Lobular Carcinoma In Situ: Biology and Management
Tari A. King
Jorge S. Reis-Filho
Lobular carcinoma in situ (LCIS) and atypical lobular hyperplasia (ALH) are relatively uncommon breast lesions, which are typically discovered in breast biopsies taken for other reasons. The first description of LCIS was reported by Ewing in 1919, who depicted this lesion as an “atypical proliferation of acinar cells” of the breast (1). The main characteristics of this lesion, however, were not thoroughly documented until 1941 in the seminal study by Foote and Stewart, in which the term LCIS was coined to refer to a spectrum of “noninfiltrative lesions of a definitely cancerous cytology” that would constitute precursors of invasive breast cancer, and be composed of a monomorphic population of dyshesive cells that expand the terminal duct-lobular units (2). A less-prominent in situ proliferation composed of cells cytologically identical to those of LCIS, and associated with a lower risk of breast cancer development, was subsequently identified and named ALH (3). In a review of 211 cases of LCIS not associated with other forms of breast cancer, Haagensen et al. (4) observed the difficulties in differentiating between LCIS and ALH, and suggested that the term LCIS not associated with invasive cancer would constitute a misnomer, given that the available evidence at that time supported the contention that these lesions would in fact constitute a “benign, non-infiltrating, special microscopic form of lobular proliferation of the mammary epithelium” (4). The term lobular neoplasia (LN) was subsequently put forward to refer to the entire spectrum of these in situ lesions, including ALH and LCIS (4). Although surgeons, oncologists, and pathologists are familiar with the concept of LCIS, the terminology and classification for these lesions, their biological significance (risk indicator vs. precursor for invasive cancer), and the best course of management following diagnosis remain controversial. This chapter will discuss the clinicopathological and molecular characteristics of LN, and the impact of recent developments on the management of these lesions.
Several of the concepts initially put forth by Foote and Stewart (2) on the biology of LCIS remain valid today. The term LCIS was chosen to emphasize the histologic similarities between the cells of LCIS and those of frankly invasive lobular carcinoma (ILC), and, importantly, was not meant to infer that the cell of origin resided in the lobules; in fact, it was acknowledged that LCIS would originate in the terminal duct-lobular unit and small ducts (2). In addition, LCIS was reported to be frequently multicentric and bilateral, and not readily identifiable on gross examination. Microscopically, the cells that constitute LCIS were thought to disseminate through the ductal system in a way akin to that of Paget’s disease; however, LCIS was almost never seen in association with true Paget’s disease of the nipple (2). Based on the frequent identification of LCIS in association with ILC and following the analogy of ductal carcinoma in situ (DCIS) and invasive ductal carcinoma (IDC), Foote and Stewart (2) hypothesized that the neoplastic cells of LCIS would still be contained within a basement membrane, and that this lesion would constitute a “hazard” (i.e., risk factor) of breast cancer development and a step along the pathway to the development of invasive cancer. Hence, based on the evidence available, simple mastectomy was suggested as the standard form of treatment (2).
Emerging data throughout the 1970s from Haagensen et al. (4) and others (5) demonstrating that the risk of breast cancer development following a diagnosis of LCIS was lower than expected for a direct precursor lesion (approximately 1% per year) and was conferred equally to both breasts generated controversy regarding the significance of these lesions and led to disparate recommendations for management, ranging from observation only to bilateral mastectomy. In current practice, a diagnosis of ALH or LCIS is typically perceived as a risk indicator rather than a precursor of subsequent carcinoma and, as such, radical treatment has fallen out of favor. Yet, observational evidence to suggest that the
risk of breast cancer development following a diagnosis of LN is higher in the ipsilateral than in the contralateral breast and compelling molecular data that demonstrate that ALH and LCIS are clonal neoplastic proliferations that commonly harbor the same genetic aberrations as those found in adjacent invasive cancers (6, 7, 8, 9 and 10) have reinstated the notion that ALH and LCIS are both non-obligate precursors and risk indicators of invasive breast cancer. Questions regarding the biology and optimal management of these lesions have returned to the forefront of breast cancer research and practice.
risk of breast cancer development following a diagnosis of LN is higher in the ipsilateral than in the contralateral breast and compelling molecular data that demonstrate that ALH and LCIS are clonal neoplastic proliferations that commonly harbor the same genetic aberrations as those found in adjacent invasive cancers (6, 7, 8, 9 and 10) have reinstated the notion that ALH and LCIS are both non-obligate precursors and risk indicators of invasive breast cancer. Questions regarding the biology and optimal management of these lesions have returned to the forefront of breast cancer research and practice.
