Modality
Signal
Contrast agent
Sensitivity [1] (mol label)
Resolution [1]
Tissue penetration
Risk
Primarily morphological/anatomical imaging modalities
MRI
Magnetic fields
Gadolinium, iron oxide nanoparticles
10−9–10−6
50 µm
Unlimited
Harmless
CT
X-rays
Iodine
10−6
50 µm
Unlimited
Radiation
US
Acoustic waves
Microbubbles
10−8
50 µm
Unlimited
Harmless
Primarily molecular imaging modalities
PET
Positrons from radionuclides
Positron-emitting radionuclides (e.g., 18F, 64Cu, 124I)
10−15
1–2 mm
Unlimited
Radiation
SPECT
γ-rays
Single photon-emitting radionuclides (e.g., 99mTc, 67Ga, 111In)
10−14
1–2 mm
Unlimited
Radiation
Fluorescence imaging
Light (NIR)
Fluorescent probes
10−12
1–2 mm
3 mm
Harmless
PAI
Acoustic waves
Probes that absorb light and emit heat
10−12
50 µm
7 cm
Harmless
As cancer research pushes toward the goal of personalized medicine, theranostics will play an increasingly important role [3]. Theranostics is the co-delivery of therapeutic and imaging agents in a single formulation [4, 5]. In this way the drug and contrast agent share the same pharmacokinetic and biodistribution profiles, and therefore imaging provides an accurate assessment of drug distribution. Regarding theranostics, drug delivery is not a black box having injected dose as input and clearance rate as output. Imaging functionality can quantify the intratumoral drug dose [6], specify the activation state of a therapeutically shielded drug [7], and indicate the intracellular uptake pathway [8]. Nanocarriers offer many advantages for drug delivery and theranostics compared to molecular systems. Encapsulation of molecular theranostic agents in nanocarriers protects them from serum degradation and rapid renal clearance, and enables the use of hydrophobic agents. It is widely recognized that nanoparticle encapsulation passively improves tumour targeting based on the enhanced permeability and retention (EPR) effect [9–11]. In addition, nanoparticle size and surface composition may be designed to avoid protein adsorption and removal by the reticuloendothelial system (RES), prolonging blood circulation, and increasing the probability of tumour uptake [12–15]. The targets of many chemotherapeutics (e.g., doxorubicin (dox), siRNA, Photofrin®) are intracellular; therefore, drug efficacy is dependent on its ability to enter the target cell. Conjugation of molecular therapeutics to targeting agents such as antibodies enhances both intracellular uptake and selectivity for tumour cells over normal cells [16, 17]. However, to maintain tumour-targeting functionality the number of therapeutic agents that can be loaded on each targeting moiety is low, and labeling can interfere with therapeutic activity. In contrast, a large number of therapeutics can be encapsulated in a single targeted nanoparticle, significantly increasing the quantity of drug delivered during each internalization event. High loading capacity for imaging agents also improves imaging contrast, and is particularly advantageous for modalities with low sensitivity such as MRI and CT. Environmental factors can also be used to differentiate tumour cells from normal cells. These factors may be internal to the tumour microenvironment (e.g., tumour acidity), or external conditions locally applied to the tumour region (e.g., light exposure). Stimuli-responsive nanoparticles are designed to respond to these environmental factors and enhance payload release [18–21]. In theranostics, actively targeted or stimuli-responsive nanoparticles are called “smart delivery” or “activatable” systems [20]. During systemic circulation, the nanoparticles are therapeutically shielded and imaging functionally silenced to minimize off-target effects and background noise. In the tumour region, the drug and imaging agent are released or activated. With nanotechnology, it is possible to integrate multiple properties in a single platform, including passive targeting, target-triggered activatable release, multimodal imaging, and therapy capabilities. Recent advances in the technological capabilities of nanotheranostic systems impact drug development as well as treatment planning at the patient bedside (Fig. 1). For example, real-time imaging of drug accumulation in the tumour region may be used to optimize drug formulation during the research and development stage, as well as to predict patient response to an administered drug dose.
