Rapid advances in radiation oncology, biology, and physics have led to an accumulation of information on the interactions of radiation with other therapeutic modalities (chemotherapy, biologic-response modifiers) and have had an impact on the understanding of normal tissue toxicities.
Radiation doses customarily deemed safe may no longer be so because, when combined with another modality, these doses can lead to severe late effects in different vital organs. Previously defined radiation tolerance doses (TD5/5 and TD50/5) remain as valuable guides, but their applicability has changed.
Emphasis is now placed on the volume of the organ irradiated, in addition to the dose; and a new construct relating global (whole organ) and focal (partial volume) injury as a function of the dose-volume histogram is presented.
Mathematical models such as the nominal standard dose, time-dose factor, and cumulative radiation effect have been supplanted by the linear-quadratic equation, using the α/β ratio and its clinical applicability to normal tissue complication probability estimates (27).
Tables 8-1 and 8-2 summarize previously defined whole- and partial-organ tolerance. Detailed information regarding a few important organs is given below.
Clinical detection: Headache, somnolence, intellectual deficits, functional neurologic losses, and memory alterations may occur during, shortly after, or—most often—as a delayed effect, at 6 months.
TABLE 8-1 Tolerance Doses (TD5/5-TD50/5) to Whole-Organ Irradiation
Organ
Single Dose (Gy)
Organ
Fractionated Dose (Gy)
Lymphoid
2-5
Testes
1-2
Bone marrow
2-10
Ovary
6-10
Ovary
2-6
Eye (lens)
6-12
Testes
2-10
Lung
20-30
Eye (lens)
2-10
Kidney
20-30
Lung
7-10
Liver
35-40
Gastrointestinal
5-10
Skin
30-40
Colorectal
10-20
Thyroid
30-40
Kidney
10-20
Heart
40-50
Bone marrow
15-20
Lymphoid
40-50
Heart
1-20
Bone marrow
40-50
Liver
15-20
Gastrointestinal
50-60
Mucosa
5-20
VCTS
50-60
VCTS
10-20
Spinal cord
50-60
Skin
15-20
Peripheral nerve
65-77
Peripheral nerve
15-20
Mucosa
65-77
Spinal cord
15-20
Brain
60-70
Brain
15-25
Bone and cartilage
>70
Bone and cartilage
>30
Muscle
>70
Muscle
>30
VCTS, vasculoconnective tissue systems.
Source: Modified from Rubin P. The law and order of radiation sensitivity, absolute vs. relative. In: Vaeth JM, Meyer JL, eds. Radiation tolerance of normal tissues. Frontiers of radiation therapy and oncology, vol 23. Basel, Switzerland: Karger, 1989:7-40.
Time course of events: Brain necrosis and gliosis require 6 to 12 months to develop.
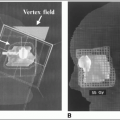
Dose/time/volume: Doses of 50 Gy to whole brain in 1.8- to 2.0-Gy fractions are generally well tolerated; in children the threshold doses are 30 to 35 Gy (7). TD5 in adults for necrosis is ≥50 Gy (54 Gy) (19); a threshold dose of 57.6 Gy was noted by Leibel and Sheline (17) using 1.8- to 2.0-Gy fractions. With focal areas, the TD50 is between 70 and 80 Gy, as evidenced by a recent Radiation Therapy Oncology Group study (22).
Chemical/biologic modifiers: Concomitant use of carmustine (BCNU) as well as temozolomide is well tolerated, but immediate subsequent use of methotrexate, intrathecally or intravenously, is of concern.
Radiologic imaging: Four stages have been described by magnetic resonance imaging, from early whitening in the periventricular region to a diffuse coalescence of white and gray matter into an intense signal region, along with loss of structure (8).
Differential diagnosis: Positron emission tomography scans indicate hypometabolic zones for necrosis and hypermetabolic zones for tumor. MR spectroscopy can also be used to help distinguish recurrence from necrosis.
Pathologic diagnosis: Establishing diagnosis is indicated only if tumor recurrence or progression is suspected. Alterations in vasculature and loss of myelination due to oligodendrocytic death are well documented.
TABLE 8-2 Normal Tissue Tolerance to Therapeutic Irradiation
TD5/5 Volume
TD50/5 Volume
Organ
1/3
2/3
3/3
1/3
2/3
3/3
Selected End Point
Kidney
50
30
23
—
40
28
Clinical nephritis
Brain
60
50
45
75
65
60
Necrosis infarction
Brainstem
60
53
50
—
—
65
Necrosis infarction
Spinal cord
5 cm
10 cm
20 cm
5 cm
10 cm
20 cm
50
50
47
70
70
—
Myelitis necrosis
Lung
45
30
17.5
65
40
24.5
Pneumonitis
Heart
60
45
40
70
55
50
Pericarditis
Esophagus
60
58
55
72
70
68
Clinical stricture/perforation
Stomach
60
55
50
70
67
65
Ulceration, perforation
Small intestine
50
—
40
60
—
55
Obstruction perforation/fistula
Colon
55
—
45
65
—
55
Obstruction perforation/ulceration/fistula
Rectum
75
65
60
—
—
80
Severe proctitis/necrosis/fistula
Liver
50
35
30
55
45
40
Liver failure
Source: Modified from Emami B, Lyman J, Brown A, et al. Tolerance of normal tissue to therapeutic irradiation. Int J Radiât Oncol Biol Phys 1991;21:109-122.
Management: This is often symptomatic treatment with analgesics and antiseizure medications, such as phenytoin (Dilantin) and barbiturates; as headaches and neurologic deficits increase, high-dose corticosteroids are used.
Follow-up: The patient is seen every day or week until relief is obtained, and then at 1- to 3-month intervals.
Clinical detection: Paresthesias (tingling sensation, shooting pain, and Lhermitte’s syndrome), numbness, motor weakness, and loss of sphincter control may progress through Brown-Séquard’s syndrome to total paraparesis and paraplegia.
Time course of events: Clinically, Lhermitte’s syndrome occurs 2 to 4 months after irradiation and persists, or returns, at 6 to 9 months. Paresis, numbness, and altered sphincter control appearing at 6 to 12 months, with progression, compose the classic onset of radiation-induced spinal cord transection (26).
Dose/time/volume: The most widely observed dose limit for the spinal cord is 45 Gy in 22 to 25 fractions. Marcus and Million (18) found that 45 Gy, conventionally fractionated, is on the flat part of the dose-response curve and yields an incidence of myelopathy of less than 0.2%. TD5 level is probably 57 to 61 Gy; TD50 is 68 to 73 Gy. No volume effect has been shown. Shortening the interval from 24 hours to 6 to 8 hours reduces spinal cord tolerance by 10% to 15%.
Chemical/biologic modifiers: Intrathecal and intravenous (IV) use of concomitant methotrexate, cisplatin (CDDP), and etoposide (VP-16) can result in enhanced neurotoxicity.
Radiologic imaging: Magnetic resonance imaging may show cord edema or atrophy, decreased intensity on T1-weighted images, or increased intensity on T2-weighted images (32). Differential diagnosis: Epidural metastasis or compression secondary to vertebral metastases must be excluded.
Pathologic diagnosis: This is not possible until postmortem.
Management: Currently, corticosteroids are prescribed, using an intensive IV schedule as with multiple sclerosis patients: methylprednisolone (Solu-Medrol) 1,000 mg IV for 3 to 5 days; followed by a gradual tapering of dose to stabilize progress.
Follow-up: Intensive nursing and rehabilitative care are essential.
Clinical detection: Cough, pink sputum, and pleuritis are common, with pneumonitis limited to the irradiation field with a geometric outline on chest film. Fibrosis can lead to decreased pulmonary function and heart failure.
Time course of events: Usually, radiation reactions occur 1 to 3 months after fractionated and single-dose therapy. When chemotherapy has been used, as in total-body irradiation and bone marrow transplantation conditioning regimens, reactions can occur during treatment.
Dose/time/volume: For single doses to the whole of both lungs, TD5 is 8 Gy; for fractionated doses of 1.8 to 2.0 Gy with limited volume (less than 30%), it is 45 to 50 Gy. Emami et al. (12) estimated the risk of 5% and 50% of radiation pneumonitis (RP) for partial lung irradiation to be 17.5 and 24.5 Gy, respectively.
Armstrong et al. (2) described a significant correlation between the occurrence of severe RP and the volume of lung receiving 25 Gy: 38% of Grade 3 toxicity occurred when more than 30% of the lung received 25 Gy or more, versus 4%. The Washington University clinical experience showed that the total lung volume receiving more than 20 Gy appeared to be a good predictive factor for pneumonitis (13).
Chemical/biologic modifiers: Actinomycin D, doxorubicin (Adriamycin), bleomycin, BCNU, interferons (α, β, and γ), and gemcitabine (3, 4) may enhance RP.
Radiologic imaging: Computed tomography should be used to confirm chest film findings of pneumonitis or fibrosis compatible with irradiation fields by comparison to high isodose-curve outlines in cross-sectional contours.
Laboratory tests: Serum surfactant apoprotein and plasma transforming growth factor-β levels are being tested and appear to correlate with clinical findings (1, 20).
Differential diagnosis: Recurrence, metastases of cancers (Hodgkin’s disease, lymphomas), pneumonia, and infiltrates such as lymphangitic spread patterns must be ruled out. Pathologic diagnosis: Tissue diagnosis rules out recurrent disease.
Management: This most often involves high-dose steroids, starting with prednisone (1 mg per kg) or dexamethasone-equivalent for pneumonitis. Symptoms clear rapidly within 24 to 48 hours.
Follow-up: The patient should be maintained on steroids for several months, after which the dose should be tapered, and the patient weaned from the drug as symptoms diminish.
Clinical detection: Electrocardiogram changes during irradiation are not due to cardiac injury related to treatment. Pericardial damage is most common, but recent interest in coronary artery disease (CAD) and cardiomyopathies has been noted, particularly in pediatric patients with long-term follow-up (5, 9, 14, 28, 31).
Time course of events: Pericardial disease (effusion) usually appears within 6 months to 1 year and may persist as a constrictive process. CAD appears 10 to 15 years after irradiation, but is associated with the usual risk factors of obesity, smoking, and hypertension.
Dose/time/volume: The incidence of pericardial disease decreases with the use of subcarinal shields blocking the major ventricles at 30 Gy. Doses should be limited to 60 Gy for less than 25% of cardiac volume and 45 Gy for more than 65% of cardiac volume (29).
Chemical/biologic modifiers: Doxorubicin (which causes cardiomyopathy) below 500 mg per m2 is recommended when combined with irradiation (15).
Radiologic imaging: Increase in size of cardiac silhouette on chest films, echocardiogram for effusion, equilibrium radionuclide angiocardiography to measure ejection fractions, and quantitative thallium scintigraphy, are all helpful in establishing cardiac injury. None is pathognomonic. Differential diagnosis: Recurrent cancers of lung and esophagus, relapsing mediastinal Hodgkin’s disease, and non-Hodgkin’s lymphoma must be excluded.
Pathologic diagnosis: Open biopsy of the pericardium is performed if necessary.
Management: If tamponade develops, pericardiocentesis becomes necessary. Preventive measures are desirable in reducing the incidence of CAD.
Follow-up: Young adults and the pediatric population should be informed that they are at high risk for CAD.
Clinical detection: Vague to intense right upper abdominal pain is followed by abdominal swelling due to hepatomegaly and ascites.
Time course of events: Anicteric ascites develops 2 to 4 months after irradiation alone; chemoirradiation-induced liver disease occurs at 1 to 4 weeks with bone marrow transplantation.
Dose/time/volume: The whole liver can receive 20 to 30 Gy, with an upper threshold of 33 to 35 Gy and onset of radiation hepatopathy at more than 35 Gy. One third to one half of the liver volume can receive more than 40 Gy without complications.
Chemical/biologic modifiers: Nitrosoureas (BCNU, lomustine [CCNU]) leads to cholestasis and necrosis.
Radiologic imaging: Enlarged liver may be detected.