EPIDEMIOLOGY AND CLINICAL FEATURES
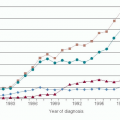
LCIS is most frequently diagnosed in women aged 40 to 55 years (4, 11). The true prevalence of LCIS in the general population, however, is difficult to estimate and likely exceeds the incidence, given that it does not present as a mass lesion nor does it have a specific radiographic appearance. Lesions diagnosed in the pre-mammography screening era were typically incidental microscopic findings in biopsies and excision specimens obtained for other reasons (2, 4). The reported incidence of LCIS in otherwise benign breast biopsy specimens ranges from 0.5% to 3.8% (4, 11), whereas population-based data reported to Surveillance, Epidemiology, and End Results (SEER) from 1978 to 1998 demonstrate an incidence of 3.19 per 100,000 women (12). It is noteworthy, however, that during this time period there was an observed four-fold increase in the number of LCIS cases reported among women over 40 years of age, with the highest incidence rate (11.47 per 100,000 person-years) in 1998 among women 50 to 59 years of age. While this trend may reflect the increasing use of mammography and image-guided biopsies during this time period (12, 13), the impact of other factors, such as the use of postmenopausal hormone replacement and more accurate pathologic diagnosis of LN based on ancillary immunohistochemical markers (see below) remains a matter of speculation. LCIS is often multifocal, with more than 50% of patients diagnosed with LCIS showing multiple foci in the ipsilateral breast. Furthermore, bilateral lesions are reported in approximately one-third of patients (14, 15). Such multifocality in a clinically non-detectable lesion is one of the reasons why planning subsequent management has proven problematic and contentious. More recent imaging series suggest that LCIS may be associated with microcalcifications (16), and LCIS has been reported to enhance on MRI (17); however, imaging criteria to differentiate LCIS from overt malignancy are lacking, and, as such, women with LCIS are frequently subject to multiple biopsies demonstrating otherwise benign findings.
The clinical characteristics of LCIS, including its multifocal and bilateral distribution, and evidence of familial clustering (18, 19) have led to the hypothesis that these lesions could be underpinned by germline genetic abnormalities. Although a hereditary form of diffuse gastric cancer and breast lobular carcinoma caused by CDH1 germline mutations (20) has been described, the potential genes involved and the pattern of inheritance of familial LCIS outside of this context remain unclear (see below).
The clinical characteristics of LCIS that support its role as a risk factor for the subsequent development of breast cancer include the cumulative long-term risk of breast cancer development that is generally conferred to both breasts, averaging 1% to 2% per year, and the observation that not all breast cancers developing after a diagnosis of LCIS are of lobular histology (reviewed in reference (21). The incidence of invasive breast cancer following a diagnosis of LCIS is steady over time (22), with a similar number of invasive lesions being reported within and after 5 years of follow-up (23). Others have also demonstrated the cumulative long-term risk, with one study reporting that over 50% of patients developed beast cancer between 15 and 30 years of follow-up (5). ALH is also associated with an increased risk of subsequent breast cancer; however, this is of a lower magnitude than that conferred by LCIS. Patients diagnosed with ALH have a four- to five-fold higher risk than the general population (i.e., women of comparable age who have had a breast biopsy performed with no atypical proliferative disease diagnosed), whereas a relative risk of 8 to 10 times is conferred by a diagnosis of LCIS (11, 24, 25). Hence, these observations suggest that the term LN, albeit helpful to describe this group of lesions collectively, may not suffice to guide the management of patients with lobular lesions, and specific classification of LN into ALH and LCIS may still be justified. It should be noted, however, that the distinctions between ALH and LCIS are subjective and, for some experts, the differences between these two categories of LN are more easily expressed in words than in actual practice (23).
The risk of breast cancer development following a diagnosis of ALH or LCIS is bilateral (14, 22, 26), which is consistent with the notion that these lesions are risk indicators; however, some have reported a higher rate of breast cancer in the ipsilateral breast (9, 21, 27), supporting a precursor role for LCIS. The histological type of breast cancer following a diagnosis of LN also differs among these reports. In studies that suggest the risk is conferred equally to both breasts, there are, similarly, an equal number of subsequent IDCs and ILCs reported to occur after a diagnosis of LCIS (22), which is consistent with the notion that LCIS would not constitute a true precursor lesion. On the other hand, in most studies that report a higher incidence of ipsilateral cancer development, the majority of the cancers are of lobular histology (8, 21, 23). This clinical observation, in parallel with SEER data demonstrating an increasing incidence of both LCIS and ILC from the late-1980s to the mid-1990s among women 50 years of age and older (12, 28), have led to renewed interest the debate over the clinical significance of LCIS.
Taken together, the current epidemiological, observational, and clinical data support the contention that LN is not only a risk indicator, but also a non-obligate precursor of invasive breast cancer. This notion is lent further credence by the striking morphologic similarities between cells of ALH or LCIS and ILC, and molecular data demonstrating the clonality between LN and synchronous invasive breast cancer (see below); in particular, the presence of concordant gene copy number and allelic abnormalities (6, 29), mitochondrial DNA mutations (7), and identical CDH1 gene mutations in matched LCIS and ILC from the same patients (10).
HISTOLOGICAL FEATURES AND CLASSIFICATION
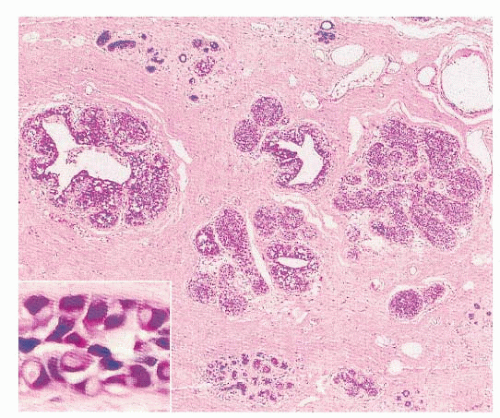
Despite the controversies surrounding the clinical implications of ALH and LCIS, their histologic features have been well characterized. The latest World Health Organization (WHO) classification of breast tumors defines LN as “a spectrum of atypical epithelial lesions originating in the terminal ductlobular unit and characterized by a proliferation of generally small, non-cohesive cells, with or without pagetoid involvement of the terminal ducts” (30). At scanning magnification, these lesions are characterized by a variable enlargement of the acini, which are filled up and, at least in part, are expanded by a proliferation of monomorphic population of dyshesive
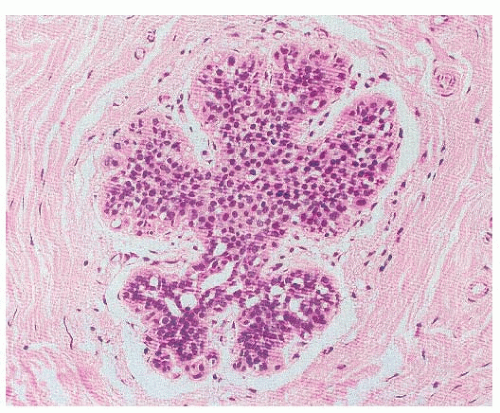
small, round, or polygonal cells, with inconspicuous cytoplasm (Fig. 22-1). In fact, the main histological characteristic of LN is that its cells are loosely cohesive, regularly spaced, and fill and distend the acini, with an overall maintenance of the lobular architecture (Fig. 22-2). Intracytoplasmic vacuoles, sometimes containing a central eosinophilic dot (known as magenta body), are usually found (2, 4, 21, 30). Despite the apparent monomorphism of the classic variant of LN, some variability in the cytomorphology between different cases, and frequently within the same case, is easily appreciated, and two cytologic subtypes have been recognized (Table 22-1). Type A cells are small, dyshesive cells with scant cytoplasm and nuclei approximately 1.5 times the size of that of a lymphocyte, whereas type B cells have more abundant, often clear cytoplasm nuclei that are approximately two times bigger than a lymphocyte nucleus, mild-to-moderate nuclear atypia (nuclear pleomorphism 1 or 2), and indistinct or absent nucleoli (21, 31). It should be noted, however, that this cytological classification scheme has not been shown to be of clinical utility and does not have a direct correlation with the risk of invasive breast cancer development. Other than an academic exercise, this classification system is only a reminder that some degree of cytologic variation can be observed in bona fide cases of classic LN. In the classic form of LN, mitoses and necrosis are uncommon. Pagetoid spread within the affected terminal duct-lobular unit, whereby the neoplastic cells extend along adjacent ducts between intact overlying epithelium and underlying basement membrane, is frequently observed (Fig. 22-3). The presence of glandular lumina is not seen in fully developed cases; it should be noted, however, that in lobules partially affected by LN, residual glandular structures can be found.
small, round, or polygonal cells, with inconspicuous cytoplasm (Fig. 22-1). In fact, the main histological characteristic of LN is that its cells are loosely cohesive, regularly spaced, and fill and distend the acini, with an overall maintenance of the lobular architecture (Fig. 22-2). Intracytoplasmic vacuoles, sometimes containing a central eosinophilic dot (known as magenta body), are usually found (2, 4, 21, 30). Despite the apparent monomorphism of the classic variant of LN, some variability in the cytomorphology between different cases, and frequently within the same case, is easily appreciated, and two cytologic subtypes have been recognized (Table 22-1). Type A cells are small, dyshesive cells with scant cytoplasm and nuclei approximately 1.5 times the size of that of a lymphocyte, whereas type B cells have more abundant, often clear cytoplasm nuclei that are approximately two times bigger than a lymphocyte nucleus, mild-to-moderate nuclear atypia (nuclear pleomorphism 1 or 2), and indistinct or absent nucleoli (21, 31). It should be noted, however, that this cytological classification scheme has not been shown to be of clinical utility and does not have a direct correlation with the risk of invasive breast cancer development. Other than an academic exercise, this classification system is only a reminder that some degree of cytologic variation can be observed in bona fide cases of classic LN. In the classic form of LN, mitoses and necrosis are uncommon. Pagetoid spread within the affected terminal duct-lobular unit, whereby the neoplastic cells extend along adjacent ducts between intact overlying epithelium and underlying basement membrane, is frequently observed (Fig. 22-3). The presence of glandular lumina is not seen in fully developed cases; it should be noted, however, that in lobules partially affected by LN, residual glandular structures can be found.
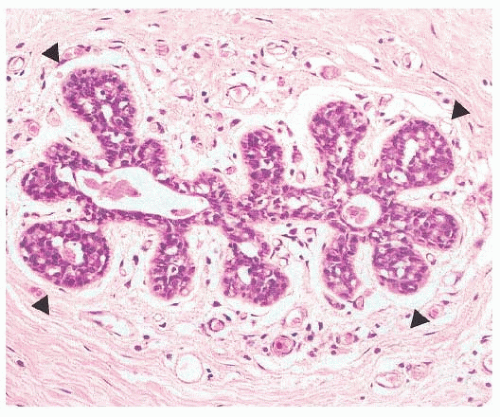
The sub-classification of LN into ALH and LCIS is quantitative rather than qualitative (30). While for a diagnosis of LCIS more than half the acini in an involved lobular unit must be filled and distended by the characteristic cells, leaving no central lumina, ALH is defined as a less well-developed and less-extensive lesion, where the characteristic cells only partly fill the acini, with only minimal or no distention of the lobule (Fig. 22-4), and glandular lumina can still be found (9, 24, 30, 32). In an attempt to standardize the diagnosis of ALH and LCIS, it has been proposed that lobular distention should be defined as eight or more cells present in the crosssectional diameter of an acinus, and that the number of acini involved in cases of ALH should account for less than half of the whole terminal duct-lobular unit (9, 24, 30, 32). Although the use of this sub-classification would be justified on the basis of the lower risk conferred by ALH than by LCIS, the differentiation between ALH and LCIS based on the criteria described above is not only arbitrary, but also subjective, prone to interobserver and intraobserver variability, and dependent on the extent of sampling of a given lesion. Therefore, the use of the term LN to encompass the whole range of changes, and remove this variability, is preferable for diagnostic purposes, particularly in core needle biopsy specimens (21, 33). In fact, in the context of diagnostic core biopsies, the term LN, with no attempt to distinguish ALH and LCIS, is recommended in the guidelines of breast cancer screening programs (e.g., the United Kingdom National Health Service Breast Screening Programme [NHS BSP]) (33).
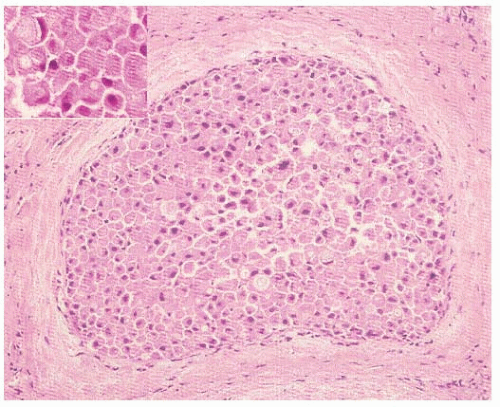
Arguably, a more relevant distinction is between the classic form of LN and the pleomorphic variant of LCIS (PLCIS), which was first identified as a distinct entity by Eusebi et al. in 1992 (34). This variant is characterized by pleomorphic cells that are substantially bigger than those of classic LN (31, 34), and by more abundant, pink, and often finely granular cytoplasm. Features of apocrine differentiation are frequently found (34, 35). As compared to the nuclei of classic LN, PLCIS nuclei are bigger (four times the size of lymphocyte nucleus), more pleomorphic, and atypical nuclei, often containing conspicuous nucleoli (Fig. 22-5). PLCIS not uncommonly presents with central, comedo-type necrosis and microcalcifications; yet, necrosis is not required for the diagnosis. Recognition of the pleomorphic subtype is important because the combination of cellular features, necrosis, and calcification can lead to difficulty in differentiation from DCIS, and potentially overtreatment, although data regarding the natural history of PLCIS are very limited. Until additional data regarding the natural history of PLCIS are available, this distinction has important implications for treatment. Whereas some advocate for a more aggressive approach to PLCIS, with treatment recommendation akin to those for DCIS, it should be noted that this approach is supported
only by molecular data demonstrating that PLCIS shares many similarities with pleomorphic ILC, not by long-term outcomes data.
only by molecular data demonstrating that PLCIS shares many similarities with pleomorphic ILC, not by long-term outcomes data.
TABLE 22-1 Cytological and Histopathological Features of Classic and Pleomorphic Lobular Carcinomas | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Additional variants of LN have been reported, including apocrine, histiocytoid, rhabdoid, endocrine, amphicrine, and the apocrine PLCIS variant (21, 30). The biological and clinical significance of these lesions also remains to be determined.
A further system for classification of LN has been proposed using the terminology lobular intraepithelial neoplasia (LIN), with subdivision, based on morphologic criteria and clinical outcome, into three grades (LIN 1, LIN 2, LIN 3), with LIN 3 representing the PLCIS end of the spectrum (36, 37). This system pre-supposes that the risk of invasive carcinoma development would be related to increasing grade of LIN. This classification system, albeit interesting and potentially sparing women from a diagnosis of “carcinoma” in the case of LCIS, is supported by limited evidence and has not been endorsed in the latest edition of the WHO classification (30).
DIFFERENTIAL DIAGNOSIS
The differential diagnosis of LN includes artifacts, benign breast lesions, and other forms of breast cancer precursors. Prolonged periods of warm ischemia and poor tissue fixation, not uncommonly seen in mastectomy specimens, can result in an artifactual discohesion of cells within a lobular unit, which can mimic ALH and LCIS (21). Benign lesions that may superficially resemble LN include foci of lactational change, where the cells harbor intracytoplasmic lipid droplets, and clear-cell metaplasia (21). More troublesome is the histologic appearance of LN colonizing other benign breast lesions, including fibroadenoma, sclerosing adenosis, and radial scar, which, clinically and radiologically, can present as a mass. Particularly well-known but rather remarkably problematic examples of LN in sclerosing adenosis may resemble IDC to the unwary, due to the distorted appearance of the residual ductal and lobular structures lined by LN cells and immersed in a sclerotic stroma (21, 30). In this context, immunohistochemical markers to demonstrate the presence
of a myoepithelial cell layer, including nuclear (e.g., p63) and cytoplasmic (e.g., smooth muscle myosin heavy chain or calponin) myoepithelial markers, and markers to ascertain the LN nature of the neoplastic cells (e.g., E-cadherin, β-catenin, and/or p120 catenin) are useful in making the distinction.
of a myoepithelial cell layer, including nuclear (e.g., p63) and cytoplasmic (e.g., smooth muscle myosin heavy chain or calponin) myoepithelial markers, and markers to ascertain the LN nature of the neoplastic cells (e.g., E-cadherin, β-catenin, and/or p120 catenin) are useful in making the distinction.
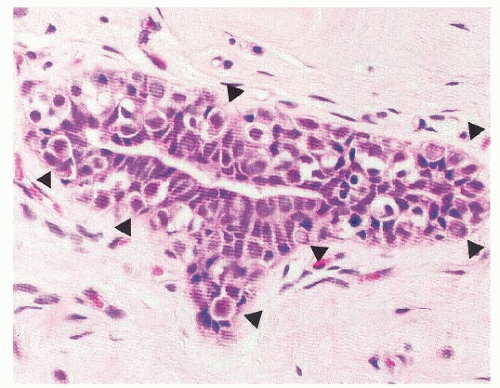 FIGURE 22-4 Atypical lobular hyperplasia. A lobular unit is focally and partially filled by characteristic cells with intracytoplasmic lumina (arrowheads). |
Differentiating low-grade, solid DCIS from LN is challenging, even in surgical excisional samples. In cases of lowgrade in situ proliferations with indeterminate features, the identification of some histologic features can be of help; the presence of a mosaic growth pattern with prominent intracytoplasmic vacuoles is suggestive of LN, whereas the presence of microacinar-like structures favors a diagnosis of solid low-grade DCIS. In this context, E-cadherin and p120 catenin are particularly helpful (see below) (38, 39). It should be noted, however, that for some lesions, the distinction between LN and solid low-grade DCIS in limited core biopsy specimens may be impossible. In addition, mixed lesions composed of bona fide LN and low-grade DCIS have been reported. Another important differential diagnosis, particularly in core biopsies, is cancerization of the lobules by DCIS. In cases with equivocal histologic features, immunohistochemistry with anti-E-cadherin and p120 catenin antibodies is helpful, given that lack of membranous E-cadherin and p120 catenin cytoplasmic staining would be consistent with a diagnosis of LN rather than cancerization of the lobules by DCIS.
The differentiation of PLCIS from high-grade DCIS can be challenging, given that both are composed of pleomorphic cells with marked cytological atypia, both often display comedo-type necrosis, and both express similar patterns of ER, progesterone receptor (PR), and HER2 expression, including lack of or reduction in ER and PR expression, and overexpression of HER2 (35, 40). Importantly, however, the neoplastic cells of PLCIS display the characteristic dyshesiveness, and these lesions consistently lack E-cadherin and β-catenin, and express cytoplasmic p120 catenin (see below).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree