81.1
Introduction
Analysis of the iliac crest bone biopsy provides information on cellular behavior and the material properties of bone matrix that cannot be obtained by any other technique. For many years now the US Food and Drug Administration (FDA) and the EMA have required bone biopsies to be performed in phase III clinical trials. This has yielded important insights into both the safety of osteoporosis medicines and their mechanism of action. This chapter will attempt to provide a comprehensive review of the effects of approved osteoporosis drugs on human iliac bone. The drugs will be considered under two categories: anticatabolic, also known as antiresorptive, and anabolic .
81.2
Anticatabolic therapies
The tissue and cellular mechanism of bone loss in postmenopausal women with osteoporosis are mainly due to an imbalance in bone remodeling, with bone resorption exceeding bone formation, resulting in a negative bone balance in each remodeling transaction. The higher the turnover and the more negative the bone balance, the greater the loss of bone mass and structural integrity. In the prevention and treatment of osteoporosis, anticatabolic therapies suppress bone resorption by decreasing the number, activity, and life span of osteoclasts and consequently reduce the bone turnover rate. This may also be accompanied by an improvement in the bone balance in each basic multicellular unit (BMU). By these actions, anticatabolic therapies are capable of maintaining or increasing bone mass, maintaining bone microarchitecture, and improving bone mineralization and mechanical properties, ultimately reducing fracture risk. The following sections summarize the findings from bone biopsy studies from major clinical trials and studies with anticatabolic agents applied in a clinical setting.
81.2.1
Calcitonin
Calcitonin is a polypeptide hormone secreted by the parafollicular cells of the thyroid gland in mammals and by the ultimobranchial glands of birds and fish. Calcitonin nasal spray, a synthetic polypeptide of 32 amino acids in the same linear sequence as calcitonin of salmon origin, is FDA-approved for the treatment of postmenopausal osteoporosis ( Chapter 79 : Long-term bisphosphonate treatment: continuation and interruption). Calcitonin increases bone mass in the spine and modestly reduces bone turnover in postmenopausal women with osteoporosis. Intranasal calcitonin has been shown to reduce the risk of vertebral fractures modestly, but efficacy on nonvertebral fractures has not been shown . The effects of calcitonin on iliac bone have been reported in numerous studies in patients with osteoporosis and, in one report, in patients with rheumatoid arthritis . The characteristics of these studies are summarized in Table 81.1 . These studies demonstrated that in iliac bone, calcitonin treatment reduced eroded surface and active resorption surface compared to baseline and mean resorption rate compared to placebo in cancellous bone. However, no significant change was observed in osteoclast number compared to baseline . While most of the studies failed to find any significant change in variables of bone formation, such as osteoblast number and perimeter, osteoid perimeter and thickness, mineralized perimeter and mineral apposition rate (MAR) , Gruber et al. suggested that the treatment did not result in a depression of osteoblastic bone formation that was commensurate with its antiresorptive action . In a novel study, Pepene et al. measured growth factors levels, including insulin-like growth factors (IGF) I and II and transforming growth factor-β1, in iliac crest bone biopsies from 170 patients with osteoporosis who had been treated with fluoride salts, hormone therapy (HT), or calcitonin . None of the treatments was shown to affect bone matrix growth factor levels.
| Reference | Subjects | Regimen | Duration (months) | Primary outcome |
|---|---|---|---|---|
| Gruber et al. |
| Calcitonin 100 MRCU daily | 24 months | Paired histomorphometry, calcium (serum, urine, and total body) |
| Marie et al. |
| Calcitonin 50 IU×5 days every third week | 6 months | Paired histomorphometry |
| Alexandre et al. |
|
| 12 months | Paired histomorphometry |
| Palmieri et al. |
| Calcitonin 40 MRCU three times a week | 2–5.5 years |
|
| Kroger et al. |
| Calcitonin 200 IU three times a week | 3 months | Paired histomorphometry |
| Gruber et al. |
| Calcitonin 100 MRCU daily | 24 months | Paired histomorphometry |
| Pepene et al. | OPTreated n =16 Control n =42 | Calcitonin 100–200 IU daily | ~9 months | Concentration of IGF-I and II, TGF-β1 in iliac bone matrix |
| Chesnut et al. |
| Calcitonin 200 IU daily | 24 months |
|
a Continuous oral phosphate therapy (Foslymar, 1500 mg/day).
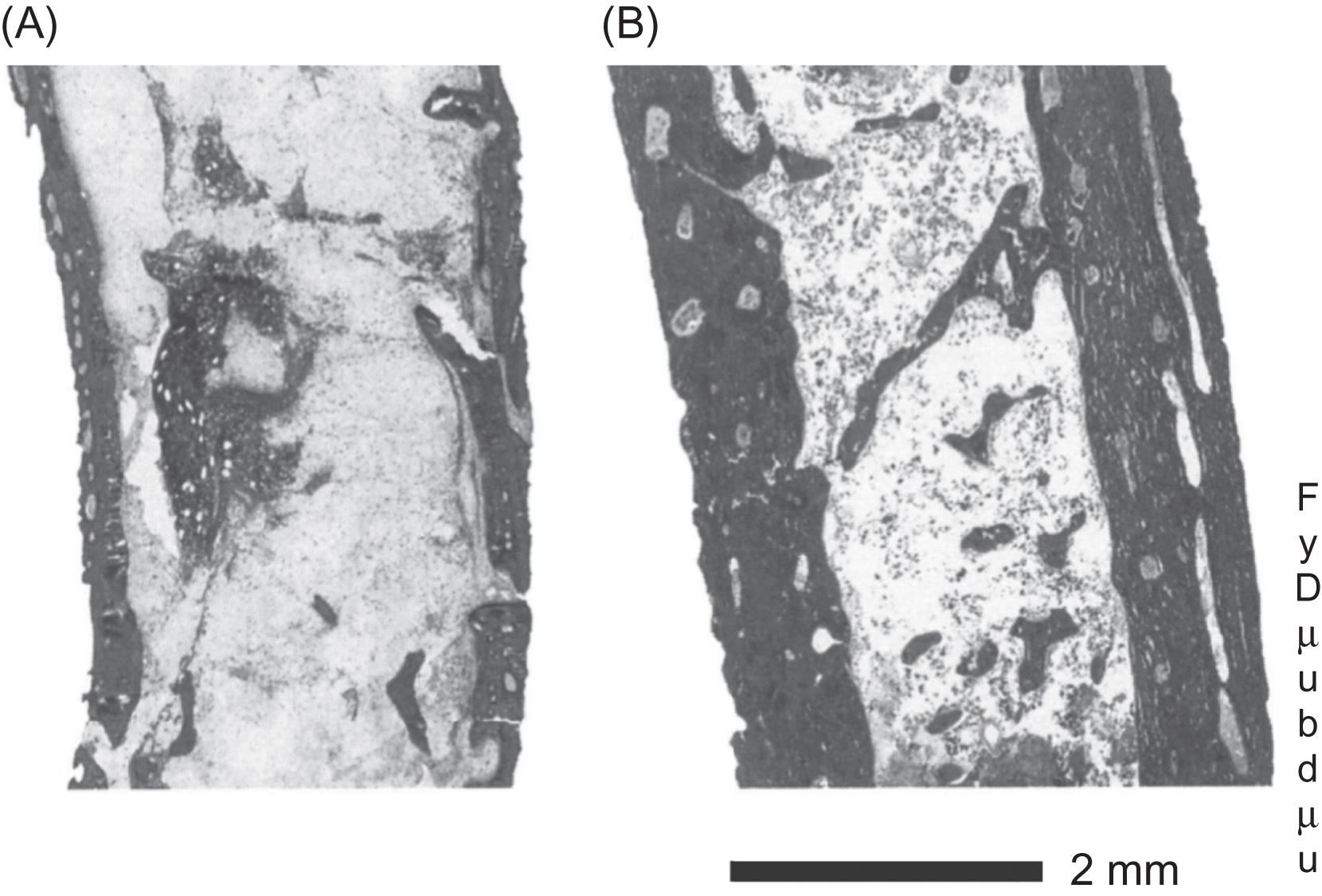
The effects of calcitonin on cancellous bone volume were reported as either no change or an increase . In one novel protocol , calcitonin was given in combination with phosphate, and the resulting improvement in cancellous bone volume was attributed to a reduction in bone resorption, accompanied by an increase in bone formation ( Fig. 81.1 ). In a later clinical trial , the so-called QUEST study, both micro-computed tomography (micro-CT) and histomorphometric analysis of iliac bone did not reveal significant differences in bone microarchitecture between calcitonin- and placebo-treated groups. The differences in the responses of bone volume and bone resorption to calcitonin treatment among those studies may reflect differences in the patient populations and the treatment regimens, as well as the relatively small number of samples in each study ( Table 81.1 ). Furthermore, information on the effects of calcitonin on bone microarchitecture and other parameters of bone quality are limited in most of these reports due to the lack of the technologies required to obtain such information at the time the studies were conducted. Using magnetic resonance imaging of the distal radius and lower trochanter of the hip, Chesnut et al. showed that cancellous bone microarchitecture was conserved in patients treated for 2 years with intranasal calcitonin compared to placebo-treated patients who exhibited significant deterioration of trabecular bone structure, despite the fact that, as noted above, such effects were not seen at the iliac crest.
81.2.2
Hormone therapy
HT, including estrogen therapy and combined estrogen/progesterone therapy, is FDA-approved for the treatment of osteoporosis in postmenopausal women (see Chapter 77 : Calcitonin in osteoporosis). There have been numerous reports on the effects of HT on iliac crest bone in postmenopausal women with osteoporosis, providing sufficient evidence to conclude that the beneficial skeletal effects of HT are predominantly due to the suppression of bone turnover, resulting in a preservation of bone volume and structure in cancellous and cortical bone ( Table 81.2 ).
| References | Subjects | Regimen | Duration (years) | Primary outcome |
|---|---|---|---|---|
| Steiniche et al. |
| Oral cyclic estrogen/gestagen | 1 |
|
| Lufkin et al. |
| Cyclic transdermal estradiol 0.1 mg/oral progesterone | 1 |
|
| Holland et al. |
| Estradiol 75 mg implant | 1 |
|
| Estradiol 75 mg implant | 1 |
| |
| Vedi et al. |
| CHT b | 2 | Paired histomorphometry |
| Eriksen et al. |
| A cyclic HRT regimen a | 2 | Paired histomorphometry |
| Vedi et al. |
| HHT c | At least 14 | Paired histomorphometry |
| Khastgir et al. |
| Cyclic estradiol 75 mg implant/oral progesterone | 6 |
|
| Khastgir et al. |
| Cyclic estradiol 75 mg implant/oral progesterone | 6 | Paired bone collagen content and cross-link, BMD |
| Vedi et al. |
| Paired histomorphometry | ||
| Paschalis et al. |
| Cyclic estradiol 2 mg/norethisterone acetate 1 mg | 2 | Mineral/matrix ratio, mineral crystallinity/maturity and collagen cross-links detected by FTIRI |
| Khastgir et al. |
| Estradiol 50 mg implant/oral MPA 5 mg daily | 3 |
|
| Boivin et al. |
| Bone mineralization |
a Trisequence; NOVO-Nordisk A/S, Copenhagen, Denmark.
b CHT with a variety of oral or transdermal formulations: Prempak C or Premarin, containing conjugated equine estrogens 0.625 mg/day. Trisequens, containing estradiol 2 mg/day. Estraderm or Estracombi, containing estradiol 50 μg/24 h.
c HHT with estradiol implant 100 mg, approximately 6-monthly on demand.
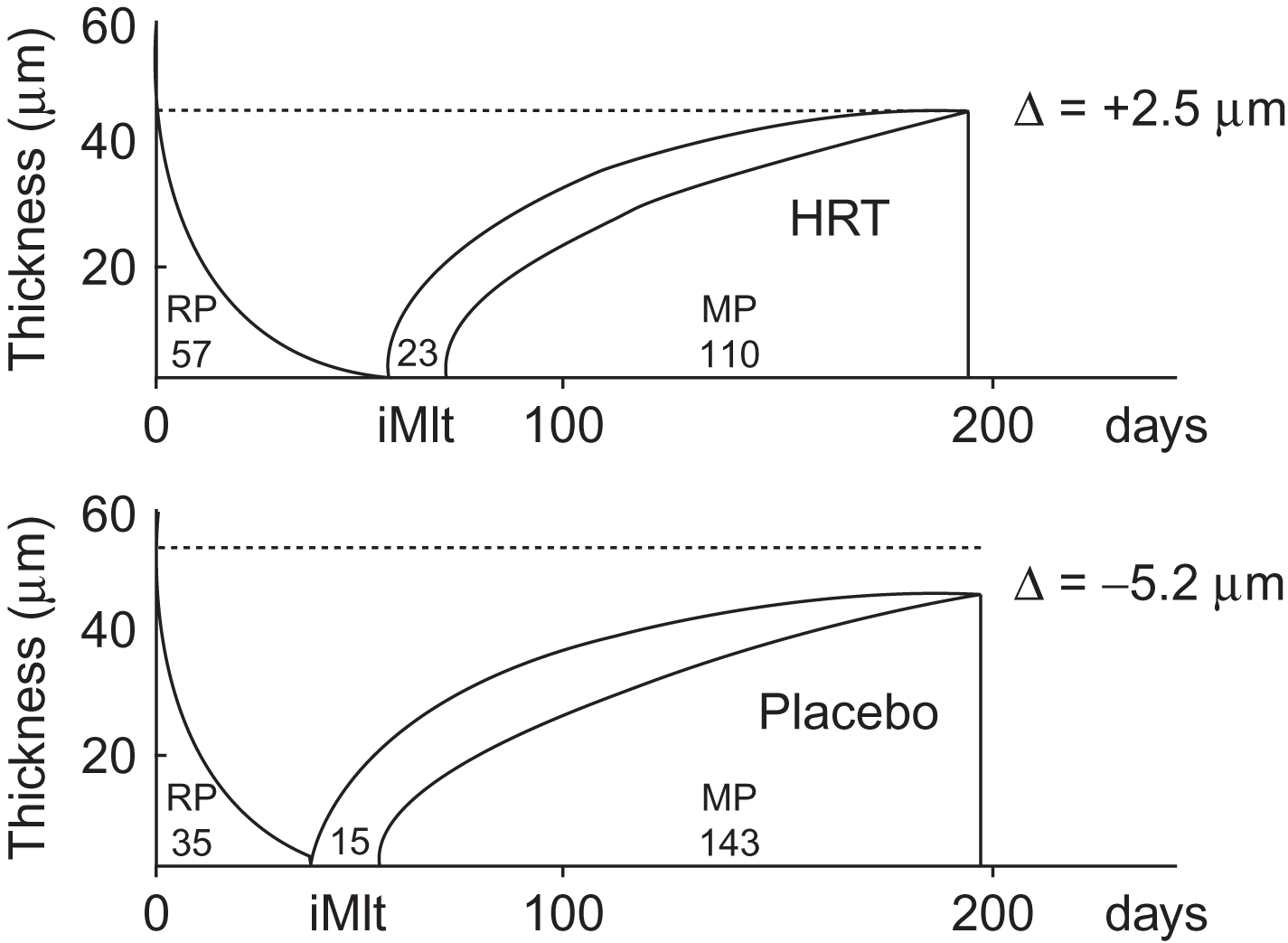
Eriksen et al. found that HT for 2 years reduced resorptive activity without significantly compromising bone formation at the BMU level. There was no difference in wall thickness between treated and placebo groups, but a pronounced decrease in resorption rate in the HT group, in contrast to a significant increase in osteoclastic erosion depth and a slight increase in resorption rate in the placebo group. It was concluded that HT reduced bone remodeling characterized by progressive osteoclastic hyperactivity in early postmenopausal women, resulting in a preservation of bone balance ( Fig. 81.2 ). Similarly, Vedi et al. reported that 2 years of HT reduced resorption cavity size. However, they found a reduction in wall width, which was interpreted as a compensatory response to the reduction in erosion depth. Steiniche et al. , in contrast, found no change in the resorption depth or wall width in biopsies from postmenopausal women treated for only 1 year with HT. Generally, HT did not significantly reduce eroded surface, except for one study , which employed high-dose estrogen.
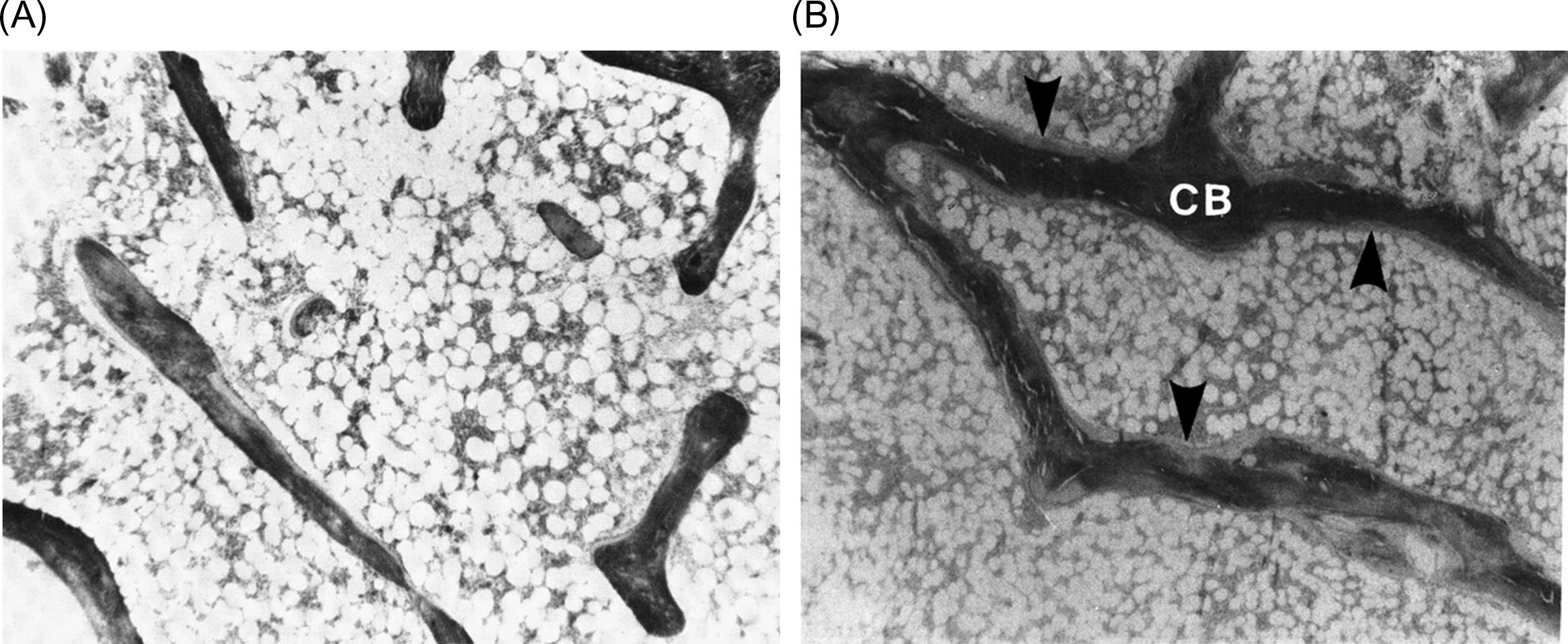
Regarding the effects of HT on bone formation, despite evidence that estrogen stimulates bone formation in animals , the possibility of anabolic action in humans remains controversial . Conventional doses of HT do not exert an anabolic effect, but result in inhibition of osteoblastic activity, which is reflected by reduced osteoid and mineralizing surfaces (MSs) and bone-formation rate, with no change or a decrease in wall width . Therefore the small increase in bone mineral density (BMD) seen with conventional HT is attributed to the closing of the remodeling space due to the suppression of osteoclastic resorption. In contrast to the effects of conventional doses of HT, an anabolic effect was demonstrated in a cross-sectional, prevention study in women given long-term, high-dose, subcutaneous estrogen implants, in which the treatment substantially increased BMD in lumbar spine and proximal femur . Iliac crest bone biopsies revealed a nonsignificant increase in cancellous bone volume accompanied by an increase in wall width and a decrease of eroded cavity area, while bone turnover was reduced, but not significantly so. The authors suggested that this anabolic action was achieved by the stimulation of osteoblastic activity . Consistent with this observation, HT delivered subcutaneously for 6 years significantly increased cancellous bone volume due to an increase in trabecular thickness and number . Wall width was also increased while bone turnover was suppressed. A similar result was also reported in young women with Turner’s syndrome treated with HT . These findings suggest that, with high doses of HT, improvement in cancellous bone volume and structure is achieved not only by the reduction in the remodeling space but also by a positive balance at the BMU level. Consistent with the histomorphometric findings, micro-CT assessment of three-dimensional (3D) bone microstructure demonstrated that HT preserves the microarchitectural integrity of cancellous bone with an improvement in the ratio of plate-to-rod-like structures .
Collagen content and its degree of maturity are important determinants of the biomechanical properties and functional integrity of the skeleton. One year of treatment with percutaneous estradiol implants resulted in an increase in the mature cross-links of hydroxylysylpyridinoline and/or lysylpyridinoline in cortical bone with a decrease in the percentage of collagen . A similar pattern was seen in cancellous bone with a significant increase in lysylpyridinoline. This finding supports a reduction in the turnover of bone collagen following HT and suggests that the formation of more mature collagen fibers may contribute to lower fracture risk. The effects of 6 years of treatment with an anabolic dose of HT have also been described . In that study, HT increased the total collagen content in both cancellous and cortical bone with an increase in mature cross-links observed only in cortical bone.
Using the technique of Fourier transform infrared microscopic imaging (FTIRI), Paschalis et al. investigated the effects of a conventional HT regimen on bone mineral and collagen properties of iliac bone in postmenopausal osteoporosis. They found that HT increased the mineral/matrix ratio, mineral crystallinity index (mineral crystallinity/maturity), and the relative ratio of collagen cross-links [pyridinoline (pyr)/dehydro-dihydroxylysinonorleucine (deH-DHLNL)]. Each of these variables is known to increase with bone tissue age. Higher crystallinity/maturity and pyr/deH-DHLNL cross-links ratio are characteristics of more mature mineral and collagen, respectively, and may simply be the consequences of reduced turnover as bone mineral and collagen mature even in the absence of direct cellular activity in biological environments . Boivin et al. used the technique of quantitative microradiography to investigate the effects of HT, at both conventional and high doses, on the degree of mineralization (DMB) of iliac bone. HT caused a dose-dependent augmentation of mean DMB due to a shift of the curves toward higher DMB values with a concomitant decrease of low DMB values, such that there was an increase in the heterogeneity of mineralization. This shift was more pronounced with high-dose HT than with conventional doses. As in the previous studies, these results are consistent with a decrease in bone turnover rate with a consequent prolongation of secondary mineralization. Given that homogeneous, denser bone tissue could favor the formation and propagation of microcracks (see later), it is important to note that the augmentation of mean DMB by HT is associated with preservation of the heterogeneity of mineralization, suggesting that the biomechanical properties of bone tissue are preserved or improved with HT .
81.2.3
Selective estrogen receptor modulators
Selective estrogen receptor modulators (SERMs) are steroid analogs that have been developed to have tissue-specific, rather than systemic effects, thus avoiding some of the adverse effects associated with HT (see Chapter 77 : Calcitonin in osteoporosis). Reports on the effects of SERMs on iliac bone biopsies are fewer than those on other anticatabolic therapies ( Table 81.3 ).
| Reference | Subjects | Regimen | Duration | Primary outcome |
|---|---|---|---|---|
| Ott et al. | PM-OP | Raloxifene | 24 months | Paired histomorphometry |
| Boivin et al. |
|
|
| |
| Weinstein et al. |
| Raloxifene 150 mg/day | 12 months |
|
| Prestwood et al. |
| Raloxifene 50 mg/day | 6 months |
|
| Wright et al. |
|
| <15 months, mean 33 months |
|
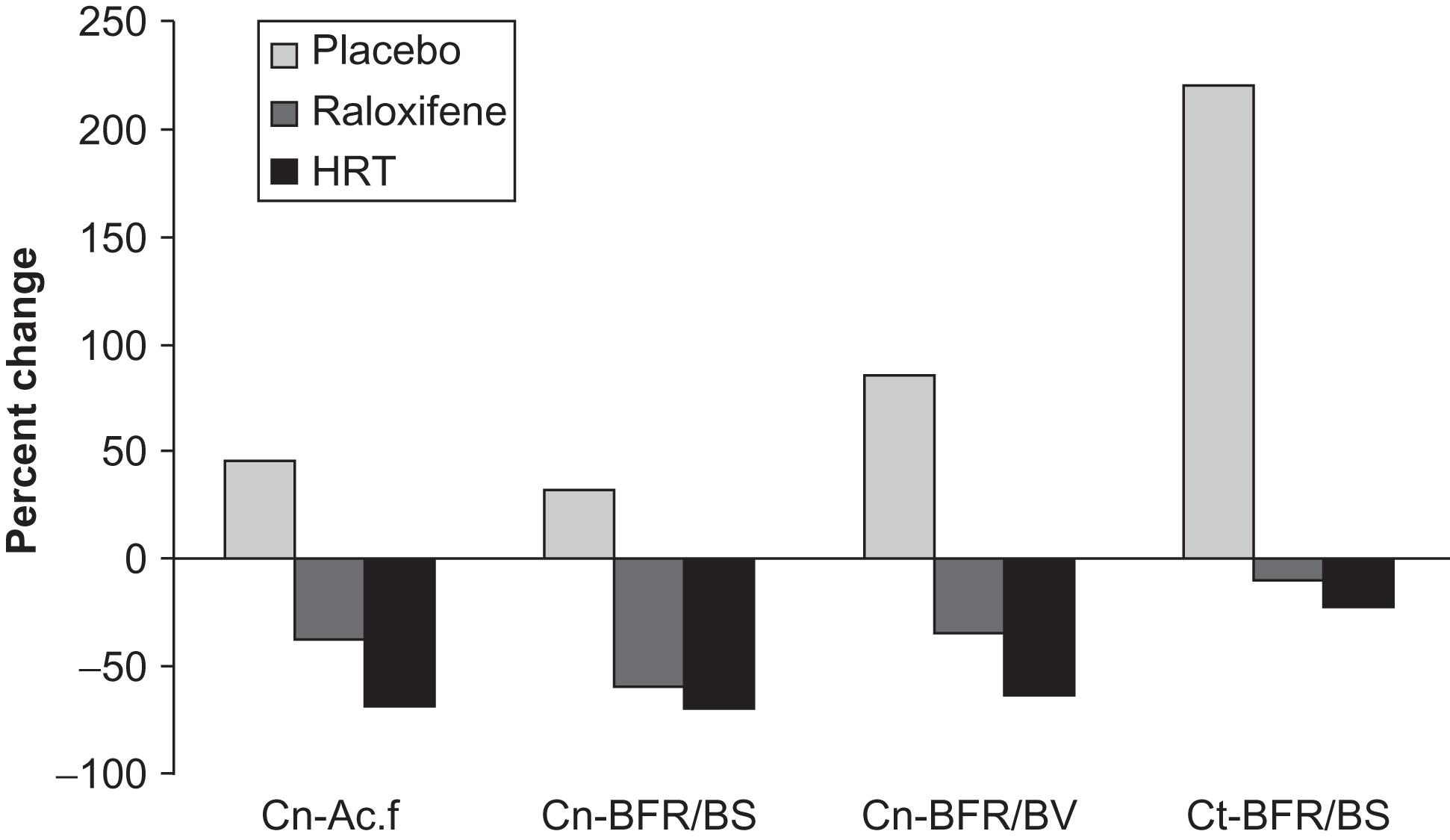
Two years of raloxifene administration in postmenopausal women enrolled in the MORE (Multiple Outcomes of Raloxifene Evaluation) trial demonstrated that the lower dose, which is the approved dose for osteoporosis management (60 mg), decreased the bone-formation rate, without changes in eroded surface and osteoclast number, while the high dose (120 mg) not only decreased the bone-formation rate but also showed a trend toward a decrease in eroded surface, osteoclast number, and osteoid surface as well as a significant decrease in urinary type I collagen excretion . Bone structure was preserved with no change in cancellous bone volume, trabecular thickness, and cortical bone width in both dosage groups compared to baseline and to the placebo group . Consistent with these findings, a 1-year trial with an even higher dose (150 mg) of raloxifene demonstrated suppression of bone turnover, as evidenced by a decrease in activation frequency and bone-formation rates on cancellous and endocortical envelopes, accompanied by preservation of lumbar spine and total body BMD ( Fig. 81.3 ). In that study the effects of raloxifene on iliac bone were compared directly with those of HT and the effects of the two agents were shown to be similar. Prestwood et al. also compared the effects of raloxifene and HT using histomorphometry. Their study used the approved dose of raloxifene (60 mg) and only lasted for 6 months. HT significantly reduced activation frequency and bone-formation rate, but raloxifene did not. Bone turnover markers were reduced by both HT and raloxifene, but to a greater extent by HT. There is currently only one study of the effects of another SERM, tamoxifen, on iliac bone. It was found that in pre- and postmenopausal women with breast cancer who had undergone mastectomy, 33 months of tamoxifen treatment resulted in a longer remodeling period, smaller resorption cavity area and reduced bone-formation rate compared to untreated patients . There were no significant differences in indices of cancellous bone structure between the two groups but there was a trend toward superior trabecular connectivity in the tamoxifen-treated subjects.
Boivin et al. subjected the iliac crest bone biopsies from the MORE trial to quantitative microradiography. They compared patients given placebo with those given raloxifene at doses of 60 or 120 mg for 2 years. All patients received calcium (500 mg/day) and vitamin D3 [400–600 international units (IU)/day]. All treatment groups showed an increase in mean DMB compared to baseline, although a dose–response effect was not seen with raloxifene and there were no significant differences in the changes in the raloxifene-treated groups compared to placebo. The raloxifene-treated patients tended to display preserved heterogeneity of mineralization compared to the placebo arm, but this effect was also not demonstrated to be statistically significant.
81.2.4
Bisphosphonates
Bisphosphonates, analogs of inorganic pyrophosphate, are potent inhibitors of bone remodeling and are used extensively in the treatment of osteoporosis (see Chapter 80 : Romosozumab for the treatment of postmenopausal osteoporosis). Histomorphometric data are available for a number of bisphosphonates, including alendronate, risedronate, pamidronate, clodronate, ibandronate, and zoledronate ( Table 81.4 ).
| Reference | Subjects | Regimen | Duration | Primary outcome |
|---|---|---|---|---|
|
| Alendronate 5 mg, 10 mg or 20 mg:5 mg/day | 24 or 36 months |
|
| Chavassieux et al. |
| Alendronate 2.5, 5, or 10 mg/day | 12 months | Histomorphometry (end point) |
| Bone et al. |
| Alendronate 1, 2.5, or 5 mg/day | 12 or 24 months |
|
| Arlot et al. |
|
| 6 or 18 months |
|
| Recker et al. |
| Alendronate | 24 or 36 months |
|
|
| Risedronate 5 mg/day | 36 months | Paired histomorphometry , micro-CT and DMB |
| Dufresne et al. |
| Risedronate 5 mg/day | 12 months | Paired micro-CT and histomorphometry |
| Borah et al. |
| Risedronate 5 mg/day | 36 and 60 months | DMB and micro-CT (triple biopsies at baseline, 3, and 5 years) |
| Zoehrer et al. (see also ) |
| Risedronate 5 mg/day | 36 and 60 months | BMDD (triple biopsies at baseline, 3, and 5 years) |
| Recker et al. |
| Ibandronate 2.5 mg/day or 20 mg intermittently | 22 or 34 months | Histomorphometry (end point) |
| Saarto et al. |
| Clodronate 1.6 g/day | 36 months |
|
| Munns et al. |
| Pamidronate 9 g/kg/year | 24 months |
|
| Recker et al. |
| Zoledronic acid 5 mg annually | 36 months |
|
| Stepan et al. |
| Alendronate, 10 mg/daily | 5 years | Microcrack density (end point) |
| Chapurlat et al. |
| Alendronate, Risedronate, or pamidronate | ≥3 years, mean 6.5 years |
|
The effects of alendronate have been investigated in patients with postmenopausal or glucocorticoid-induced osteoporosis . In these studies, biopsies were taken from the treatment and placebo groups at the end of the study period. Evidence for the suppression of bone remodeling with alendronate treatment comes from data indicating a reduction in osteoid surface and thickness, MS, bone-formation rate, and activation frequency. MAR was unchanged, and this, coupled with the decrease in osteoid thickness, was taken to indicate that alendronate suppresses bone turnover without inhibition of bone mineralization over 2–3 years of treatment. Although the primary action of bisphosphonates on the skeleton is to inhibit osteoclastic resorption, alendronate, like other anticatabolic agents, had little if any effect on histomorphometric variables of bone resorption, including eroded surface and volume, osteoclast number, and erosion depth. This is in sharp contrast to the marked reductions seen in biochemical markers of bone resorption and probably reflects the fact that the primary histomorphometric variables reflecting resorption are static (i.e., no temporal markers) as opposed to the formation variables, which are dynamic (i.e., time-stamped with tetracycline) in nature. Following 2 years of alendronate treatment, there was a significant increase in wall thickness of cancellous bone packets, accompanied by a trend toward a decrease in erosion depth, which resulted in a positive bone balance (wall thickness minus erosion depth), but these effects were not seen in patients treated for 3 years . In addition, there was no difference in cancellous bone volume between placebo- and alendronate-treated groups. However, in a later study using both two-dimensional (2D) histomorphometry and 3D micro-CT, Recker et al. reported that cancellous bone volume, trabecular thickness, and trabecular number were both higher in patients treated with alendronate for 2–3 years than in placebo-treated controls and that trabecular spacing was lower ( Fig. 81.4 ). The assumption is that cancellous bone microarchitecture declined in the placebo arm and that this was prevented by alendronate, although without baseline biopsies, we cannot be certain that this was the case. Despite the fact that the pathogenesis of glucocorticoid-induced osteoporosis is quite different from that of postmenopausal osteoporosis, alendronate had quite similar effects in both conditions. Chavassieux et al. studied the effects of a 1-year treatment with several doses of alendronate (2.5, 5, or 10 mg/day) or placebo on men and women who had been exposed to long-term glucocorticoid therapy. Biopsies were obtained at end point. Alendronate reduced MS, activation frequency, and bone-formation rate. Osteoid thickness and volume were also significantly reduced in the alendronate-treated patients but MAR was unaltered. As in postmenopausal osteoporosis, no significant differences were noted between alendronate- and placebo-treated patients in variables reflecting bone resorption. Cancellous bone volume and its components (trabecular thickness, number, and separation), as assessed by 2D histomorphometry, were also similar in the alendronate- and placebo-treated arms. The effects of alendronate on bone-remodeling variables in cancellous bone of glucocorticoid-treated patients were mirrored on the endocortical surface . Despite the fact that bone formation was already compromised by the glucocorticoids in these patients, alendronate treatment did not completely suppress remodeling, with the average reduction in remodeling variables (approximately 70%) being somewhat less than that seen in postmenopausal osteoporosis (approximately 90%) .
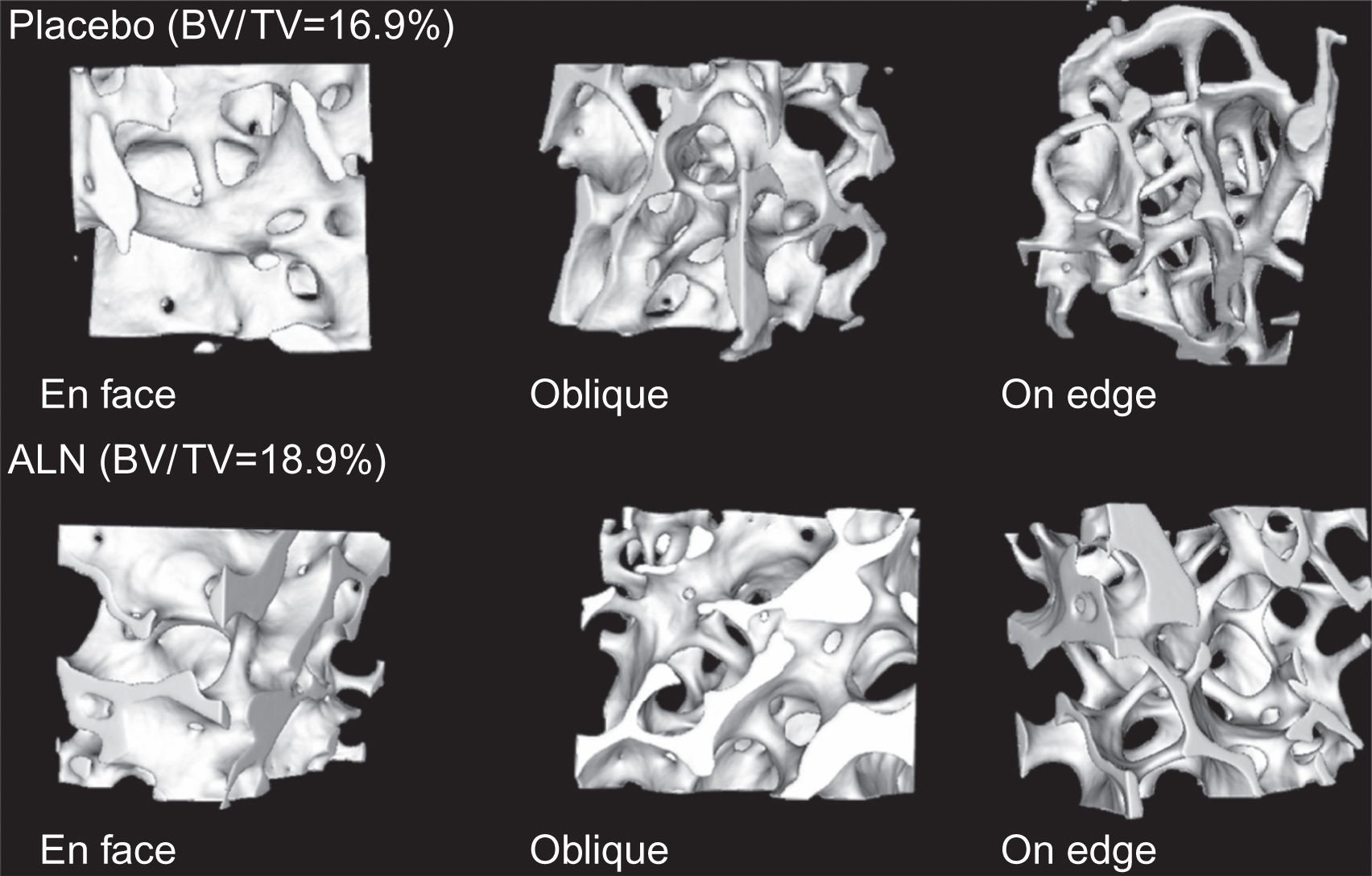
The effects of alendronate on matrix mineralization have been studied in both animal models and humans. It was theorized that, as the “life span” of osteons is increased as a consequence of the reduced bone turnover, the duration of secondary mineralization is extended . This hypothesis was supported by studies in which mineralization was assessed by either quantitative microradiography or quantitative backscattered electron microscopy in minipigs, baboons, and humans . Two or 3 years of alendronate treatment in postmenopausal women with osteoporosis increased the mean DMB and shifted the distribution of mineralization levels in cancellous and cortical bone toward the highest values ( Fig. 81.5 ). These effects were accompanied by decreased activation frequency, and prolonged formation period, but unchanged bone volume . Since a decrease in the remodeling space, an increase in bone balance per remodeling cycle, and an increase in mineralization have all been associated with alendronate treatment, a computer simulation of bone remodeling was used to estimate the relative contributions of focal bone balance and mineralization on BMD. The model predicted that mineralization might be a larger contributor to the BMD change caused by alendronate than changes in the focal bone balance .
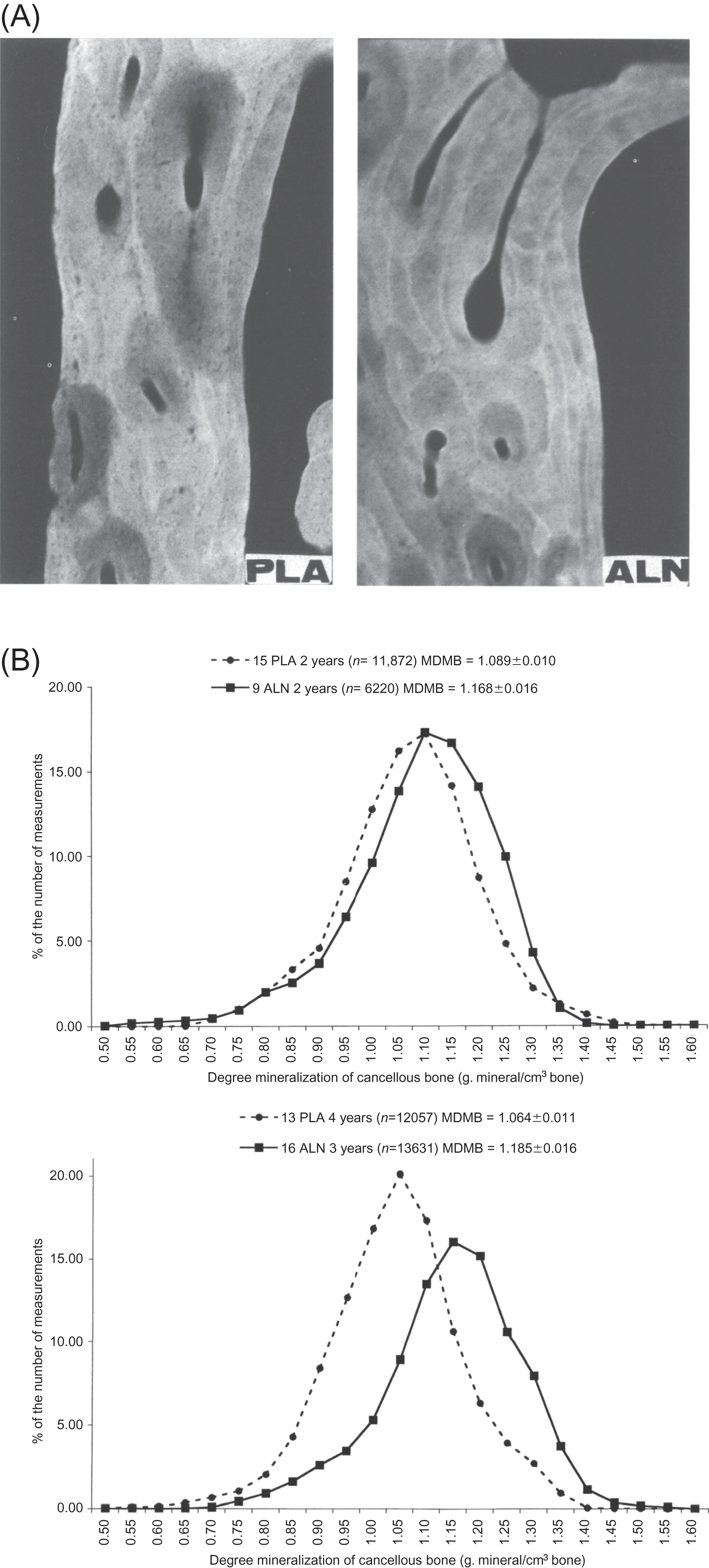
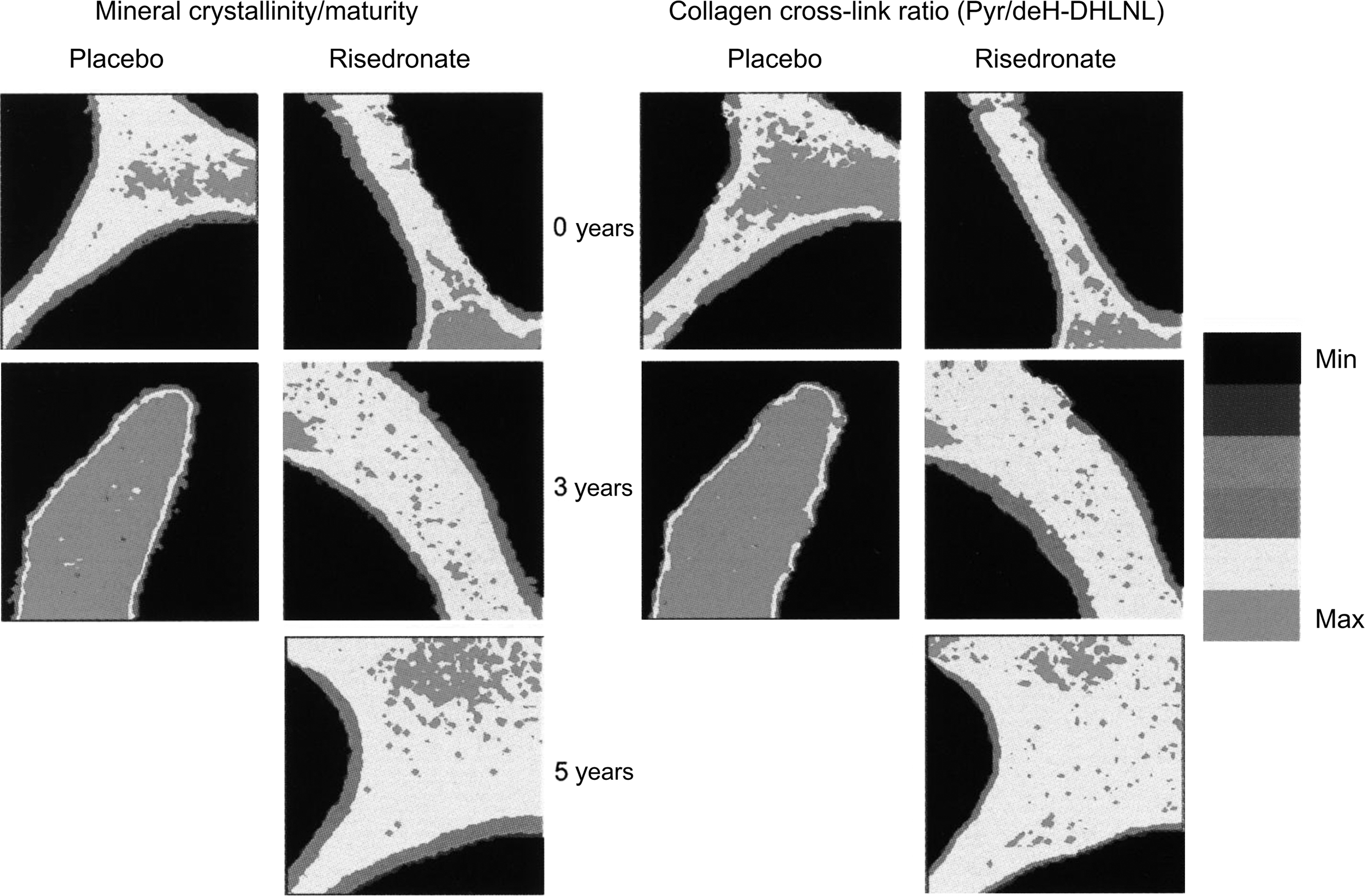
A paired biopsy design was used to study the effects of risedronate treatment . Similar to the action of alendronate on iliac bone, 3 years of treatment with risedronate in postmenopausal women with osteoporosis caused a moderate reduction in bone turnover as evidenced by decreased MS, bone-formation rate, and activation frequency, with normal bone mineralization as demonstrated by unchanged osteoid thickness and mineralization lag time, and a trend toward an improvement in bone balance . Although no significant change was observed in eroded surface and depth, there was a significant decrease in resorption rate after risedronate treatment and a significant increase in erosion depth was found in the placebo-treated women. As for alendronate, risedronate treatment was without effect on indices of cancellous bone structure as assessed by conventional histomorphometry . However, risedronate was shown to preserve trabecular microarchitecture using micro-CT . There were no significant changes in 3D structural variables compared to baseline in risedronate-treated women, while trabecular architecture deteriorated significantly in a subset of placebo-treated women who had higher bone turnover at baseline . Furthermore, there was a significant correlation between baseline bone turnover and bone loss in the placebo group, providing evidence that the higher the turnover in postmenopausal women, the more rapid the subsequent structural deterioration. One year of risedronate therapy in early postmenopausal women resulted in a reduction of bone turnover of the same magnitude as that seen following 3 years of treatment, and the deterioration of trabecular architecture in early postmenopausal women was also prevented by 1 year of the bisphosphonate . Using micro-CT with synchrotron radiation, Borah et al. showed that the reduction of turnover by risedronate was associated with an increase in the DMB and a reduction in the ratio of low-to-high-mineralized bone fractions. These findings were later extended in seven patients who consented to sequential triplicate biopsies at baseline and following 3 and 5 years of treatment . The findings verified that risedronate treatment results in sustained effects over 5 years on bone mineralization and trabecular architecture. The data further demonstrated that risedronate treatment resulted in a mineralization distribution similar to that found in premenopausal women. Similarly, 10 years of alendronate treatment in the Fracture Intervention Trial (FIT) Long-Term Extension (FLEX) trial resulted in a mineralization density similar to that seen with 5 years of alendronate treatment followed by 5 years of placebo treatment . These are important observations as they mitigate widespread, although unsupported, concern that prolonged treatment with bisphosphonates may lead to hypermineralization of bone tissue, which could increase its brittleness . The effects of risedronate on two other material characteristics of bone have also been investigated using the technique of Fourier transform infrared imaging . Following 3 years of risedronate treatment, there was no change in the mineral maturity/crystallinity or the collagen cross-link ratio, whereas both of these variables increased significantly in the placebo-treated patients ( Fig. 81.6 ). The authors interpreted this to mean that risedronate treatment halts the aging of bone tissue in untreated osteoporosis.
Zoledronic acid is a third-generation aminobisphosphonate. The Health Outcomes and Reduced Incidence with Zoledronic acid Once Yearly-Pivotal Fracture Trial (HORIZON-PFT) demonstrated that 3 years of annual intravenous zoledronic acid 5 mg infusions significantly reduced the risk of various fracture types in patients with postmenopausal osteoporosis. In a substudy of the HORIZON-PFT by Recker et al., 152 patients receiving zoledronic acid treatment or placebo underwent iliac crest bone biopsy at 3 years. The analysis of bone structure by micro-CT demonstrated a higher trabecular bone volume and number, and lower trabecular separation with a trend toward improvement in connectivity density in the zoledronic acid treatment group compared to the placebo group, indicating preservation of trabecular structure with zoledronic acid. Following an extended search protocol, histomorphometric analysis found that tetracycline labels were present in 81 of 82 biopsies from patients treated with zoledronic acid and in all placebo-treated subjects. However, the MS was 0.45% in the zoledronic acid-treated subjects compared to 4.79% in placebo. Furthermore, 21 out of 59 subjects in the zoledronic acid group had insufficient double tetracycline label to allow their inclusion in the assessment of MAR, whereas there were only 4 out of 52 such subjects in the placebo arm. Perhaps as a result of this imbalance, MAR was significantly higher in the zoledronic acid group than in placebo (see later). Driven primarily by the reduced MS, zoledronic acid treatment reduced activation frequency and the bone-formation rate compared to placebo. These results indicate a potent reduction of bone turnover by zoledronic acid.
The mechanism of action of bisphosphonates on reducing fractures is to suppress bone remodeling and subsequently increase bone strength . Given that one of the functions of bone remodeling is thought to be to remove skeletal microdamage occurring in response to mechanical loading, the amount of microdamage accumulation is a consequence of the balance between microdamage formation and its removal by bone remodeling . A concern was therefore raised about the possible accumulation of microdamage with bisphosphonate treatment, particularly for the longer term. Although data from animal studies showed an increase in microdamage accumulation with bisphosphonates, the implications of increased microdamage on fracture risk with bisphosphonate treatment are uncertain as these studies also showed an increase in vertebral bone strength and stiffness (see Chapter 37 : Osteoporosis in childhood and adolescence, for a comprehensive discussion of the effects of bisphosphonates on the material properties of bone). Indeed, some of the principal proponents of the microdamage hypothesis have concluded, “it is not clear that microdamage accumulation in bone under normal physiological circumstances is even a relevant biomechanical concern for living bone” . The available human reports showed that bisphosphonate treatment generally does not seem to increase microdamage accumulation significantly . However, alendronate-treated patients with low BMD had a higher accumulation of microdamage in the iliac crest biopsies than those with higher BMD and this difference was not present in untreated patients . Furthermore, microdamage density was significantly higher in the patients treated with alendronate for a mean of 5 years . One important limitation to these studies, however, is that the iliac crest is probably not the most appropriate site to study microdamage accumulation . Nevertheless, studies in animal models continue to support the view that treatments that offer improvement in cancellous bone volume and microarchitecture with minimal microdamage accumulation have a net beneficial effect on bone strength . There are a number of other studies on the effects of different bisphosphonates, such as etidronate, clodronate, pamidronate, and ibandronate on iliac bone. The data obtained in patients with osteoporosis treated with these bisphosphonates are broadly similar to those obtained with alendronate, risedronate, and zoledronic acid . Of note, however, are the dramatic improvements in bone structure and turnover seen in children with osteogenesis imperfecta treated with bisphosphonates ( Fig. 81.7 ).