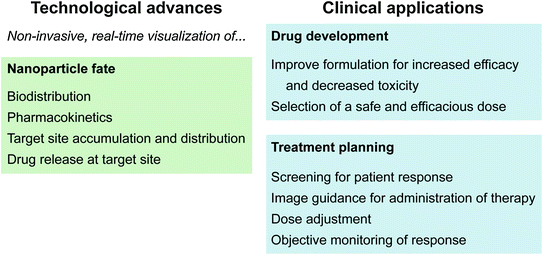

Fig. 1
Recent technological advances in nanotheranostics and their clinical applications for drug development and treatment planning
Various nanocarriers have been investigated for theranostics, including lipid nanoparticles, polymer conjugates, micelles, and dendrimers, as well as inorganic nanoparticles such as silica, gold, quantum dots, iron oxide, and carbon nanotubes [22–24]. Among them, lipid nanoparticles are in the most advanced stage of development and have shown favorable biocompatibility and biodegradation in comparison to inorganic nanoparticles [25]. Extensive knowledge has been obtained on the exploitation of these platforms for cancer therapy, particularly for their ability to enhance therapeutic efficacy over free drugs through improved encapsulation, prolonged circulation half-life, and sustained or triggered release [16, 26–28]. This chapter focuses on recent developments in advanced lipid theranostic nanomedicine from the perspective of the “all-in-one” or the “one-for-all” approach.
2 Engineering Theranostic Lipid Nanoparticles by Assembling Multiple Functional Components—The “All-in-One” Approach
The design paradigm of “all-in-one” is the most common approach for assembling multifunctional lipid nanoparticles, where the advantages of multifunctionality and theranostics are achieved by combining multiple components that each possesses a specific singular function, such as therapeutic activity, imaging contrast, targeting, clearance avoidance, and triggered drug release. Here, we will review lipoprotein nanoparticles and liposomes as representatives of the “all-in-one” approach.
2.1 Lipoprotein Theranostics
Lipoproteins are normally comprised of a hydrophobic lipid core and an outer shell of phospholipids and amphipathic apolipoproteins that confer water solubility and precise size control [29]. As natural cholesterol transporters, lipoproteins integrate many advantages for drug delivery [30–36]. For example:
1.
As endogenous carriers, they can escape recognition as foreign entities by the human immune system and clearance by RES [37].
2.
Their long blood circulation time provides favorable systemic drug delivery without the need for a polyethylene glycol (PEG) coating to improve steric stability.
3.
Their natural structure enables stable systemic delivery of hydrophobic bioactive compounds [38]. This permits various drug loading approaches, including core loading, surface loading, and protein conjugation.
4.
Apolipoprotein interaction with the lipid layer encourages a consistent and reproducible number of apolipoproteins per particle and a narrow size distribution, overcoming concerns of polydispersity in size and also providing ligand-targeted delivery [8].
Lipoproteins have been employed for delivery of many chemotherapeutics including dox, paclitaxel, siRNA, hypericin, valrubicin, curcumin, statin, etc. [39–48], and there are many comprehensive reviews available on their uses for drug delivery [35, 37, 38, 49–51]. This chapter focuses on the expansion of their development for theranostic applications.
2.1.1 Building Imaging Functionality for Lipoprotein Nanomedicine
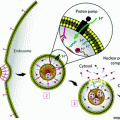
Lipoproteins have a rich history as imaging contrast agents due to their ability to transport large loads of hydrophobic agents and ease of loading. Three main strategies have been explored to functionalize lipoproteins: (1) surface loading—noncovalent intercalation of the imaging agent within the surface of the lipoprotein; (2) covalent modification—conjugating the imaging agent onto the surface of the apolipoprotein or onto the phospholipid headgroup; (3) and core loading through reconstitution—encapsulating hydrophobic agents in the nanoparticle core (Fig. 2a) [35]. Table 2 provides an overview of the imaging modalities demonstrated to date. These modalities generally focus on tracking different aspects of lipoprotein fate. MRI, CT, and PET monitor lipoproteins during systemic circulation to determine their biodistribution and ability to accumulate in the tumour region. Fluorescence imaging is widely used to determine the cell delivery mechanism [30, 53–56]. Core-loaded fluorophores permit low background fluorescence imaging by means of a “smart delivery system” approach that takes advantage of the well-established phenomenon of aggregation-induced chromophore quenching [68]. The intracellular uptake mechanisms of low-density lipoprotein (LDL) and high-density lipoprotein (HDL) have been disclosed using fluorescence microscopy. LDL enters cells through the LDL receptor (LDLR)-mediated endocytosis pathway [69], while HDL interacts with scavenger receptor class B member 1 (SR-B1) to drive direct transport of the payload molecules into the cell cytoplasm without internalization of the entire particle [8].
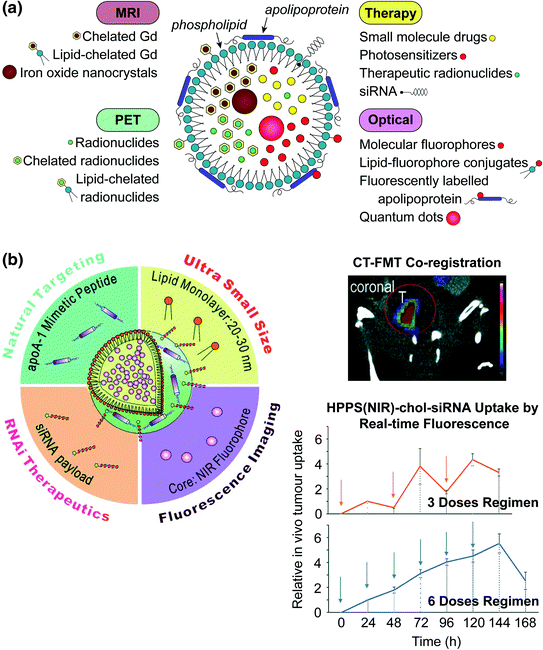

Fig. 2
a Schematic representation of the strategies available for incorporating imaging and therapeutic agents in lipoproteins. Hydrophobic molecules such as drugs, photosensitizers, and fluorophores, as well as inorganic nanoparticles (e.g., iron oxide, QD) are core encapsulated through reconstitution. Gadolinium and radionuclides are core encapsulated with and without assistance of chelation (e.g., by diethylene triamine pentaacetic acid (DTPA)). Imaging agents are incorporated into the nanoparticle shell through lipid conjugation or fluorescence labeling of the apolipoprotein. Cholesterol-conjugated siRNA is embedded within the lipid layer though noncovalent intercalation. b Left HDL-mimetic lipoprotein nanoparticle “HPPS” incorporating inherent targeting functionality, gene therapy, and fluorescence imaging in a compact spherical structure. Right, top CT-FMT co-registered images for visualizing siRNA delivery in a prostate tumour model; bottom siRNA delivery quantified longitudinally by FMT. Adapted with permission from Lin et al., copyright Wiley-VCH (2014) [52]
Table 2
Imaging modalities incorporated in lipoproteins for visualizing biodistribution, metabolism, fate, and cytosolic delivery
Modality | Label | Lipoprotein type | Purpose | References |
|---|---|---|---|---|
MRI | Gd-DTPA | LDL | Biodistribution, metabolism | [59] |
Gd-DTPA-PE | LDL, HDL | Imaging arteriosclerotic plaques | ||
SPION | LDL, HDL | Tumour accumulation, metabolism kinetics | ||
CT | Poly-iodinated triglyceride | LDL | Tumour accumulation | [63] |
Au nanocrystals | LDL, HDL | Tumour accumulation | ||
PET/SPECT | 99mTc | LDL | Biodistribution, tumour accumulation | [66] |
68Ga-DTPA | LDL | Metabolism | [67] | |
111In-DTPA | LDL | Metabolism | [67] | |
Fluorescence imaging | Fluorescent probes | LDL, HDL | Delivery function | |
Quantum dots | HDL | Delivery function | [62] |
Beyond natural lipoproteins, many advanced lipoprotein nanocarriers have been developed to overcome the significant challenges that limit the use of lipoproteins as general theranostic nanoplatforms. For example, the lipoprotein rerouting approach developed by Zheng et al. demonstrates a plausible approach to redirecting lipoprotein theranostics to other cancer associated biomarkers beyond their normal receptors that are only overexpressed in a limited number of cancers [31]. Furthermore, original lipoprotein nanocarriers were natural structures directly isolated from human donors, and faced issues of purity, quantity, length of processing time, as well as safety. New synthetic lipoproteins have been developed, including reconstituted lipoproteins that use isolated apolipoproteins (recombinant or naturally derived) to construct the nanoparticle [41], and lipoprotein-mimetic nanoparticles constrained by apolipoprotein-mimetic peptides [70]. These synthetic systems possess the known functions ascribed to native lipoproteins, including natural targeting, enzyme/pathway activation, intracellular uptake, and lipid transfer. More importantly, their controlled synthesis offers advantages of uniform size, zeta potential, and stable drug loading (core and surface loading). The formulation can be adjusted for the desired phospholipid composition, hydrophobic core moiety, payload, and protein or peptide type. Synthetic lipoproteins may therefore significantly simplify the scale-up process for manufacturing lipoproteins for human use, as well as accelerate the clinical translation of lipoprotein drug delivery.
The development of multifunctional and theranostic lipoproteins has been reliant on the advancement of methods for encapsulating imaging agents. Allijn et al. developed a sonication method for core-labeling native LDL particles with a range of diagnostically active nanocrystals or hydrophobic agents, allowing for detection of LDL delivery from the anatomical level (whole-body imaging by conventional and spectral CT and MRI), to the microscopic level (by fluorescence imaging), and down to the nanometer scale for subcellular localization (by transmission electron microscopy (TEM)) [57, 58]. Using additional well-developed reconstitution methods [53], a range of photosensitizers (e.g., phthalocyanine, naphthalocyanine, pyropheophorbide, bacteriochlorin-e6) have been stably incorporated within lipoprotein particles for effective targeted delivery of PDT agents [30, 34, 54, 71, 72]. Their intrinsic NIR fluorescence can be tracked through fluorescence imaging, thus providing a noninvasive tool to assess in vivo tumour accumulation and guide laser placement for effective PDT. Zheng et al. recently developed a synthetic lipoprotein termed HPPS (HDL phospholipid scaffold) [43, 70, 73], comprised of a lipid shell with a hydrophobic core, constrained by an amphipathic peptide which functionally mimics the apolipoprotein ApoA-1 to confer HDL-like structure and function [74]. HPPS is easily synthesized by sonication and modified for incorporation of functional payloads. Both the hydrophobic core and lipid shell can be replaced with hydrophobic [72] or amphipathic [75] therapeutic or imaging agents [70, 73], making it a versatile platform for theranostics.
Benefiting from their inherent cell uptake mechanism, lipoproteins are useful nanocarriers for treatments requiring cell delivery to be effective, such as gene therapy. SR-B1-mediated delivery is particularly advantageous as it does not involve endosomal entrapment typical of receptor-mediated endocytosis, thus minimizing degradation of siRNA [8, 37, 51, 52, 76, 77]. Cholesterol-conjugated siRNA (chol-siRNA) can be stably loaded into the HDL shell, protecting it from rapid renal clearance. The siRNA is selectively delivered to tumour cells that upregulate SR-B1, significantly improving cell uptake compared to free siRNA. Adding additional imaging functionality to track siRNA delivery would be useful for determining the optimal therapeutic regimen and predicting patient response. Therefore, a 30 nm fluorescent HDL-like nanoparticle was developed for siRNA delivery (HPPS(NIR)-chol-siRNA), with a NIR fluorescent core and siRNA payloads intercalated within the particle membrane (Fig. 2b). In vitro and in vivo fluorescence hyperspectral imaging demonstrated that the NIR core is a suitable surrogate for fluorescently labeling therapeutic siRNA [52]. Directly labeling siRNA itself is disadvantageous as labeling can affect therapeutic activity. Using this system and a CT-FMT (fluorescence molecular tomography) co-registration approach, the authors were able to evaluate the efficacy of two different treatment regimens in vivo: (1) administration of three doses every other day; compared to (2) administration of six smaller daily doses. Monitoring the real-time fluorescence uptake curve demonstrated that six daily doses gives a continuous increase in siRNA accumulation compared to three larger doses spaced further apart in time, thus guiding the treatment regimen to achieve efficacious RNAi therapy in an orthotopic PC3 tumour model.
