Chapter 72 Lipid Disorders
INTRODUCTION
The use of highly active antiretroviral therapy (HAART)1–3 is frequently associated with side effects. Among the most significant of these is lipodystrophy, manifested as an abnormal distribution of body fat. Characteristically fat is lost subcutaneously, particularly from the face, limbs, and buttocks, and visceral fat may be increased.4–6 In addition to these changes in fat distribution, a large proportion of patients develop insulin resistance (see Chapter 70) and elevated plasma lipid concentrations. The combination of dyslipidemia, insulin resistance, and visceral fat accumulation closely resembles the abnormalities seen in ‘the metabolic syndrome’7 and has given rise to the concern that HIV-1-infected patients treated with potent combination antiretroviral therapy (ART) may be at increased risk of developing premature coronary artery disease (CAD). In this chapter we discuss the evidence so far with respect to this concern, discuss the differential effects of antiretroviral drugs on plasma lipid concentrations, review current hypotheses with respect to the pathogenesis of dyslipidemia, and address recommendations for its treatment and prevention. However, this cannot be discussed without first addressing the effects HIV infection itself has on plasma lipid concentrations and cardiovascular risk.
DYSLIPIDEMIA AND CAD DURING THE NATURAL COURSE OF HIV INFECTION
Early in the natural course of HIV infection, as seen in other infections and chronic inflammatory conditions, plasma total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C) and low-density lipoprotein cholesterol (LDL-C) concentrations slightly decrease.8,9 This decrease is followed by a decrease in the size of LDL particles leading to an increase in more atherogenic small dense LDL particles (LDL type B).10 Late in the infection, when patients progress to a clinical diagnosis of AIDS, triglyceride concentrations may increase as well.9 This latter increase is probably caused by both an increased production of triglyceride-rich VLDL particles and a decreased clearance of VLDL by the liver. This correlates closely with circulating concentrations of interferon-α which is strongly related with disease progression in untreated HIV-1-infected patients.11 Consistent with this notion, both triglycerides and interferon-α levels were described to decrease in patients with advanced HIV disease using zidovudine monotherapy.12 Recently, Riddler et al published a longitudinal study of investigated lipid profiles in HIV seroconverters (Table 72-1).13 This study confirmed the previous findings from cross-sectional studies showing decreases in total, LDL, and HDL-C following seroconversion but prior to the initiation of HAART in asymptomatic HIV-infected individuals with a mean CD4-cell count of 307/μL at the time of second measurement.
Table 72-1 Plasma Lipid Changes After HIV-Seroconversion
| Pre Seroconversion | Post Seroconversion | |
|---|---|---|
| Total-C (mg/dL) | 201 (5.26 mmol/L) | −30 (−0.78 mmol/L) |
| HDL-C (mg/dL) | 51 (1.35 mmol/L) | −12 (−0.31 mmol/L) |
| LDL-C (mg/dL) | 122 (3.13 mmol/L) | −22 (−0.57 mmol/L) |
Plasma lipid changes following HIV-1 seroconversion. Mg/dL: milligram per deciliter. mmol/L: millimol per liter.
Total-C: total cholesterol; HDL-C: high density lipoprotein cholesterol; LDL-C low density lipoprotein cholesterol.
Several cases and a single case-control study concerning CAD in patients with HIV infection have been published in the pre-HAART era. A small series of eight necropsies was described by Tabib in 1992 which demonstrated significant coronary lesions in deceased young HIV-infected patients (23–32 years old).14 None of these patients had a known positive family history for coronary heart disease (CHD) or other known risk factors. All eight subjects showed fibrosis of their coronary arteries with foam cells, generally widely disseminated occluding 40–50% of the coronary arterial lumens similar to the lesions generally seen in older populations and quite distinct from the concentric pattern of the accelerated forms of (postgrafting) coronary disease. These results were confirmed in a relatively small case-control study using ultrasound showing an increased incidence of asymptomatic plaques in peripheral arteries in HIV-1-infected individuals compared to matched controls.15
DYSLIPIDEMIA AND CARDIOVASCULAR RISK DURING HAART
Soon after the introduction of HAART several case reports were published suggesting that patients receiving HAART were prone to the development of premature CAD.5,16–18 This concern led to numerous studies addressing the question as to whether HAART is an independent risk factor for CAD. When addressing this question, it is very important to take into account the prevalence of known cardiovascular risk factors in the HIV-infected population being studied. For instance, the Data collection of Adverse effects of anti-HIV Drugs (D:A:D) study,19 found that HIV-1-infected patients (both treated or therapy-naive; n = 17 852 patients) showed an above average prevalence of various well-known risk factors for CAD. For instance, 50% of all HIV-infected individuals were current smokers compared to 30–40% in the general population. Furthermore, 8% of patients at the time of enrollment into this study had hypertension, 22% had elevated TC, 28% elevated triglyceride concentrations, and 25% were 45 years of age or older.
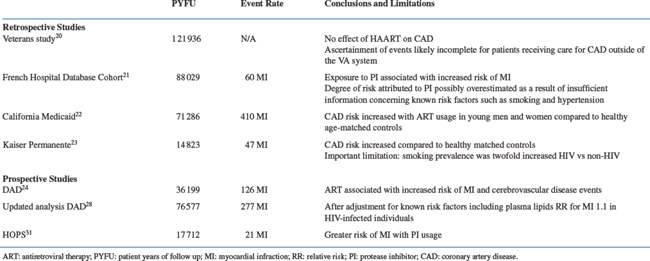
Retrospective Studies
The largest retrospective analysis of whether HAART results in an increased incidence of CAD is a study by Bozzette et al who extracted data from the Master Veteran Record National Database and VA National Patient Care databases in the United States (Table 72-2). These anonimized databases contain information on virtually all patients receiving care within the VA system.20 In this study 36 766 HIV-1-infected patients (almost exclusively men) were followed for an average of 40 months from 1993 to 2001 (total patient-years of follow-up (PYFU) 121 936 years). The authors concluded that the overall incidence of cardiovascular and cerebrovascular events did not change over time. Furthermore, the authors did not find an increased incidence of CAD in the patients taking protease-inhibitor (PI)-based HAART compared to patients that did not. One important issue that might have biased the interpretation of the results is the fact that only patients admitted to a VA clinic were identified as cases. Since patients who receive ambulatory care within the VA system for acute coronary events are often admitted to hospitals outside of the system, this may have led to an underestimation of the true incidence of CAD. Furthermore, the duration of exposure to PI was relatively short (16 months). Another large study was published by Mary-Krause et al21 utilizing the national French Hospital Database among 34 976 French HIV-1-infected men with a total of 88 029 PYFU. This study did find a relation between more prolonged use of PI and the occurrence of myocardial infarction (MI). Exposure to PI was associated with a relative hazard for MI of 2.56 (95%CI 1.03–6.34). The standardized morbidity ratio of men exposed to PI for >30 months compared to the general French male population, was 2.9 (range 1.5–5.0). The California Medicaid database consisting of 28 513 HIV-1-infected patients with 71 286 PYFU found that the overall incidence of CAD was increased for young men and women.22 In 18–33-year-old men the relative risk was 2.16–6.76 (P < 0.0001), whereas in 18–44-year-old women this was 1.53–2.47 (P < 0.011) compared to matched healthy uninfected controls. The relatively small Kaiser Permanente registry study looked at discharge diagnoses in 4159 HIV-1-infected individuals with 14 823 PYFU.23 They found an increased age-adjusted event rate of CAD (6.3 per 1000 patient-years) in HIV-1-infected individuals compared to HIV negative (3.7 per 1000 patient-years, P <0.01). Of note is the fact that in those HIV-positive the prevalence of smoking was increased twofold in HIV-positive patients compared to the HIV-negative persons. In this latter study PI-usage was not identified as being an independent risk-factor for CAD.
Prospective Studies
Currently, there are two large prospectively designed cohort studies that have reported data regarding the incidence of coronary and cerebrovascular events in HIV-infected individuals (Table 72-2).
The largest cohort is the D:A:D.24 This multicontinental study included cohorts from Europe, Australia, and the United States and consisted of 23 468 patients who had been included into the study between December 1999 and April 2001. During 36 199 PYFU, 126 MIs occurred. The incidence of MI increased with longer exposure to combination ART (adjusted relative rate per year of exposure, 1.26 (95% confidence interval (CI), 1.12–1.41); P <0.001). This implies a 26% increase in the relative risk of having an MI per additional year of HAART usage. This effect was independent of a number of known CAD risk factors which were also significantly associated with MI, including older age (adjusted relative rate per additional 5 years, 1.38 (95% CI, 1.26–1.50); P <0.001), history of current or former smoking (adjusted relative rate, 2.17 (95% CI, 1.30–3.62); P =0.007), previous cardiovascular disease (adjusted relative rate, 5.84 (95% CI, 3.51–9.72); P <0.001) and male gender (adjusted relative rate, 1.99 (95% CI, 1.04–3.79; P =0.04). A higher total serum cholesterol level, a higher triglyceride level, and the presence of diabetes were also associated with an increased incidence of MI. As noted before the prevalence of known cardiovascular risk factors was high in this population (56% of the total population were either current or former smokers). A substudy from D:A:D demonstrated differential effects of different types of regimens on plasma lipoprotein profiles in previously therapy-naive HIV-1-infected patients.25 In the majority of patients first-line therapy consisted of a backbone of two nucleoside reverse transcriptase inhibitors (NRTIs) with the addition of either a PI, or a non-nucleoside reverse transcriptase inhibitor (NNRTI). The PI-based regimens resulted in a potentially more atherogenic lipid profile compared to both therapy-naive and NNRTI-based regimes. This differential effect is similar to the results previously found in a prospective randomized clinical trial comparing PI with NNRTI-based therapy.26,27 A recent update from D:A:D showed that PI exposure was associated with an increased risk of MI which was markedly similar to that found in the initial analysis for exposure to combination ART as a whole (RR 1.16 (1.10–1.23), P =0.0001).28 This effect was not seen for NNRTI exposure. The effect of PI on the increased risk of MI could partly, but not fully, be explained by the dyslipidemia known to be induced by this drug class.
The original analysis within D:A:D with regard to MI has also been extended to cardiovascular and cerebrovascular disease events (CCVE).29 The endpoint of this analysis was the occurrence of a first CCVE during prospective follow-up, defined as the first of any of the following events: acute MI, performance of an invasive cardiovascular procedure (specifically coronary artery angioplasty, bypass, or carotid artery endarterectomy), stroke, or death from other CCVE. A total of 268 events occurred in 207 patients over 36 145 PFYU. There were 134 MI among 126 patients (first event in 126 patients), 87 invasive cardiovascular procedures in 82 patients (first event in 39 patients), 41 strokes in 39 patients (first event in 38 patients), and six patients died from CCVE (first event in four patients). The multivariate analysis showed results similar to the original analysis which only looked at cases of first MI. Smoking, older age, a previous history of CCVE, a positive family history of CCVE or male gender were independently associated with an increased prevalence of CCVE. After controlling for these variables the incidence rate of CCVE increased by 26% per additional year of exposure to combination ART (RR per year of exposure, 1.26; 95% CI, 1.14–1.38; P = 0.0001). This analysis was not adjusted for current or previous antiretroviral agents used by patients. Additional analyses tested the association between CCVE and a number of possible metabolic and physiological causative factors. Factors independently associated with the risk of CCVE from these analyses were (time-updated): cholesterol (RR 1.11; 95% CI 1.03–1.19 per mmol higher; P = 0.008); triglycerides (RR 1.30; 95% CI 1.12–1.51 per log2 mmol higher; P = 0.0006); diabetes mellitus (RR 2.22; 95% CI 1.46–3.37; P = 0.0002); and hypertension (RR 1.79; 95% CI 1.25–2.56; P = 0.001). Adjustment for these factors in multivariable models that also included the risk factors for CCVE, led to a small reduction in the RR associated with exposure to ART, although this variable remained significantly associated with CCVE in all analyses (relative rates ranging from 1.21 to 1.25 in the individual analyses). A similar effect was recently reported within D:A:D for risk factors for a first MI, now including 277 cases of first MI during 76 577 PYFU.30 The relative rate of MI per additional year of exposure to HAART, after adjusting for age, gender, history of smoking, prior CAD, and family history, was 1.17 (95% CI 1.08–1.26) in this updated analysis. After also adjusting for time-updated changes in total and HDL cholesterol as well as triglycerides, the relative rate was reduced to 1.10 (95% CI 1.01–1.19). This again suggests that the association between HAART use and risk of MI may partly, but not completely, be explained by the effect of treatment on lipids.
The second large prospective cohort study is the HIV Outpatient Study (HOPS) consisting of 5672 patients with 17 712 PYFU.31 The majority of patients were men (82%), and the incidence of MI rose over time. A total of 21 MIs occurred. Nineteen events were in patients on PI-containing regimens resulting in an odds ratio of 1.42 per 1000 person-years of observation, compared to only 2 in patients who did not use a PI (0.46 per 1000 person-years of observation). Controlling for known CAD risk factors showed similar results. These findings suggest the risk of CAD is increased in patients using PI-based therapy.
Surrogate Endpoints
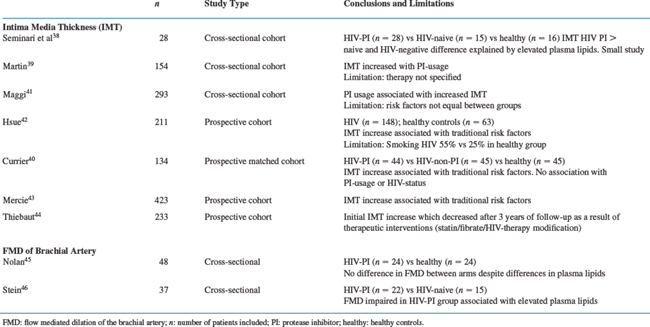
In recent years several noninvasive imaging tools have been introduced for assessing atherosclerosis progression/regression in the general population, as potential surrogates for clinical endpoints (Table 72-3). A recent paper by Sankatsing et al provides an extensive overview of these different techniques.32
The technique most frequently utilized in HIV-infected individuals is the measurement of carotid intima-media thickness (IMT) by high-resolution B-mode ultrasonography. This noninvasive technique allows for real-time in vivo imaging of all stages of atherosclerosis and offers a good reproducibility under standardized conditions. Three large observational studies have shown that carotid IMT in the general population is an independent risk factor for CAD and can be used as a surrogate marker for cardiovascular disease.33–35 In numerous intervention studies with statins conducted in the general population, carotid IMT has proven to be a reliable parameter to assess efficacy of lipid-lowering therapy.36,37 Moreover carotid IMT is strongly correlated with other cardiovascular risk factors such as LDL-C and HDL-C and hypertension. However, a potential problem may lie in the great variability between available measurement protocols.
Following the report by Seminari et al in 2002 of a small cross-sectional study suggestive of increased carotid IMT values in patients with PI-induced hyperlipidemia,38 several larger studies have been published with different results. The key issues when interpreting the results of all of these studies are whether the effects seen on carotid IMT directly result from any of the individual antiretroviral agents being used, or are secondary to therapy-induced lipid changes, as well as to the influence of HIV itself, the immune system and other known CAD risk factors.
A recent single-center cross-sectional cohort study by Martin et al examined 154 patients and found that carotid IMT was significantly correlated with known cardiovascular risk factors including systolic blood pressure, plasma lipoprotein and glucose concentrations and age.39 Before adjustment for such cardiovascular risk factors the cumulative exposure to PI but not NRTI or NNRTI was correlated with carotid IMT (P = 0.02). Unfortunately, the authors did not specify the exact number of patients in the different treatment groups making it very hard to interpret these results. After adjustment for known cardiovascular risk factors the cumulative exposure to lopinavir/ritonavir (RTV) remained correlated with carotid IMT (P = 0.01). Interestingly, in 35 patients without a history of prior cardiovascular events, who either had carotid IMT values greater than 0.75 mm and/or a Framingham risk score greater than 3, the authors performed myocardial perfusion scintigraphy during exercise and did not identify a single case who would qualify for a cardiovascular intervention.
A prospective matched cohort study by Currier et al compared HIV-infected individuals using PI-based therapy for more than 2 years (n = 44), with those who did not (n = 45) and with an HIV-uninfected control group (n = 45). All were matched for age, sex, ethnicity, smoking status, blood pressure, and menopausal status.40 This study found an association between carotid IMT and traditional CAD risk factors but not with HIV infection or with the use of PI.
A cross-sectional cohort study of 293 patients by Maggi et al concluded that PI-treated patients (n = 105) had a higher prevalence of premature carotid lesions. However, patients were not matched for cigarette smoking and family history of cardiovascular disease which represents an important potential bias.41 Hsue et al in a prospective study demonstrated that carotid IMT was increased in 148 HIV-infected individuals compared to healthy controls and was clearly related to traditional risk factors.42 Again, there was a significant difference in the percentage of smoking between the groups (HIV-infected 55% vs 25% in non-HIV-infected, P <0.01). In another large prospective cohort study with 1 year follow-up (n = 346) Mercie et al likewise concluded that traditional risk factors were associated with an increase in carotid IMT.43 In light of the latter two studies Thiebaut et al published a prospective cohort study with 3 years of follow-up in 233 HIV-infected individuals (86% using HAART at baseline) that clearly showed that although carotid IMT initially increased at 12 months it normalized to baseline values at 36 months of follow-up.44 This decrease seemed to be largely driven by increased prescribing of statins and/or fibrates and/or switching to more lipid-friendly antiretroviral regimens which resulted in a decrease in total and LDL cholesterol, which may have been the result of an increased awareness among physicians concerning CAD risk in patients with HIV.
A very early sign of atherogenesis which precedes the occurrence of atherosclerotic lesions is endothelial dysfunction which can be measured using flow-mediated dilatation (FMD) of the brachial artery and is associated with all known risk factors such as hypertension, smoking, hypercholesterolemia and diabetes mellitus. Two small studies showed contrary results with respect to the effect of PI on FMD.45,46 Related to this, a study by Dressman et al in mice suggested that PI may not only affect endothelial atherosclerosis indirectly through effects on lipids, but also directly through other mechanisms.47 Mice in this study were treated with the PI RTV, indinavir or amprenavir at both low and high doses for 8 weeks. At high doses plasma triglycerides and TC levels increased (RTV > indinavir > amprenavir), whereas at low doses no changes in plasma lipoproteins were observed in that study. When the ascending aortas from these mice were examined, extensive atherosclerotic lesions were observed in both groups, albeit more so at high rather than low doses of PI. Therefore, the development of atherosclerosis in these mice seemed at least in part independent of the induction of dyslipidemia. The direct effect of PI was mediated by an up-regulation of the scavenger receptor CD36 in macrophages. The administration of PI to CD36 knock-out mice did not result in atherosclerosis.
With respect to the possible direct effects of PI on endothelial function in humans, 4 weeks of treatment with indinavir in healthy HIV-negative men resulted in significantly impaired endothelial function in the absence of significant effects on plasma lipoprotein concentrations.48 The authors speculate that this effect was induced by an impairment of nitric oxide production at the level of the endothelium which was previously demonstrated in vitro.49 The administration of indinavir in these healthy men did result in statistically significant reduction in insulin sensitivity. Based on earlier studies, this probably results from a direct impairment of the activity of the glucose transporter GLUT-4 by indinavir.50–55 However, since the observed changes in insulin concentrations were relatively small and not correlated with changes in endothelial function the authors suggested that an as yet unexplained direct effect of PI on endothelial function may exist. Several recent in vitro studies demonstrated that another PI RTV decreases endothelium-dependent vasorelaxation in cultured porcine carotid arteries.56–59 This seemed to be mediated by an increase in superoxide anion production resulting in increased oxidative stress. Bonnet et al, in a cross-sectional case-control study of endothelial function and carotid IMT in HIV-infected children (including a large proportion of therapy-naive patients), reported endothelial function to be impaired in both treatment-naive and treated HIV-infected children compared to age- and sex-matched healthy children, whereas no changes in carotid IMT were found.60 No differences were found between treated and therapy-naive children. Since most of the traditional risk factors for CAD are not present in children, this strongly suggests that HIV infection itself may contribute to the development of endothelial dysfunction. Indirect evidence for this is also provided by the results of a recent AIDS Clinical Trials Group (ACTG) study A5152s.61 In this large randomized clinical trial which compares PI-sparing (2 NRTIs + efavirenz) with NNRTI-sparing (2 NRTIs + lopinavir/RTV) and NRTI-sparing (efavirenz + lopinavir/RTV) therapy in previously antiretroviral-naive patients endothelial function was prospectively assessed in a subgroup of 82 patients. Although the investigators remain blinded to the actual treatment, significant improvements in endothelial function as measured by FMD of the brachial artery were seen following 24 weeks of therapy in each of the treatment arms. Interestingly, although significant differences were observed between treatments with respect to changes in plasma lipoproteins, no consistent correlation could be found between changes in FMD and changes in any lipoproteins. This again suggests that HIV infection per se may have an effect on endothelial function which may be reversed by suppressing HIV at least over the short term.
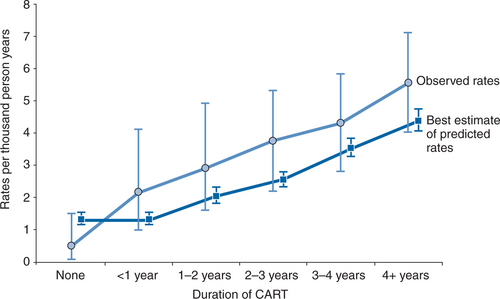
As indicated before, an updated analysis from the D:A:Dstudy suggests that the association between HAART use and risk of MI may partly, but not completely, be explained by the effect of treatment on lipids.30 This may also explain why the rate of MI which was observed in D:A:D was consistently higher than the one predicted when using the Framingham algorithm62 (Fig. 72-1). Another potential explanation may be that the D:A:D population is younger than the one on which the Framingham algorithm has been based, and many of the same CVD risk factors are known to carry a higher risk in younger compared to older individuals. Importantly, both curves are parallel which suggests that algorithms such as Framingham can reasonably be used clinically for broadly assessing CAD risk in patients with HIV until an algorithm has been developed which is specific to HIV-infected populations. However, given that changes in plasma lipid profiles do explain at least some of the incremental risk of MI, differences between individual drugs and ART regimens on lipids are important to address.
Antiretroviral Drugs/Drug Classes: Effects on Plasma Lipids
Soon after the first indication that ART might be associated with hyperlipidemia the measurement of plasma lipid profiles was incorporated in many randomized clinical trials. As briefly mentioned before it became clear that different antiretroviral regimens differ with respect to their effects on plasma lipids. For instance, patients at time of entry into the D:A:D study who were on first-line regimens including PIs had higher TC and triglyceride concentrations as well as higher TC over HDL cholesterol ratios than did antiretroviral-naive patients.25 In contrast, NNRTI-based therapy showed a potentially more cardio-protective plasma lipoprotein profile, by virtue of being associated with higher levels of HDL-C. Similar results were reported by Young et al from the Swiss HIV cohort study.63
NON-NUCLEOSIDE REVERSE TRANSCRIPTASE INHIBITORS
Treatment with a nevirapine-containing regimen in a trial conducted in antiretroviral-naive patients was associated with a 49% increase in HDL cholesterol and a decrease in the ratio of TC over HDL-cholesterol when compared to an indinavir-based regimen as well as a regimen consisting of three nucleosides, in the absence of significant differences in antiviral potency.26 The study which has provided the best data comparing the effect of nevirapine and efavirenz is the 2NN study. In this trial, again conducted in previously ART-naive patients, both nevirapine- and efavirenz-containing regimens showed increases in HDL-C and a reduction in the ratio of total over HDL cholesterol, with these changes being most profound in the nevirapine-treated patients.64 Both in the 2NN and D:A:D study, efavirenz-based therapy was associated with a higher plasma triglyceride concentration, whereas this was not the case for nevirapine-containing regimens.25 Treatment with delavirdine resulted in a 13.2% increase in HDL-C after 8 weeks of treatment, whereas no changes were observed in PI-treated patients.65
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree