred twenty-six patients were enrolled in the trial. CRT resulted in significant improvement in 5-year locoregional control (48% vs. 25%; p = .002), DFS (27% vs. 15%; p = .01), and survival (23% vs. 16%; p = .05). This improvement in efficacy was accompanied by a significant increase in acute mucositis (grade ≥2) from 39% to 71% (p = .005). Severe acute cutaneous and hematologic toxicity and worse nutritional status were also significantly more prevalent in the patients who received combined-modality therapy. Severe late toxicity, primarily cervical fibrosis, occurred in 27% of the combined-modality patients and in 12% of those treated with RT alone (p = .04). Severe dental complications were twice as frequent in the combined-modality patients (37% vs. 18%; p = .01).
Wendt et al.42 conducted a multi-institutional trial of CRT versus RT for patients with unresectable stage III/IV head and neck cancer. CRT patients received three cycles of cisplatin, 5-FU, and leucovorin during a 7-week period. Cisplatin was given as a 60 mg/m2 bolus. 5-FU was given as an initial 350 mg/m2 bolus followed by a 4-day continuous infusion of 350 mg/m2/day. Leucovorin was also given for 4 days at 100 mg/m2/day. Radiotherapy was given as three cycles of 23.4 Gy at 1.8 Gy bid in both treatment arms. It coincided with the chemotherapy on the CRT arms. Planned treatment breaks were given between the cycles of treatment to ameliorate treatment-induced mucositis. The cumulative dose of radiation therapy in both treatment arms was 70.2 Gy in 7 weeks.
One-third of the 270 patients enrolled had oropharynx primary tumors. CRT doubled both 3-year local control (35% vs. 17%; p < .004) and survival (49% vs. 24%; p < .003) (Fig. 40.2). As in the GORTEC 94-01 oropharyngeal trial, confluent mucositis was significantly higher (38% vs. 16%; p < .001) with the use of CRT.
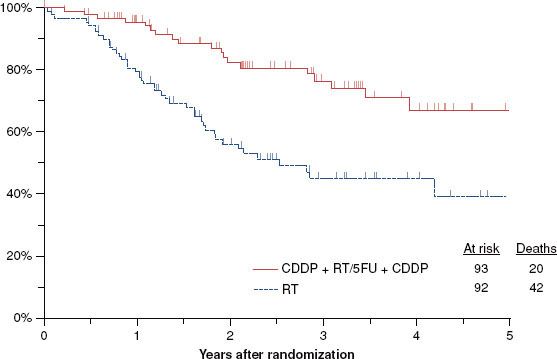
Treatment of advanced nasopharynx carcinoma with radiation and concurrent chemotherapy was the subject of an intergroup study in which patients in both arms received conventionally fractionated RT (1.8 to 2.0 Gy/day) to a total dose of 70 Gy.43 Those patients who were randomized to concurrent chemotherapy also received three cycles of cisplatin during RT at a dose of 100 mg/m2. After the completion of RT, they received an additional three cycles of cisplatin at 80 mg/m2 as well as 4-day continuous infusions of 5-FU at 1,000 mg/m2/day. All patients had stage III/IV, M0 disease. In spite of the initial plan of enrolling 270 patients, the trial was terminated early when an interim analysis demonstrated the superiority of the combined-modality regimen. One hundred ninety-three patients were enrolled, and the median follow-up is 2.7 years. Three-year progression-free survival favored the combined-modality patients (69% vs. 24%; p < .001). Similarly, 3-year survival was 78% versus 47% (p = .005) in favor of the patients who received concurrent chemotherapy (Fig. 40.3). Table 40.1 summarizes the data from the trials of conventionally fractionated irradiation and concurrent chemotherapy.
FIGURE 40.2. Data from the Meta-Analysis of Chemotherapy in Head and Neck Cancer (MACH-NC), which demonstrates that adding concurrent chemotherapy to radiotherapy provides a significant improvement in survival but that induction chemotherapy does not. A: Concomitant chemotherapy. B: Induction chemotherapy.
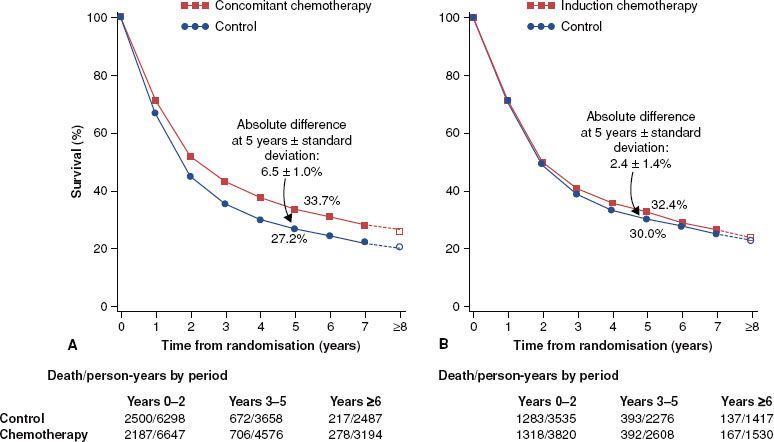
FIGURE 40.3. Three-year survival in the Intergroup Nasopharynx Carcinoma Trial was significantly better (p = .005) for those patients who received concurrent cisplatin and postirradiation adjuvant cisplatin/5-fluorouracil (78%) than for those who received radiotherapy alone (47%). These results led to early trial closure.
One must ask not only whether CRT is more effective than conventionally fractionated RT, but also whether it is superior to hyperfractionated or accelerated fractionation irradiation as these strategies represent the most efficacious single-modality treatment strategies. A prospective randomized trial from Duke University provides some insight into this issue.44 Patients with locally advanced head and neck cancer were randomized to hyperfractionated irradiation alone versus split-course hyperfractionation with concurrent CDDP/5-FU chemotherapy. Patients in the RT-alone arm received 1.25 Gy bid continuous course to 75 Gy in 6 weeks, whereas those patients on the CRT arm received 1.25 Gy bid split course to 70 Gy in 7 weeks. Chemotherapy was given during weeks 1 and 6 of irradiation (CDDP 12 mg/m2/day é 5 days; 5-FU continuous infusion was 600 mg/m2/day é 5 days).
The time–dose aspects of the RT in the combined-modality arm are similar to those of the previously discussed trials. The time–dose characteristics of the RT in the control arm were similar to certain aspects of both the concurrent boost arm (decreased treatment time) and the hyperfractionation arm (increased total dose) of RTOG 90-03. Most importantly, these characteristics made the RT more intensive in the control arm than in the experimental CRT arm. This study design was intentional because a primary objective of the trial was to determine whether a lower dose of RT with concurrent chemotherapy would be superior to maximally intensive/effective RT alone.
Fifty-four percent of the patients presented with unresectable disease, and approximately 40% of the primaries were located in the oropharynx. One hundred sixteen patients were enrolled, and the updated median follow-up now exceeds 5 years. Locoregional control was 70% versus 44% (p = .006), favoring the combined-modality patients. An unpublished update of the 5-year survival revealed superiority in the combined-modality patients (42% vs. 27%; p = .04) (Fig. 40.4). Confluent (grade 3) mucositis was seen in approximately 75% of the patients in both arms, the main difference being that the mean time to resolution of mucositis was 50% longer in the patients receiving radiation and concurrent chemotherapy (6 vs. 4 weeks).
Jeremic et al.45 evaluated hyperfractionated irradiation (1.1 Gy bid to 77 Gy) with or without concurrent low-dose daily cisplatin (6 mg/m2) in stage III/IV patients. One hundred thirty patients were enrolled; primary tumors originated in the oropharynx in approximately one-third of the population. Fifty-nine percent presented with T3 or T4 primaries, and 80% had nodal involvement. Five-year locoregional control (50% vs. 36%; p = .04), progression-free survival (46% vs. 25%; p = .007), and overall survival (46% vs. 25%; p = .007) (Fig. 40.5) were all significantly improved with the addition of concurrent chemotherapy. Of note, the distant metastasis-free survival was also improved in the concurrent therapy patients (86% vs. 57%; p = .01).
A German multicenter trial also confirmed that CRT is superior to maximally intensive single-modality irradiation.18 Three hundred eighty-four patients, 93% of whom had either stage III or IV oropharyngeal or hypopharyngeal primaries, were enrolled. As in the Duke trial, the total dose of RT delivered in the CRT arm was lower than that in the RT control arm. RT patients received 77.6 Gy in 6 weeks (14 Gy at 2 Gy once daily followed by 63.6 Gy at 1.4 Gy twice daily), and CRT patients received 70.6 Gy during 6 weeks (30 Gy at 2 Gy per day followed by 40.6 Gy at 1.4 Gy twice daily). Chemotherapy consisted of mitomycin-C (10 mg/m2) on days 5 and 35 and 5-FU given as a single bolus of 350 mg/m2 and a 5-day continuous infusion of 600 mg/m2/day. Two-year survival was significantly better in the combined-modality arm (54% vs. 45%; p = .05), as was locoregional control (61% vs. 45%; p = .001). Acute and chronic toxicity were equivalent in the two treatment populations.
A French cooperative group (FNLCC-GORTEC) tested a related concept in 163 patients with technically unresectable carcinomas of the oropharynx and hypopharynx.46 RT was administered at 1.2 Gy twice daily to a total dose of 80.4 Gy/46 days to oropharyngeal primary tumors and 75.6 Gy/44 days to hypopharyngeal primary tumors. The experimental arm received the same RT and concurrent CDDP (100 mg/m2) on days 1, 22, and 43 of RT. Three 5-day cycles of continuous infusion 5-FU were also administered. The first cycle was 750 mg/m2/day and the second and third cycles were 430 mg/m2/day. Three-year DFS favored the concurrent chemoradiation arm (48% vs. 25%; p = .002), as did overall survival (38% vs. 20%; p = .04). Post hoc subset analyses demonstrated that the larger (and statistically significant) benefit was confined to the patients with oropharyngeal carcinomas. However, the trial was not designed to compare treatment efficacy in these two different primary sites of origin.
A three-armed randomized trial from the University of Vienna compared conventionally fractionated RT (2 Gy daily to 70 Gy) against continuous hyperfractionated accelerated RT with and without mitomycin C (V-CHART + MMC and V-CHART, respectively).47 Radiotherapy was given as an initial 2.5-Gy fraction followed by 1.65 Gy twice daily to a total dose of 55.3 Gy in 17 days. MMC was given as a 20 mg/m2 bolus on day 5 of RT. Of the 239 patients enrolled, 85% had T3/4 primary tumors, and 79% had nodal involvement.
Three-year actuarial locoregional control was 48% for V-CHART + MMC versus 32% for V-CHART and 31% for conventional fractionation (CF) (p = .05 and.03, respectively). Survival including death from all causes was also improved to 41% in the V-CHART + MMC arm as compared with 31% for V-CHART and 24% for CF (p = .03).
The incidence of confluent mucositis was 90% in both experimental arms as compared with 33% in the CF arm. The median time to complete resolution of mucositis was 6 to 7 weeks in all three arms. Grade 3/4 hematologic toxicity, primarily thrombocytopenia, developed in 18% of the V-CHART + MMC patients. Table 40.2 summarizes the data from the trials that used modified fractionation and concurrent chemotherapy and includes the RTOG 90-03 and EORTC 22791 data as a point of reference for optimally delivered RT alone.
FIGURE 40.4. Five-year locoregional control in the Duke University randomized trial of continuous course accelerated hyperfractionation (44%) versus split-course accelerated hyperfractionation and concurrent cisplatin/5-fluorouracil (p = .01). Extended follow-up has demonstrated that the survival benefit of combined-modality treatment is also statistically significant.
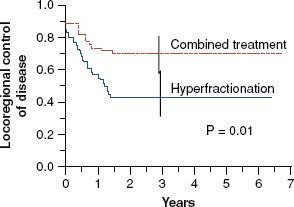
FIGURE 40.5. Five-year survival in the Yugoslavian trial of hyperfractionated irradiation alone (25%) or with daily low-dose concurrent cisplatin (46%) ( p = .007).
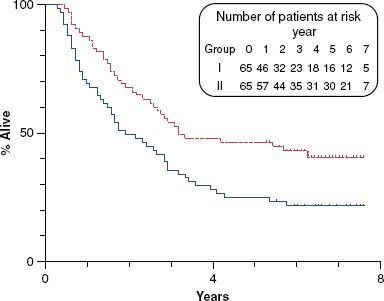
TABLE 40.2 RANDOMIZED TRIALS OF ACCELERATED OR HYPERFRACTIONATED RADIOTHERAPY AND CONCURRENT CHEMOTHERAPY IN ADVANCED HEAD AND NECK CANCER