2.2 Liposome Theranostics
Among all lipid nanoparticles, liposomes have demonstrated significant advances both as drug delivery and diagnostic tools, and represent the nanocarriers furthest along in clinical application [78–80]. Liposomes are spherical nanocarriers comprised of a self-assembled lipid bilayer and an aqueous core [81]. Liposomes have been extensively studied for drug delivery owing to their unique structure that allows for loading of hydrophilic therapeutic agents within the core, as well as hydrophobic agents within the bilayer. Recent reviews of the experimental development and clinical use of liposomes as drug delivery vehicles have focused on the emerging trend of multifunctionality (Fig. 3a) [16, 26–28]. Inclusion of functional components transforms conventional liposomes into stealth, ligand-targeted, and triggered-release liposomes. More recently, liposomes have been recognized as suitable nanocarriers for theranostics because they have ample room for both therapeutic and diagnostic agents. Inclusion of molecular and nanoparticle imaging agents can be achieved by core encapsulation, embedding in the lipid bilayer, or conjugation/adsorption to the liposome surface [84], thus enabling integration of multiple single-modality components for advanced nanotheranostics. Following this strategy, liposome theranostics has high potential for clinical translation as all of the assembled therapeutic and imaging agents, as well as the nanocarrier itself, are already approved by regulatory agencies and are well-established in the clinic. Below are highlighted the recent developments in liposome theranostics.
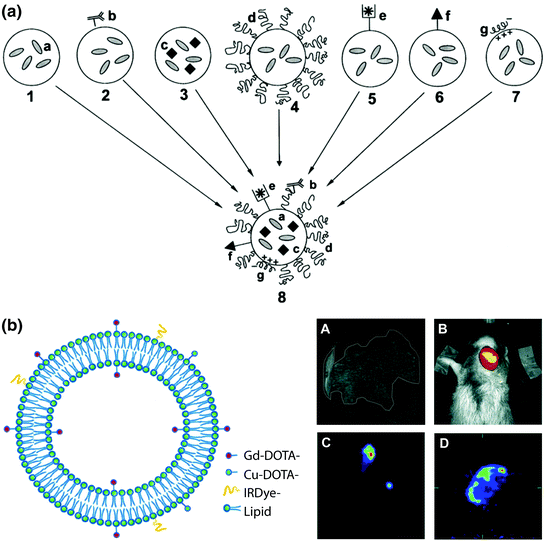

Fig. 3
Multimodal lipid nanotheranostic systems incorporating multiple single-modality agents. a Schematic representation of the “holy grail” for multifunctional liposome design, combining a small molecule drugs, b ligand targeting, c nanocrystals, d PEGylation, e chelated radionuclides, f cell-penetrating peptides, and g siRNA. Adapted with permission from Torchilin, copyright Elsevier (2006) [82]. b Left theranostic liposome with MRI, PET/SPECT, and fluorescence imaging modalities provided by inclusion of amphipathic chelators (DOTA-lipid) and fluorophores (IRdye-lipid) combined with core loading of hydrophilic agents. Right In vivo imaging of SCCHN tumour xenograft model by A MRI using Gd(III)-liposomes, B NIR fluorescence imaging using IRdye-Gd-liposomes, C SPECT using 99mTc-Gd-liposomes, and D PET using 64Cu-Gd-liposomes. Adapted with permission from Li et al., copyright American Chemical Society (2012) [83]
2.2.1 Building Imaging Functionality for Liposome Nanomedicine
Visualizing nanoparticle biodistribution and pharmacokinetics can help researchers better assess how effectively a nanocarrier accumulates in the tumour region and avoids RES clearance, and compare their design with other formulations. For example, Grange et al. recently reported liposomal dox labeled with an amphipathic form of gadolinium (Gd(III)-DOTAMA(C18)2) for MRI [85]. The authors prepared a targeted formulation using a peptide ligand that binds to the vascular factor neural cell adhesion molecule (NCAM), which is upregulated in Kaposi’s sarcoma, a highly angiogenic disease. Using MRI, the authors showed that the NCAM-targeted liposome exhibits approximately 50 % overall enhanced tumour accumulation compared to the untargeted form, which is primarily retained in the blood. Ex vivo TEM and in vitro confocal imaging indicate that this result is due to enhanced cell uptake by NCAM targeting. The lipophilic Gd(III) remained sequestered in the tumour cell membrane, enabling monitoring of the tumour response longitudinally. Using MRI, the authors were able to conclude that despite faster clearance, NCAM ligand targeting enhances liposome accumulation in Kaposi’s sarcoma and improves tumour response.
Following administration of a chemotherapeutic, patient response is assessed to optimize the subsequent dose or, in the case of a poor tumour response, to consider alternate options. For example, in breast cancer treatment, tumour response is primarily determined based on tumour staging and hormonal status. In nanomedicine, however, individual differences in tumour vasculature significantly impact delivery of the nanocarrier and must be considered. Poor vascular permeability limits tumour uptake and intratumoral distribution and is associated with faster tumour growth and poor therapeutic outcome. To provide clinicians with a tool to assess vascular permeability, Karathanasis et al. co-encapsulated dox and the CT contrast agent iodixanol in a liposome to be used for mammography [86]. In an animal study, the authors administered the liposome, once a week for 2 weeks, and found that the sum of the CT signal enhancement within the tumour region over the 2 weeks was correlated with overall survival. This relationship was present despite large heterogeneity in the two CT signals within the same animal. Using this nanotheranostic, the authors were able to predict the therapeutic outcome based on nanoparticle accumulation, and could in the future adjust the administered dose to accommodate for individual differences in vascular permeability.
MRI “smart delivery” systems are the main class of activatable theranostic liposomes reported for tracking intracellular uptake. For MRI labels such as Gd(III), the MR signal (T1 relaxivity) changes based on its environment and interaction with water molecules. Liposome-encapsulated gadolinium cannot interact with water molecules as water cannot easily permeate the lipid bilayer. In this case the T1 relaxation enhancement is minimal. Following release of the gadolinium label, interaction with water molecules increases T1 relaxivity and appears as positive contrast, or a bright spot, on the MR image. MRI contrast enhancement has been quantitatively correlated with therapeutic payload release from activatable liposome systems. Viglianti et al. co-loaded the hydrophilic MRI label MnSO4 and dox in the liposome core [87]. Using T1-weighted images, the authors developed an algorithm for quantifying dox release and MRI contrast in a rat oral fibrosarcoma model. The model was confirmed both ex vivo using liquid chromatography and by histology. A follow-up study was conducted by Tagami et al., replacing toxic MnSO4 with gadolinium using diethylene triamine pentaacetic acid (DTPA) as a chelating agent [88]. As in the first study, the release profile of dox (measured by fluorescence) and MRI contrast agent were identical, and MRI could be used to monitor drug release. Both of these studies envisaged activatable MRI as a tool for treatment planning during delivery of temperature-sensitive liposomes (TSL). TSLs such as ThermoDox® are under clinical study to selectively improve drug release at the tumour site [89–91]. The lipid content is chosen to provide a melting temperature of 40–43 °C. Co-delivery of heat (e.g., by focused ultrasound) destabilizes the lipid membrane leading to rapid release of the therapeutic agent. Due to variations in tissue structure and composition, thermal gradients can form leading to nonuniform drug distribution. Activatable MRI could be used to monitor the drug distribution in real time and adjust heat delivery to provide sufficient and uniform release.
Combining multiple imaging modalities significantly increases the power of nanotheranostics for drug development and treatment planning (i.e., multimodal imaging). As outlined in Table 1, each imaging modality suffers from technical disadvantages such as low resolution for PET/SPECT, sensitivity for MRI and CT, or tissue penetration depth for fluorescence imaging, and not all modalities are suitable for every desired application. Imaging both the systemic- and micro-distribution of nanocarriers requires the integration of noninterfering modalities to provide both high spatial resolution and sensitivity [92]. To achieve this goal, Li et al. reported a liposomal dox formulation with MRI, PET/SPECT, and fluorescence imaging functionality (Fig. 3b) [83]. During liposome synthesis, some of the lipid shell is replaced with an amphipathic chelator (DOTA-DSPE) and an MRI agent (Gd(III)-DOTA-DSPE). Postsynthesis the liposome is labeled with 64Cu for PET though metal chelation with DOTA-DSPE. Fluorescence imaging functionality is provided by postinsertion of an amphipathic fluorophore (IRdye-DSPE) into the outer shell of the lipid bilayer. For SPECT, 99mTc is encapsulated in the aqueous core. Dox is also encapsulated postsynthesis, or alternatively a therapeutic radionuclide (186Re/188Re) can be encapsulated for radiotherapy. The authors found that this formulation and synthesis procedure was more robust than other multistep encapsulation methods (<20 nm size change with loading), and has a high loading capacity for both imaging and therapy (>90 % efficiency for all imaging components and 65 % for dox). With high-resolution MRI, the authors followed liposome biodistribution and intratumoral micro-distribution in a xenograft model of head-and-neck squamous cell carcinoma. Using sensitive PET/SPECT and fluorescence imaging, tumor distribution was monitored quantitatively and longitudinally, respectively.
3 Engineering Theranostic Lipid Nanoparticles Using Intrinsically Multifunctional Components—The “One-for-All” Approach
The “all-in-one” approach for combining multiple functionalities (Fig. 3a) has been described as the “holy grail” for lipid nanomedicine [82, 93, 94]. Despite the advantages of the many advanced nanotheranostics investigated in the lab, there is a disparity between these and the simplistic nanocarriers seen in the clinic [27, 28, 95]. While transferrin conjugated and thermally sensitive liposomes are undergoing clinical trials, the only theranostic nanoparticles currently under investigation are inorganic iron oxide nanoparticles for magnetic heating and MRI contrast [96, 97].
Transitioning theranostic nanoparticles from the lab to the clinic is hindered by the current paradigm of the “all-in-one” approach [98]. Inclusion of functional units increases the complexity and cost of synthesis and purification, and may be justified due to the benefits they provide. However, physical entrapment of therapeutic and imaging agents generally leads to an increase in particle polydispersity, and this random effect is amplified if more than one agent is encapsulated. Polydispersity raises regulatory concerns because nanoparticle size and composition is uncontrolled, leading to unpredictable in vivo behavior [99, 100]. Furthermore, adding imaging agents to lipid nanoparticle drug formulations does not generally provide imaging capabilities above the threshold required for high-quality images. It is particularly difficult to achieve a therapeutic payload in lipid-nanoparticle complexes. Radiolabeling has been recognized as a possible solution because PET is a highly sensitive technique [101]. With a careful design and controlled synthesis, most regulatory challenges can be overcome.
Complementary to the “all-in-one” approach is the emerging paradigm of the “one-for-all” approach where nanoparticle components are intrinsically multifunctional. We will discuss the “one-for-all” approach using porphysomes as a representative “one-for-all” nanoparticle, and how this concept might overcome the regulatory hurdles facing theranostic lipid nanomedicine.
3.1 Porphysome Theranostics Using Porphyrin-Lipid Technology
Porphyrins are chromophores widely studied for their versatility and numerous applications in medicine and technology [102, 103]. Porphyrins have long been utilized clinically for their theranostic capabilities including fluorescence imaging and photosensitization [104, 105]. Porphyrins used in photodynamic therapy generate cytotoxic singlet oxygen as well as fluorescence upon excitation, which can be used to monitor tumor accumulation and intracellular uptake for treatment planning. In addition, photobleaching is a surrogate measurement for therapeutic activity and has been used to quantify the results of adjusting treatment parameters such as light dosimetry [106]. Encapsulated porphyrins form nonfluorescent aggregates which emit absorbed light as heat, enabling their use for hyperthermia and photothermal ablation as well as PAI [107]. Depending on their molecular structure, porphyrins can be stable metal chelators for MRI and PET/SPECT [108]. Metal chelation can also affect the photonic properties, including shifting the absorption spectrum or stabilizing thermal generation.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree