Chapter 9 Lamivudine
STRUCTURE
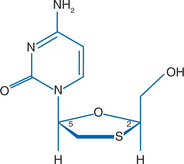
Lamivudine (3TC, Epivir) is the negative or cis enantiomer of 2′-deoxy-3′-thiacytidine that has antiviral activity against human immunodeficiency virus types 1 and 2 (HIV-1, HIV-2) and hepatitis B virus (HBV). This compound is a pyrimidine nucleoside analog that contains a sulfur atom in place of the 3′ carbon of the ribose ring (Fig. 9-1). 3TC was originally synthesized in a racemic mixture (BCH-189), and this racemic mixture was subsequently separated into positive and negative enantiomers. 3TC, the negative enantiomer, has its ribose ring in a position opposite to the ribose ring position in physiologic nucleosides and most nucleoside analogs.
MECHANISM OF ACTION AND IN VITRO ACTIVITY
Like all nucleoside analogs, 3TC must be metabolized to its triphosphorylated form, 3TC-triphosphate, to be an active antiviral compound. 3TC-triphosphate is a reverse transcriptase (RT) inhibitor that competes with deoxycytidinetriphosphate (dCTP), an endogenous nucleotide, for binding in the HIV RT binding site. Incorporation of 3TC-triphosphate into the elongating DNA molecule results in irreversible chain termination as 3TC lacks the 3′-hydroxyl group required for the 5′-3′ linkage required for DNA synthesis.1 As mentioned above, 3TC was originally synthesized as a racemic mixture (BCH-189), and this mixture has potent activity in vitro against HIV-1 with a mean 50% inhibitory dose (IC50) of 0.73 μM in an MT4 cell line assay.2 The mixture was active against zidovudine (ZDV)-resistant isolates and had less cytotoxicity than ZDV.2 When BCH-189 was separated into the positive and negative enantiomers, both compounds were discovered to have anti-HIV-1 activity.3,4 The positive enantiomer, (+)-2′-deoxy-3-thiacytidine, has significantly more cytotoxicity than 3TC in vitro, and 3TC appeared to have more antiretroviral activity,4,5 with a median effect in the nanomolar range in some experiments.3 3TC has been tested against laboratory strains of HIV-1 and HIV-2 in a variety of lymphoid cell lines, and the IC50 ranged from 4 to 670 nM.6 3TC was also highly active against HIV-1 isolates in peripheral blood mononuclear cell assays (IC50 2.5–90 nM).6 In these experiments the IC50 for cytotoxicity was typically 1000-fold higher. That 3TC is more active than its positive enantiomer has been ascribed to the resistance of 3TC to cleavage from the 3′ terminals of RNA/DNA complexes by 3′-5′ cellular exonucleases.5,7 In a series of experiments, Skalski and colleagues showed that the positive enantiomer has more inhibitory activity than 3TC-TP against the HIV RT, although a novel cellular exonuclease removed the positive enantiomer at a two- to sixfold higher rate.5 This group also showed that 3TC was more readily phosphorylated in the cell than the positive enantiomer. In addition to in vitro activity against HIV-1 and HIV-2, 3TC inhibits the HBV8 and has antiviral activity in patients with chronic active hepatitis B.9,10
Lamivudine has been shown to be synergistic in vitro with a variety of antiretroviral agents in inhibiting HIV-1. Against ZDV-sensitive isolates and in some studies against ZDV-resistant isolates11 3TC has been shown to be synergistic or additive with nucleoside analogs (ZDV,11–13 stavudine,11,12 didanosine12), protease inhibitors,11 and the non-nucleoside RT inhibitors (NNRTIs).11,14 Three-drug combinations of 3TC/ZDV/saquinavir,11 3TC/ZDV/d4T,11 3TC/ZDV/nevirapine,11 3TC/ZDV/delavirdine (DLV),14 and 3TC/ZDV/indinavir,15–17 have also been shown to be synergistic or additive in vitro. 3TC/ZDV/efavirenz (EFV) has proved to be a particularly effective regimen delaying virologic failure and leading to a shorter time to viral load suppression when compared to three NRTI (nucleoside RT inhibitor) regimens (3TC/ZDV/ABC) and regimens of ddI/d4T/NFV or ddI/d4T/EFV as initial therapy.18,19 Four-drug combination regimens (3TC/ZDV/ABC/EFV or EFV and nelfinavir combined with 3TC/ZDV and either ddI or d4T) have not proved to provide additional benefit when compared with a three-drug (3TC/ZDV/EFV) regimen as initial therapy in time to virologic failure and CD4 changes.20,21 Two of the three NRTI combinations listed by the 2006 recommendations of the International AIDS Society-USA Panel (3TC/ZDV, 3TC/ABC and FTC/TDF) for use with either an NNRTI or ritonavir (RTV) boosted PI as initial antiretroviral regimens include 3TC22 A trial of 3TC/ZDV/EFV versus FTC/TDF/EFV confirmed the noninferiority of the latter combination to the lamivudine containing regimen.23
It is of note that 3TC interferes with phosphorylation of zalcitabine (ddC),24 most likely because these agents are both cytosine analogs. These two agents may be antagonistic against HIV-1 replication, as has been shown in vitro for stavudine (d4T) and ZDV, which are both thymidine analogs.11 Neither combination is recommended as a component of highly active antiretroviral therapy (HAART).25
PHARMACOKINETICS
Favorable oral bioavailability was demonstrated during the initial in vivo studies of 3TC. When single doses of 3TC over a range of five doses (0.25–8.0 mg/kg) were given intravenously and orally to adult men who were HIV-infected, the bioavailability was 82%.26 Food has no significant effect on the extent of 3TC absorption.27 Other studies have shown similar oral bioavailability of tablet and oral solution formulations of 3TC,28 although intrasubject variability in the bioavailability of the tablet was seen. The bioavailability of 3TC in infants, which was 66% in one study, is somewhat less than that seen in adults.29
In a phase I/II multiple-dose study, 97 subjects with acquired immunodeficiency syndrome (AIDS) or advanced HIV (median CD4+ T-lymphocyte count 128 cells/mm3) were administered 3TC at 0.5, 1.0, 2.0, 4.0, 8.0, 12.0, and 20.0 mg/kg twice daily in sequential cohorts.30 Pharmacokinetic parameters obtained at steady state after 2 weeks on therapy showed dose linearity with peak concentrations well above the in vitro IC90 of HIV-1, especially at the higher doses. The half-life of 3TC in serum was 3–4 h. The pharmacokinetic parameters did not change after 24 weeks of continuous dosing. Other studies, which examined single doses of 3TC, have shown that the half-life in plasma was substantially longer than in the study of Pluda et al,30 ranging from 8 to 11 h.28,31 3TC clearance is dependent on weight and renal function and is not influenced by gender, disease stage, CD4+ T-lymphocyte count or race.32 3TC has low protein binding in plasma and freely crosses the placenta and into breast milk33; it appears to be concentrated in the male genital tract.34 Similar to other drugs in its class, it appears to cross the placenta by simple diffusion and is thought to concentrate in the amniotic fluid through fetal urinary excretion.35 3TC clearance is prolonged in neonates compared to that in infants and older children.33 3TC is excreted predominantly by the kidney, with 70% excreted unchanged in the urine.26 Dose adjustment is required with significant renal impairment (creatinine clearance <50 mL/min).31,36 3TC is cleared by hemodialysis, though given the large volume of distribution of 3TC no increase in dose is required once an individual with chronic renal failure begins dialysis.36 3TC pharmacokinetics are unchanged in individuals with moderately to severely impaired hepatic function (cirrhosis).37
Lamivudine enters the cell by passive diffusion8 and appears to be phosphorylated more efficiently in resting lymphocytes than in activated cells.38 At steady state ∼15–20% of 3TC in peripheral blood mononuclear cells is in the triphosphate form (3TC-TP) and 50–55% is in the diphosphate form, (3TC-DP), making the conversion of 3TC-DP to 3TC-TP the rate-limiting step during intracellular metabolism.39 The intracellular half-life of 3TC-TP is ∼12–16 h39,40 compared to 1.0 and 2.6 h for ZDV-TP and ddC-TP, respectively. Once-daily dosing of 300 mg of 3TC is now recommended as an acceptable dosing interval owing to these properties.
In a dose-range phase I/II study of 3TC, serum and cerebrospinal fluid (CSF) samples were obtained from some subjects and the CSF/serum drug concentration ratio was found to be low (0.06) similar to what was previously reported for ddI and ddC.41 The concentration of 3TC in CSF relative to serum in nonhuman primates was significantly higher (41%), though levels in ventricular CSF in these animals was similar to those seen in humans.42 In children the CSF/serum ratios were more than 0.141 and therefore higher than reported by van Leeuwen et al41 in adults.43 It is of note that because 3TC has such favorable pharmacokinetics, reaching high levels in serum, the absolute concentrations of 3TC in CSF are as high or higher than the two thymidine nucleoside analogs d4T and ZDV.44 In addition, CSF concentrations of nucleoside analogs are relatively stable over time, unlike plasma concentrations. Therefore, CSF/serum drug ratios are highly time dependent, with higher ratios when sampling is done later in the dosing interval.44
The penetration of 3TC into male genital secretions has also been examined. In 70 samples from nine men on 3TC and ZDV followed over time, the median seminal fluid/blood plasma 3TC concentration ratio was 9:1, demonstrating marked accumulation of 3TC in this compartment.34 When the steady-state relation between the seminal fluid–blood plasma 3TC concentration was examined relative to the timing of drug ingestion, the 3TC concentrations in semen were remarkably constant over time, suggesting that 3TC is either actively taken up or trapped in this compartment.45 The semen/blood concentration ratios for 3TC are higher than for other nucleosides,34,45,46 NNRTIs,46,47 and single protease inhibitors45,48,49 described to date. Whether these high concentrations of 3TC affect the likelihood of HIV-1 sexual transmission relative to other antiretroviral agents is not known.
TOXICITY
Some of the toxicity seen with nucleoside analogs results from their affinity for human DNA polymerases, although for most nucleoside analogs the affinity for this enzyme is less than that for HIV-1 RT. The relative affinity of the NRTIs for specific human DNA polymerases varies by agent and may explain in part their differing toxicities. Significant attention has been given to the potential mitochondrial toxicity of nucleoside analogs, which may relate to their affinity for DNA polymerase-γ.50–52
Lamivudine has limited cytotoxicity in vitro,4–6 possibly owing to the low affinity of 3TC-TP for human DNA polymerases.8,53 For each of these human enzymes the positive enantiomer has greater affinity than 3TC,8 and these enzyme affinities offer a likely explanation for the differences in cytotoxicity between these enantiomers. There was no evidence of a neuropathic effect of 3TC in an in vitro model of neuron toxicity,54 and the potential for hematologic toxicity as measured in vitro is low.55
Significant toxicity that is clearly attributable to 3TC is uncommon (the toxicities are: neutropenia, headache, and nausea). Dose-limiting toxicity was not observed in early studies of 3TC monotherapy.26,30,41 At doses higher than currently recommended, neutropenia was observed in a small number of subjects, and a general downward trend in absolute neutrophil counts was seen.30,41 The subjects in these studies had relatively low CD4+ T-lymphocyte counts, and individuals with low counts may be at greater risk of hematologic toxicity, as has been shown for ZDV.56 In comparative trials, the addition of 3TC to ZDV in a double-blind placebo-controlled trial appeared to have no significant adverse effects other than those seen in subjects who were given ZDV alone.57 In this study subjects given 3TC alone had significantly higher hemoglobin levels than the ZDV- and ZDV/3TC-treated subjects. In large clinical trials in subjects with more advanced disease and previous ZDV therapy for an average of 2 years, severe adverse effects of 3TC/ZDV were also uncommon.58,59 In the study in which 3TC (at two doses)/ZDV was compared to ZDV alone, nausea was the most common adverse effect and was seen more commonly in the 3TC/ZDV arms (10% vs 5% of subjects), though this difference was not significant.59 Neutropenia occurred more commonly in subjects receiving 3TC (300 mg twice daily)/ZDV, but again the differences were not significant between arms. The number of subjects with severe neutropenia was the same in the 3TC (150 twice daily)/ZDV arm and the ZDV monotherapy arm. There were no episodes of pancreatitis and only one episode of peripheral neuropathy among the 223 subjects randomized. In a larger study of subjects with relatively advanced HIV disease (median CD4+ T-lymphocyte count ∼210 cells/mm) in which two doses of 3TC/ZDV were compared with ddC/ZDV, the differences in cumulative moderate and severe toxicity between the treatment arms were not significant.58
The contribution of 3TC to antiretroviral adverse effects that may be due to mitochondrial toxicity (e.g., lipoatrophy and lactic acidosis) is not known. Among patients with hepatitis B treated with 3TC, there was no evidence of mitochondrial toxicity on liver biopsy after 6 months of therapy, albeit the 3TC dose was lower than is commonly used to treat HIV.60 3TC is a preferred antiretroviral in patients with risk factors for hepatotoxiciy of antiretroviral therapy (Cirrhosis, obesity, female gender, a prior hepatotoxicity and co-infection with hepatitis B and C).61–63 Some observational cohort studies have suggested that elevated lactate levels are more common in subjects treated with 3TC and d4T in contrast to other combinations,64,65 but this finding has not been consistently observed.66 An observational study found a higher rate of subcutaneous lipoatrophy and a greater increase in serum lactate at one year in those taking the nucleosides ddI/d4T versus 3TC/ABC.67 Lamivudine has been used successfully to rechallenge patients with prior mitochondrial toxicities (symptomatic hyperlactatemia, lactic acidosis) to NRTIs regimens, including those that included 3TC.68 In a patient with prior symptomatic lactic acidosis, reintroduction of 3TC to the antiretroviral regimen must await full recovery, preceded by careful weighing of risks and benefits, and followed by close monitoring of symptoms and lactic acid levels.22
Episodes of pancreatitis have been reported in pediatric patients receiving 3TC in clinical trials.69 However, these HIV-infected children and infants had advanced HIV disease and had received or were currently receiving concomitant medications that are associated with pancreatitis. Increased frequency of pancreatitis has not been observed in subjects receiving 3TC in controlled trials in adults.
ANTIRETROVIRAL ACTIVITY AND CLINICAL EFFICACY
Multiple trials of 3TC activity and efficacy have been completed. Initially, trials of 3TC monotherapy demonstrated the antiretroviral activity of this agent. Subsequent trials have evaluated 3TC in combination with other antiretroviral agents. Some trials have evaluated the effect on the CD4+ T-lymphocyte count or virologic suppression as the primary endpoint, and others have investigated the clinical efficacy of 3TC-containing regimens. The next few sections will examine the evolution of the use of 3TC in the therapy of HIV infection.
3TC MONOTHERAPY
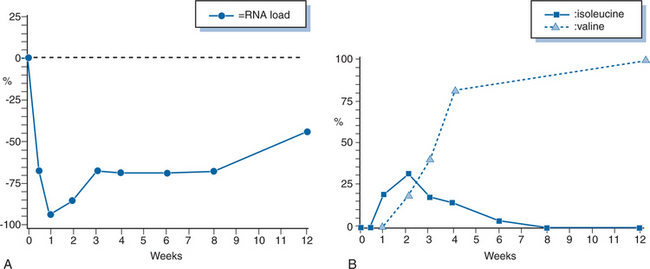
Trials of 3TC as a single agent were predominantly small studies that were undertaken to evaluate the pharmacokinetic parameters of the drug.30,41,70 An initial dose-range study of patients with advanced HIV disease showed only modest evidence of virologic or immunologic activity though HIV-1 RNA was not measured at that time.30 Short-term increases in CD4+ T-lymphocyte counts were observed at higher doses (8 and 12 mg kg−1 day−1) but subsequently decreased to baseline after 20 weeks. A similar study performed in Europe tested doses of 3TC ranging from 0.5 to 20.0 mg/kg and showed small, short-lived increases in CD4+ T-lymphocyte counts.41 There were also changes in other surrogate markers of HIV infections, such as p24 antigen, β2-microglobulin, and neopterin levels. These changes persisted for a longer duration than the changes in CD4+ T-lymphocyte counts, though there was no clear dose–response effect on these parameters. Schuurman and colleagues demonstrated that 3TC administered as a single agent resulted in a rapid decrease in serum HIV-1 RNA levels with an average decrease of more than 95% in 2 weeks (Fig. 9-2A).70 This study was also one of the first to demonstrated in vivo emergence of resistance to 3TC (see below). More recent trials of 3TC monotherapy have focused on comparing its antiretroviral activity to that of emtricitabine.71,72
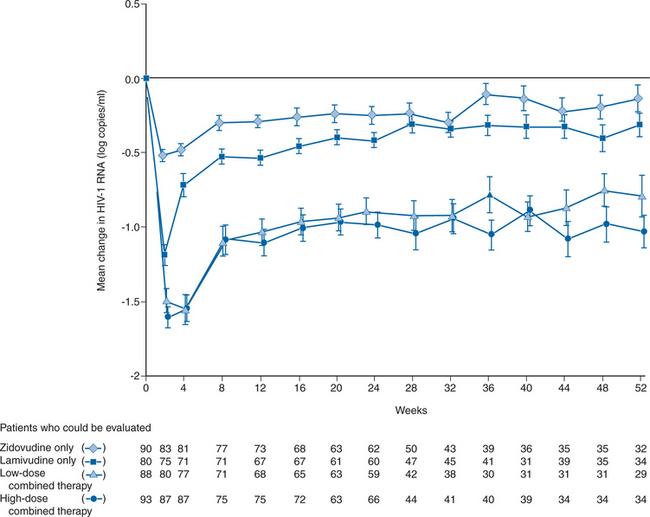
Monotherapy with 3TC was also studied in 90 subjects given 300 mg twice a day in a larger clinical trial done primarily to evaluate the combination of 3TC/ZDV.57 Lamivudine monotherapy resulted in a peak mean increase in CD4+ T-lymphocyte count of 35 cells/μL above baseline in subjects with CD4+ T-lymphocyte counts of 200–500 cells/mm3. Mean CD4+ T-lymphocyte counts remained above baseline for ∼8 months, an effect similar to that observed with ZDV monotherapy in the same study. HIV-1 RNA levels were decreased initially by a mean of 1.2 log10 copies/mL and remained below baseline through 52 weeks of observation (Fig. 9-3). HIV-1 RNA levels were reduced to a significantly greater extent with 3TC than with ZDV monotherapy.57
The limitations of 3TC monotherapy became apparent relatively early during laboratory and clinical studies of the drug. Resistance developed rapidly in vitro, with the initial effect of 3TC on CD4+ T-lymphocyte counts and p24 antigen levels being modest and transient.30,73 In addition, the synergistic interactions of nucleoside analogs in vitro were being noted,13,74,75 and the potential advantages of combination antiretroviral therapy were being recognized.76,77
Several trials have shown continued antiviral activity of 3TC despite the presence of an M184V mutation (confers high-level phenotypic drug resistance) in the HIV RT gene.22,70,78–81 A randomized 48-week open-label pilot study of 3TC monotherapy in patients with 3TC resistant virus showed better immunological (defined as first CD4 < 350) and clinical (defined as occurrence of CDC grade B or C event) as when compared to complete therapy interruption.82 In addition, the strategy of continuing 3TC monotherapy showed benefit in the form of more favorable rates of CD4 decline, viral rebound and recovery of HIV-1 replicative capacity.82 Based on the available data, 3TC continues to contribute antiviral activity despite existing resistance, and 3TC monotherapy is undergoing study as a ‘bridging’ option in HIV-1 multidrug resistant patients until the arrival of two or more active antiretrovirals.22,78–82
DUAL NUCLEOSIDE THERAPY
The use of 3TC in combination with ZDV resulted from a convergence of observations and treatment concepts. As outlined, the modest effect of 3TC in vivo and the emergence of 3TC resistance in vitro were apparent. The clinical toxicity of 3TC was minimal, however. Importantly, it was noted that when the mutation associated with 3TC resistance (discussed in the next section) was added to HIV-1 variants resistant to ZDV, these variants regained sensitivity to ZDV.83 In additional experiments, it was shown that resistance to a 3TC-like compound (FTC) developed more slowly in vitro in the presence of FTC and ZDV.83
Antiretroviral Activity and Effect on CD4+ T-Lymphocyte Count
The combination of lamivudine and ZDV was evaluated in two studies of patients who had received less than 4 weeks of ZDV and no other antiretroviral therapy. Both were randomized, double-blind, placebo-controlled multicenter trials comparing 3TC/ZDV with ZDV monotherapy. 3TC monotherapy was also evaluated in one of the studies.57 Eron and colleagues evaluated subjects with CD4+ T-lymphocyte counts of 200–500 cells/mm3 who remained on their original blinded therapy assignment through 52 weeks.75 Primary study endpoints included the change (from baseline) of CD4+ T-lymphocyte counts and the plasma HIV-1 RNA levels. The primary metric for analysis of immunologic and HIV-1 RNA endpoints was the time-weighted area under the curve (AUC) of all postbaseline measurements minus the baseline value.84 Sustained CD4+ T-lymphocyte increases were seen during 52 weeks of the 3TC/ZDV combination at two doses of 3TC (150 and 300 mg twice daily); there was a peak mean increase of 79 and 78 cells/μL, respectively, with little trend toward baseline over time.57 By week 52 the difference between the mean CD4+ T-lymphocyte count for either combination arm and ZDV monotherapy (200 mg three times a day) was more than 100 cells/mm3. The combination arms showed a mean peak effect on HIV-1 RNA in plasma of almost 1.6 log10 copies/mL, whereas the peak effect of ZDV was 0.5 log10 copies/mL. The median time-weighted change from baseline over 24 weeks were decreases of 1.1 and 1.2 log10 copies/mL for the low- and high-dose 3TC/ZDV combinations, respectively, compared to a decrease of 0.3 log10 copies/mL for ZDV monotherapy (Fig. 9-3). It is of note that in a subset of patients who began the study with a plasma HIV-1 RNA level of 20 000 copies/mL or more and therefore had the opportunity to experience a 2 log10 copies/mL decrease from baseline (the lower limit of detection of the HIV-1 RNA assay was 200 copies/mL) the combination of ZDV with either dose of 3TC showed a median peak decrease of more than 2.0 log10 copies/mL (100-fold reduction from baseline). Over the 52 weeks of the blinded therapy phase, the 3TC/ZDV combination had a persistent ∼10-fold inhibitory effect on HIV-1 RNA levels. Clinical endpoints were also examined in this study, though the size of the study limits the interpretation of this information. Significantly fewer Centers for Disease Control and Prevention (CDC) class B and C endpoints occurred during 52 weeks in patients on combination therapy compared with patients on ZDV alone (J J Eron, unpublished observations).
Katlama et al studied subjects with CD4+ T-lymphocyte counts of 100–400 randomized to one of two treatment arms: (1) ZDV 200 mg every 8 h/placebo; (2) ZDV 200 mg every 8 h/3TC 300 mg every 12 h. The initial study period was 24 weeks, after which time patients were offered open-label 3TC and ZDV and were followed for an additional 24 weeks. In this study the peak mean change in CD4+ T-lymphocyte counts from baseline was 85 cells/mm3 at 8 weeks for ZDV/3TC and 34 cells/mm3 at week 4 for ZDV monotherapy.85 At 24 weeks the mean CD4+ T-lymphocyte count was 78 cells above baseline for patients on 3TC/ZDV and had decreased to nine cells below baseline in subjects on ZDV. Among the subjects who completed 24 weeks of study, 97% opted to continue on open-label 3TC and ZDV. Over the subsequent 24-week period the positive CD4+ T-lymphocyte effect of 3TC/ZDV persisted, though it declined somewhat to a mean of 48 cells above baseline at week 48. Using an immune-based capture technique,86 HIV-1 RNA levels in plasma were evaluated in 29% of the patients. This assay measures only intact HIV-1 particles, and so a potentially smaller log reduction may be seen with this assay compared to other assays that measure HIV-1 RNA in plasma. The combination showed a 0.8 log10 copies/mL decrease at week 24. This antiretroviral effect persisted at 1.1 log10 copies/mL below baseline at week 48, though only a small number of samples were available at this time point. Despite 24 weeks of previous ZDV monotherapy the addition of 3TC to this group resulted in similar decreases in HIV-1 RNA at week 48.
These studies of 3TC and ZDV in antiretroviral treatment-naive patients yielded remarkably similar results. The 3TC/ZDV combination produced significant and prolonged effects on both CD4+ T lymphocyte counts and HIV-1 RNA levels in plasma over the 48–52 weeks of the study. These effects were clearly superior to ZDV monotherapy and were obtained with no significant increase in adverse events when compared to ZDV alone.57,85
The 3TC/ZDV combination was also studied in patients who had undergone previous ZDV treatment. One study compared the addition of 3TC to ZDV versus continuing ZDV alone in 223 subjects with CD4+ T-lymphocyte counts of 100–400 cells/mm3 who had been treated with ZDV for more than 6 months. Subjects either continued ZDV 200 mg three times daily or received 3TC at 150 or 300 mg bid/ZDV. Most (54%) of the subjects were asymptomatic, with a mean CD4+ T-lymphocyte count for the group of 251 cells/mm3. The mean duration of previous ZDV therapy was 24 months. There were significant increases in CD4+ T-lymphocytes in the patients treated with ZDV/3TC, with a mean increase in CD4+ T-lymphocytes of ∼40 cells/mm3 above baseline at 24 weeks with either dose of 3TC and remained at ∼30 cells/mm3 above baseline through 48 weeks.59 A mean decrease in plasma HIV RNA of ∼1 log10 copies/mL was observed in either combination-therapy arm over 24 weeks.
In a study conducted in North America in ZDV-experienced patients with CD4+ T-lymphocyte counts of 100–300 cells/mm3, the addition of two doses of 3TC (150 and 300 mg twice daily) were compared to adding ddC.58 Altogether, 254 subjects with a median duration of previous ZDV treatment of 20 months, a median CD4+ T-lymphocyte count of 214 cells/mm3, and a median plasma HIV RNA level of 4.7 log10 copies/mL were studied. Adding 3TC to ZDV resulted in significant increases in the CD4+ T-lymphocyte counts above baseline compared to adding ddC. The median change in CD4+ T-lymphocyte count after 52 weeks on 3TC (150 mg twice daily)/ZDV was an increase of 43 cells/mm3, whereas subjects on ddC/ZDV experienced a decrease of 30 cells/mm3. The effects on plasma HIV-1 RNA levels were similar in all three treatment arms, with median decreases of 0.4–0.5 log10 copies/mL at year. There were fewer new AIDS events in the 3TC/ZDV-treated arms than in the ZDV/ddC-treated group, but this trend did not reach statistical significance. These two studies of the addition of 3TC to the treatment of ZDV-experienced patients demonstrated significant immunologic and virologic effects. They also showed that the adding 3TC to the regimen of patients already receiving ZDV has a less potent effect than initiating the two agents simultaneously.
There has been evidence demonstrated that 3TC and ZDV can have a prolonged, though incomplete, antiretroviral effect in selected patients, even in the presence of likely 3TC resistance. In subjects treated with 3TC/ZDV for 2 years, the drugs were discontinued at the completion of the study period; the patients were then observed for 2 weeks before starting alternative antiretroviral therapy. Despite 2 years of treatment, these subjects experienced a rapid rise in HIV-1 RNA off therapy with the increases ranging from twofold to 50-fold87 over the 2-week period; this increase suggests that a significant antiretroviral effect of 3TC/ZDV was present up to 2 years in some patients. Multiple studies have demonstrated the persistence of antiviral activity against HIV-1 and provide the rationale for recommending the continuation of 3TC or FTC in subsequent therapeutic regimens despite documented resistance to this drug, particularly in the setting of multiple regimen failures.22,78–81
The combination of 3TC with other nucleosides has also been evaluated. Detailed comparisons of the effects of several nucleoside regimens on HIV-1 RNA levels and CD4+ T-lymphocyte counts have been carried out by the AIDS Clinical Trials Group (ACTG). ACTG 306 enrolled subjects naive to previous treatment and who had CD4+ T-lymphocyte counts of 200–600 cells/mm3. The patients were randomized to one of two treatment limbs: one didanosine (ddI)-based and one stavudine (d4T)-based.88 In the ddI limb subjects were randomized to ddI monotherapy, ddI/3TC, or ZDV/3TC. In the d4T limb subjects were randomized to d4T monotherapy, d4T/3TC, or ZDV/3TC. After 24 weeks subjects on either ddI or d4T monotherapy were also given 3TC. Altogether, 299 subjects were enrolled. The median baseline plasma HIV RNA levels were ∼10 000 copies/mL, and the median CD4+ T-lymphocyte counts were ∼400 cells/mm3. Using an HIV RNA assay with a lower limit of detection of 50 copies/mL, the mean log10 copies/mL decreases in RNA on the ddI limb at 24 weeks were 1.3, 1.2, and 1.4 for ddI, ddI/3TC, and ZDV/3TC, respectively.
None of the comparisons between treatment arms were significant. For the d4T limb the adjusted mean decreases in plasma HIV-1 RNA at 24 weeks were 0.5, 1.6, and 1.6 log10 copies/mL for d4T monotherapy, d4T/3TC, and ZDV/3TC, respectively. The difference between d4T and d4T/3TC was highly significant (P = 0.001), and the difference between d4T/3TC and 3TC/ZDV was not (P = 0.77). At 48 weeks the addition of 3TC to d4T and to ddI resulted in additional 0.44 and 0.79 log10 copies/mL adjusted decreases, respectively. After 48 weeks the decreases in HIV RNA ranged from 1.2 log10 copies/mL (d4T/3TC) to 1.8 log10 copies/mL (ddI/delayed 3TC) with both d4T/3TC and ZDV/3TC at 1.5 log10 copies/mL. None of the comparisons within arms were significant. During 48 weeks the CD4+ T-lymphocyte count increases were not significantly different between arms, with increases of 77–118 cells/mm3. These results suggest that during 48 weeks in subjects with a relatively low viral load (mean HIV RNA at baseline ∼10 000 copies/mL) 3TC/d4T is at least as effective at suppressing HIV RNA levels and increasing CD4+ T-lymphocyte counts as 3TC/ZDV. Data suggest that the genotypic resistance patterns for d4T and ZDV are similar, and therefore a mutational interaction between 3TC and d4T resistance similar to the 3TC–ZDV interaction may occur.89 The antiviral activity of 3TC/ZDV compared to ddI/3TC and ddI monotherapy was more difficult to interpret, as activities over 24 and 48 weeks were quite similar. On the one hand, the similarity of the 3TC/ddI and ddI arms suggests that adding 3TC to ddI affords little benefit. On the other hand, adding 3TC to ddI after 24 weeks resulted in a 0.8 log10 copies/mL decrease and supports the opposite conclusion. Resistance data from this study may shed further light on the utility of the ddI/3TC combination. The overall results from this study must be interpreted with some caution. The viral load of all the subjects at baseline was low, and the results may be less applicable to individuals with high viral loads. In addition, although the censoring of change in HIV RNA levels by the lower limits of the HIV RNA assays was taken into account, these methods may be imperfect and more censoring may have occurred in the dual nucleoside arms, which perhaps narrowed the differences.
The 3TC/d4T combination has also been investigated in an open-label study.90 In treatment-naive subjects the antiretroviral effect of 3TC/d4T over a 6-month period was similar to effects seen with ZDV/3TC in previous studies and in the ACTG 306 trial (median decrease of 1.66 log10 copies/mL).88 The effect of 3TC/d4T was much less potent in nucleoside treatment-experienced subjects who had not received 3TC or d4T, with a median decrease in HIV RNA of 0.55 log10 copies/mL at 24 weeks.
Effect on Disease Progression and Mortality
The effect of 3TC in combination with other nucleoside analogs on HIV disease progression and mortality has now been clearly demonstrated. After completion of four of the initial studies (reviewed in previous sections) that primarily examined antiretroviral efficacy, a meta-analysis of these studies was performed in an attempt to analyze effects on disease progression.91 This analysis, which combined data from 972 subjects, showed a beneficial effect of 3TC/ZDV on disease progression compared to the control arms in various studies. There was a 49% reduction in all clinical events with ZDV/3TC therapy and a 66% reduction in progression to new AIDS events. This difference was seen when subjects were divided into subsets by treatment history, presence of symptoms, or CD4 cell counts. Few deaths occurred in these studies, and the number of AIDS-defining endpoints was limited. These factors, coupled with the retrospective design of the analysis, limited interpretation of the results.
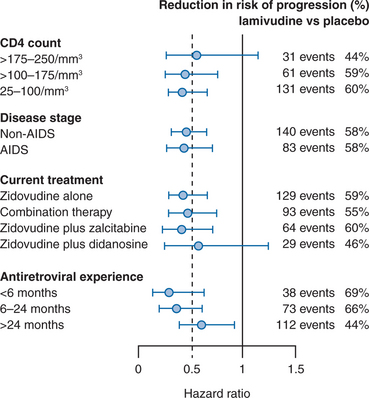
To examine the effect of 3TC on disease progression, a large randomized, placebo-controlled trial of the addition of 3TC to ZDV-containing nucleoside regimens was undertaken.92 This study, referred to as the CAESAR trial to represent the locations (Canada, Australia, Europe, South Africa) where the study took place, enrolled subjects receiving ZDV alone or in combination with ddI or ddC and with a CD4+ T-lymphocyte count of 25–250 cells/mm3. More than 1800 subjects were randomized to receive placebo, 3TC (150 mg twice daily), or 3TC/loviride (100 mg three times daily), an NNRTI. Subjects were randomized in a 1:2:1 fashion so half the subjects added 3TC alone to their ZDV-containing regimen. The median duration of antiretroviral treatment prior to study entry was 28 months, and most subjects (62%) were on ZDV alone at baseline. The study was terminated prematurely at a second interim analysis because of the statistically significant benefit of 3TC in reducing the relative risk of disease progression compared to placebo. In the final intent-to-treat analysis, the relative reduction in risk of progression to AIDS or death was 57% (relative hazard (RH) = 0.43) for 3TC-containing arms compared to placebo. There was also a 60% reduction in mortality for the 3TC-containing arms compared to placebo. The addition of loviride to the 3TC regimen appeared to offer no benefit in the overall analysis of clinical progression. When the relative risk reduction of 3TC compared to placebo was examined for various subgroups (e.g., CD4+ T-lymphocyte count, AIDS vs non-AIDS, treatment at study entry, duration of antiretroviral experience), a consistent risk reduction of ∼50% was seen (Fig. 9-4). The greatest risk reduction was seen in subjects who had received less than 6 months of previous antiretroviral therapy. The CAESAR study clearly demonstrated that the antiretroviral effects of 3TC that had been seen in earlier studies are translated into clinical benefit, as suggested by smaller studies91,93 and seen with other antiretroviral therapies.94 Future studies of combination regimens would soon eclipse the outcomes achieved with dual NRTI regimens.
3TC IN HAART COMBINATIONS
3TC in Combination with NNRTIs
Antiretroviral Effects and Effects on CD4+ T-Lymphocyte Counts
Effect on Disease Progression and Mortality
The International AIDS Society-USA Panel and the Department of Health and Human Services Guidelines Panel 2006 recommendations for choice of initial HIV treatment regimen suggest the utilization of 2 NRTIs combined with either an nNRTI or a PI boosted with low-dose RTV.22 The continued relevance of 3TC’s and FTC’s role in contemporary HIV management is validated by its inclusion in two of the three recommended choices for the dual nNRTI component of initial HAART regimens (3TC/ABC, 3TC/ZDV).22
3TC in Combination with Protease Inhibitors
Antiretroviral Effects and Effects on CD4+ T-Lymphocyte Counts
The demonstration of enhanced antiretroviral effect and clinical benefit of combination nucleoside therapy,94,95 especially ZDV and 3TC,57,92 in conjunction with the clinical development of protease inhibitors96–99 has dramatically altered the design of clinical research trials and ultimately of recommendations for clinical practice.100,101 HAART regimens, which most commonly consist of two nucleosides in combination with a RTV boosted protease inhibitor, or an NNRTI is the standard of care in developed countries.22,25,102
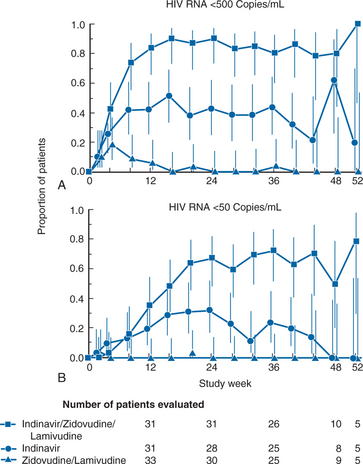
Multiple trials of 3TC in combination with ZDV or d4T and an unboosted protease inhibitor demonstrated the potent activity of these combinations. One of the earliest of these studies was a trial that compared 3TC/ZDV/indinavir with ZDV/3TC and with indinavir alone.103 In this double-blind study, 97 subjects who had previously received ZDV for at least 6 months (median previous treatment for 30 months) were randomized to have 3TC added to their ZDV, to have 3TC and indinavir added, or to switch to indinavir alone. Subjects had CD4+ T-lymphocyte counts of 50–400 cells/mm3 and a plasma HIV-1 level of more than 20 000 copies/mL. The median CD4+ T-lymphocyte count at entry was 144 cells/mm3, and the median HIV-1 RNA level was 43 000 copies/mL. Subjects treated with the three-drug regimen had a more profound antiretroviral response than previously described. HIV RNA levels decreased to less than 500 copies/mL (the limit of quantification of the assay) in 90% of subjects after 24 weeks of therapy (Fig. 9-5). No subject who received only 3TC/ZDV had this level of response, whereas 43% of subjects on indinavir monotherapy were below quantification levels at 24 weeks. Approximately 70% of subjects on the three-drug therapy after 24 weeks were below detection limits when a more sensitive assay that could quantify HIV RNA in plasma to ∼50 copies/mL was used (Fig. 9-5). CD4 cell changes were not significantly different between the indinavir arm and the three-drug therapy arm, although both were superior to ZDV/3TC. Follow-up of subjects on the three-drug therapy arm is ongoing, and two-thirds of subjects originally assigned to three-drug therapy have had their HIV RNA levels remain at less than 50 copies/mL through 3 years of therapy.16,104 Similar results (although for a shorter time period) were seen with d4T/3TC and indinavir.105
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree