Heather L. Cox, Gerald R. Donowitz
Linezolid and Other Oxazolidinones
The oxazolidinones are a class of antimicrobial agents prepared completely by organic synthesis. In 1978, a patent was issued to E. I. du Pont de Nemours and Company for a series of 5-(halomethyl)-3-aryl-2-oxazolidinones that had antimicrobial activity against plant pathogens. Further manipulation of the molecule led to the development of linezolid, which displayed activity against human pathogens.1 A number of oxazolidinones are being investigated. Only linezolid (Zyvox) is approved by the U.S. Food and Drug Administration (FDA) for clinical use.
Chemical Structure
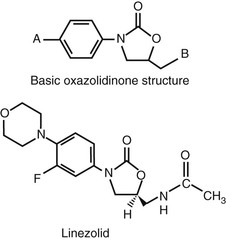
The basic molecular structure of the oxazolidinones is shown at the top of Figure 32-1. Initial chemical manipulation included the incorporation of a piperazine moiety into the basic structure at site A. Antibacterial activity was increased by the addition of a hydroxyacetyl group to the heterocyclic nitrogen at site B. Further antibacterial activity was derived by fluorine substitution at the phenyl 3 position. Only the enantiomers with a 5S acetamidomethyl configuration have antibacterial activity. The molecular structure of linezolid is shown at the bottom of Figure 32-1. The unique chemical structure of the compound makes cross-resistance with older compounds such as β-lactams and vancomycin, as well as newer agents such as quinupristin-dalfopristin and daptomycin, unlikely.
Mechanism of Action
The oxazolidinones are inhibitors of protein synthesis and are bacteriostatic against most bacteria. The mechanism of action is believed to be unique, involving inhibition of the earliest steps of bacterial protein synthesis.2 These agents bind to the 50S ribosome at its interface with the 30S unit, thereby preventing the formation of the 70S initiation complex. Some data have shown that production of the 50S subunit may also be inhibited in some bacteria.3 Binding is competitively inhibited by chloramphenicol and lincomycin, which suggests either shared or overlapping binding sites.2 It has been shown that oxazolidinones do not inhibit the formation of initiator transfer RNA (N-formylmethionyl-tRNA). The ribosomal peptidyltransferase center appears to be the major site of drug action.4
Antimicrobial Activity
Linezolid has consistent activity against the majority of clinically important gram-positive organisms, including Staphylococcus aureus (methicillin-susceptible and methicillin-resistant [MRSA] strains), coagulase-negative staphylococci, Enterococcus faecium and Enterococcus faecalis (vancomycin-susceptible and vancomycin-resistant strains), and streptococci (including penicillin-resistant strains of Streptococcus pneumoniae) (Table 32-1).2,5–8 The distribution of minimal inhibitory concentrations (MICs) for most of these organisms is unimodal and typically ranges from 0.12 µg/mL or less to more than 8 µg/mL for staphylococci, enterococci, and streptococci.6,7 Although linezolid is usually bacteriostatic, it is bactericidal against strains of S. pneumoniae. However, tolerant strains have been reported.5,9
TABLE 32-1
In Vitro Susceptibility of Common Aerobic Gram-Positive Organisms to Linezolid
| ORGANISM | MIC90 (µg/mL) | SUSCEPTIBLE (% of strains) |
| Staphylococcus aureus | ||
| Methicillin-susceptible | 2 | 100* |
| Methicillin-resistant | 1-2 | 99.8-100 |
| Coagulase-negative staphylococci | ||
| Oxacillin-susceptible | 1 | 99.6-100 |
| Oxacillin-resistant | 1 | 98.3-98.4 |
| β-Hemolytic streptococci | 1 | 100 |
| Streptococcus pneumoniae | 1 | 100* |
| Viridans group and other streptococci | 1 | 99.8-100 |
| Enterococci† | 1-2 | 99.6-99.7 |
* A resistant strain has been reported.
† Includes vancomycin-susceptible and vancomycin-resistant strains.
MIC90, minimum concentration at which 90% of strains are inhibited.
Other gram-positive organisms appear to be susceptible to linezolid, although fewer strains have been evaluated. These include Corynebacterium spp., Listeria monocytogenes, Bacillus spp., Micrococcus spp., Erysipelothrix rhusiopathiae, Leuconostoc spp., Rhodococcus equi, Pediococcus spp., and many strains of Actinomyces spp.5,10
Linezolid has demonstrated activity against Neisseria spp., inconsistent activity against Haemophilus influenzae,2,11 and virtually no activity against other gram-negative bacteria. Resistance in organisms such as Escherichia coli appears to be related to an efflux pump that effectively removes the drug from within the bacterial cell.2 Linezolid has in vitro activity against Bacteroides fragilis, Clostridium spp. including Clostridium difficile, Fusobacterium, and anaerobic cocci, but clinical information is sparse.5,12
Mycoplasma pneumoniae and Ureaplasma urealyticum are usually resistant to linezolid. Mycoplasma hominis is susceptible.13 Linezolid has activity against a wide number of strains of Nocardia with MIC90 values of 1 to 4 µg/mL.14 Virtually all strains tested have proved susceptible. Finally, linezolid has demonstrated in vitro activity against Mycobacterium tuberculosis, Mycobacterium avium complex, Mycobacterium marinum, and strains of rapidly growing mycobacteria.2,15–17
Significant synergy of linezolid with other antimicrobial agents has not been consistently documented. However, when used with gentamicin, linezolid demonstrated bactericidal rather than bacteriostatic activity against streptococci. This finding was not observed with staphylococci.18
Pharmacology
Absorption after ingestion is rapid, with peak serum levels occurring after 1 to 2 hours. Bioavailability approaches 100%. Intravenous or oral dosing at 625 mg twice daily maintains mean serum concentrations at the MIC90 or higher for target pathogens throughout the dosing interval.19 The most common dosing regimen of 600 mg intravenously or orally twice daily generates peak serum levels of 15.1 and 21.2 µg/mL, respectively.20 These concentrations represent data from small numbers of healthy volunteers and likely do not represent significant differences between formulations.
Linezolid is 31% bound to plasma proteins.20 Penetration into various body sites has been documented in small numbers of patients. Trough cerebrospinal fluid concentrations in patients after neurosurgery and in a patient with meningitis ranged from 1.46 to 7.0 µg/mL21,22 with cerebrospinal fluid/plasma ratios greater than 1.21 Peak cerebrospinal fluid concentrations of 3.12 to 12.5 µg/mL have been documented in patients with meningitis.22,23 Concentrations adequate to treat most relevant pathogens are achievable in pulmonary epithelial lining fluid, alveolar cells, pancreatic secretions, and bone.24
Linezolid is metabolized by oxidation and appears to interact minimally with cytochrome P-450 enzymes. Urinary excretion accounts for approximately 85% of drug elimination, with 30% to 40% of drug excreted unchanged. Fecal excretion of its two major metabolites accounts for most of the remaining drug.19,20 The elimination half-life in adults is approximately 5.5 hours. No dose adjustment has been suggested for patients with renal or hepatic insufficiency. However, because linezolid and its metabolites are removed by dialysis, administration after hemodialysis is suggested.20 Similarly, continuous renal replacement modalities also remove linezolid but no routine change in dosage has been recommended.25
The approved dose of linezolid for adults and adolescents is 600 mg intravenously or orally every 12 hours for serious infections, with a dose of 400 mg every 12 hours for adults with uncomplicated skin and soft tissue infections. Pharmacokinetic/pharmacodynamic parameters most predictive of efficacy are the time above MIC (T > MIC) and the ratio of the area under the concentration-time curve (AUC) to the MIC (AUC/MIC).19 A study of intermittent versus continuous infusion of linezolid in 18 critically ill patients showed that administration by continuous infusion was more likely to achieve proposed targets of T > MIC exceeding 85% and AUC/MIC values between 80 and 120. However, the study was not designed to determine clinical efficacy.19,26
Linezolid does present some potential for drug-drug interactions. When administered to healthy volunteers in combination with rifampin, a 32% decrease in the linezolid AUC was observed.27 Conversely, the addition of clarithromycin produced a more than threefold increase in the linezolid AUC in a patient receiving treatment for extensively drug-resistant M. tuberculosis.28 Although the clinical significance of these interactions remains unclear, some have proposed that they may be explained by linezolid acting as a P-glycoprotein substrate and that therapeutic drug monitoring may be warranted.28,29 Drug-drug interactions with serotonergic and adrenergic agents are discussed later in the chapter under “Untoward Reactions.”
Clinical Use
Linezolid has been approved by the FDA since April 2000 for use in a variety of clinical situations involving gram-positive organisms that may or may not be resistant to more commonly used agents. Current FDA-approved indications include (1) infections with vancomycin-resistant Enterococcus faecium, including those with associated bacteremia; (2) nosocomial pneumonia caused by Staphylococcus aureus (methicillin-susceptible and methicillin-resistant strains) and Streptococcus pneumoniae; (3) uncomplicated skin and skin structure infections caused by S. aureus (methicillin-susceptible strains only) and Streptococcus pyogenes; (4) complicated skin and skin structure infections, including diabetic foot infections (without osteomyelitis) caused by S. aureus (methicillin-susceptible and methicillin-resistant strains), S. pyogenes, and Streptococcus agalactiae; and (5) community-acquired pneumonia caused by S. aureus (methicillin-susceptible strains) and S. pneumoniae (including cases with concurrent bacteremia).20 Therapy for infections caused by specific organisms is described in the following paragraphs.
Staphylococcus aureus Including MRSA
Linezolid has been shown to be as effective or superior to comparators in patients with complicated skin and soft tissue infections, including susceptible strains of staphylococci.10 It has also been evaluated for therapy of a variety of infections with MRSA. A single open-label study of patients with suspected or proven MRSA-complicated skin and soft tissue infections showed better outcomes with linezolid versus vancomycin; however, adverse effects were significantly more common among linezolid-treated patients.10 Linezolid has also been compared with vancomycin for the treatment of nosocomial pneumonia. In pooled data from two randomized, double-blind studies, linezolid in combination with aztreonam proved equivalent to vancomycin plus aztreonam for treatment of nosocomial pneumonia in which S. aureus (both MSSA and MRSA) and S. pneumoniae were the most commonly isolated gram-positive pathogens.30 Subgroup analysis revealed that linezolid was associated with significantly higher clinical cure and improved survival when considering only those treated for MRSA infection; however, the methodology involved in the subgroup analysis has been questioned.31 In a subsequent randomized, double-blind, multicenter study of linezolid versus dose-optimized vancomycin for the treatment of hospital-acquired or health care–associated MRSA pneumonia by the same investigators, significantly higher cure rates were observed in the linezolid arm. More patients randomized to vancomycin were mechanically ventilated and had MRSA bacteremia at baseline, while the median serum trough concentration at day 3 was suboptimal at 12.3 µg/mL. The incidence of adverse effects was similar, although nephrotoxicity occurred more frequently in the vancomycin group, particularly in the setting of baseline glomerular filtration rates greater than 50 mL/min. No difference in all-cause 60-day mortality was observed.32
Although levels of evidence vary, guidelines for the treatment of MRSA infections in adults and children recommend linezolid as initial or alternative therapy for complicated skin and soft tissue infections, pneumonia, bone and joint infections, and central nervous system infections and for select patients with persistent bacteremia and/or vancomycin treatment failure.33 Linezolid has been used as therapy in cases of MRSA endovascular infection with mixed results.34 Animal models have suggested that linezolid serum concentrations exceeding those achievable with present dosing regimens are required for successful therapy.35 Lower than expected concentrations may also play a role in clinical failure.36
Increasing MICs to vancomycin have been observed to lead to poorer clinical outcomes even with MRSA strains considered to be vancomycin susceptible.37 Consequently, the role of vancomycin versus other agents as first-line therapy for MRSA infections has been questioned. Data would suggest that vancomycin should be viewed as first-line therapy for MRSA infections where MICs of the organism are less than 2 µg/mL. MICs greater than or equal to 2 µg/mL or lack of clinical response to vancomycin could be cause to use alternative agents including linezolid for MRSA infections.38,39
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree