Introduction
The evaluation of short stature in children requires a skilled interaction between the clinician and the laboratorian in order to provide a cost-effective and expedient diagnostic assessment of the infant or child . Based initially on growth velocity, the evaluation can be targeted to use laboratory and economic resources appropriately. Growth is a complex process. This chapter focuses on linear growth emphasizing the growth-hormone/insulin-like-growth-factor I axis (GH/IGF-I axis).
Definition of short stature
In order to discuss short stature, we must define the term “short stature.” Children’s heights are judged according to their sex and age by plotting height versus age on a sex-appropriate growth chart. Many growth charts provide a series of lines that define various percentiles. Fig. 14.1A depicts a simplified growth chart depicting the 3rd, 50th, and 97th percentiles for boys. For example, a boy at the 75th percentile for age is taller than 75% of boys his age, whereas he is shorter than 25% of boys his age.

After 18–24 months of age, most children grow along, or near to, a particular height percentile. Prior to this time, approximately one-third of children accelerate to higher percentiles, one-third decelerate to lower percentiles, and one-third grow along a percentile similar to their percentile at birth. Birth weight is related, in part, to fetoplacental nutrition, and fetal and maternal health. In general, larger mothers give birth to larger babies.
Short stature is generally defined as height below the third or fifth percentiles for age and sex. The chart below depicts the correlation between percentile and the number of standard deviations (SDs) below the mean that the percentile corresponds to:
Although not typically used by pediatricians or family practitioners, some growth charts depict height as the number of SDs that the child is above or below the mean in place of percentiles. In addition to height and weight growth charts, pediatric endocrinologists often use growth velocity charts ( Fig. 14.1B ). In such charts, the rate of growth in centimeters per year is plotted against age. These charts are sex-specific. Growth velocity is maximal during infancy. In the first year of life, height increases by ~50% with growth velocities as high as 25 cm/year. After infancy, growth velocity declines typically to ~6 cm/year until puberty. During puberty, the growth rate rises again to 10–12 cm/year for a few years. This provides the adolescent “growth spurt.” A patient’s height velocity should be compared with sex- and age-specific norms.
To assess linear growth, growth velocity must be known in addition to absolute height. Accurate height measurements require a stadiometer. A stadiometer is an apparatus for measuring height using a vertical ruler (preferably mounted on a wall) where a sliding horizontal bar is adjusted to rest on the top of the patient’s head. The child’s back should be flat against the wall with their head looking forward and their feet together. Two to three measurements should be taken and averaged. From 3 to 6 months later at the same time of the day on the same stadiometer, the child should be remeasured to determine height velocity (growth in cm/year). The more the child’s height deviates from normal, the greater is the likelihood that the child is affected with a significant medical condition. If the growth velocity is below the lower limit of normal and especially if the growth velocity approaches zero, a serious disease is likely to be present.
More boys than girls are evaluated for short stature. This likely reflects two factors: (1) as a society, height is more of a concern in males than in females, and (2) constitutional delay in growth and maturation (CDGM; e.g., delayed maturation) is more common in boys than in girls. Most children with short stature do not have identifiable disease or disordered GH secretion. If the height is between 2 and 3 SDs below the mean, in only 8% of children is an organic (medical) etiology identified. In contrast, if the height is between 3 and 4 SDs below the mean, 50% of cases display an organic etiology.
Socially, short stature could be “functionally” defined as “not being as tall as one wants to be” or “having a child that is not as tall as the parent wants the child to be.” There are social implications to height that are often referred to as “heightism” . It is interesting to note that the upper classes in England are taller than the lower classes, executives are taller than their subordinates, US Presidents are, in general, taller than their piers, taller people earn higher incomes, and taller people are often considered to be more successful than people of lesser height. An alternative view is that “height is measured in more than inches” and that social stature does not equal linear height . Nonheight “stature” includes virtues such as integrity, honesty, and the ability to accomplish tasks. The societal economic cost of GH treatment is also of concern .
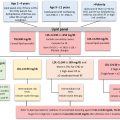
Psychologically, short stature can impair self-perception and self-confidence , although debate remains as to the frequency and severity of such effects . Medically, short stature may be a manifestation of a serious underlying medical illness (e.g., malnutrition) and thus would require critical medical evaluation. Appropriate therapy can alleviate or minimize many forms of short stature. However, an incorrect diagnosis could lead to inappropriate therapy, whereas improper therapy may be harmful or lead to the misuse of medical and/or financial resources. Assuming that a child has short stature, growth velocity is used to classify the child into one of two categories: short stature with a normal growth velocity (curve A of Fig. 14.2 ) versus short stature with a subnormal growth velocity (curve B of Fig. 14.2 ).
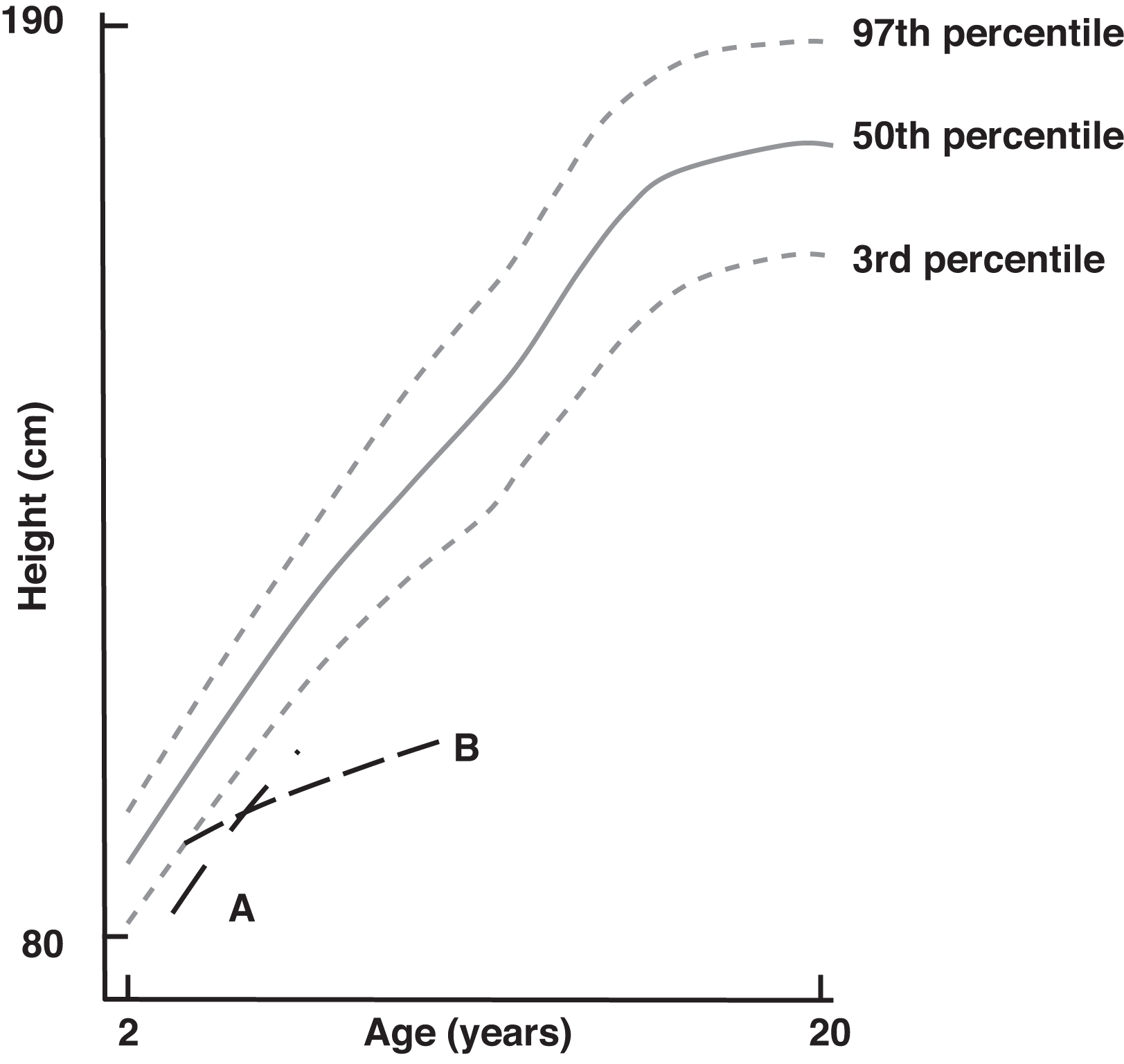
Short stature with normal growth velocity
There are three conditions that generally cause short stature with normal growth velocity: (1) familial short stature, (2) constitutional delay in growth and adolescence (e.g., delayed maturation), and (3) primordial short stature [which includes children that were small for gestational age (SGA)].
Familial short stature
Familial short stature reflects the child’s family history and inherited genetic potential . If the child’s parents are short, it is likely that the child will also be short. In such cases, the parental heights need to be plotted to determine their adult percentiles. As a rule of thumb, the child’s expected height is the mean of the parents’ heights plus 2 inches for boys or minus 2 inches for girls. Bone age is commonly used as a measure of biologic age. Bone age is a radiologic assessment of biologic maturity carried out by comparing the ossification and shape of the bones in the left wrist and hand to a standardized image atlas . Bone age in infants is assessed by looking at the shape and the degree of ossification of growth centers in the long bones, especially in the knees.
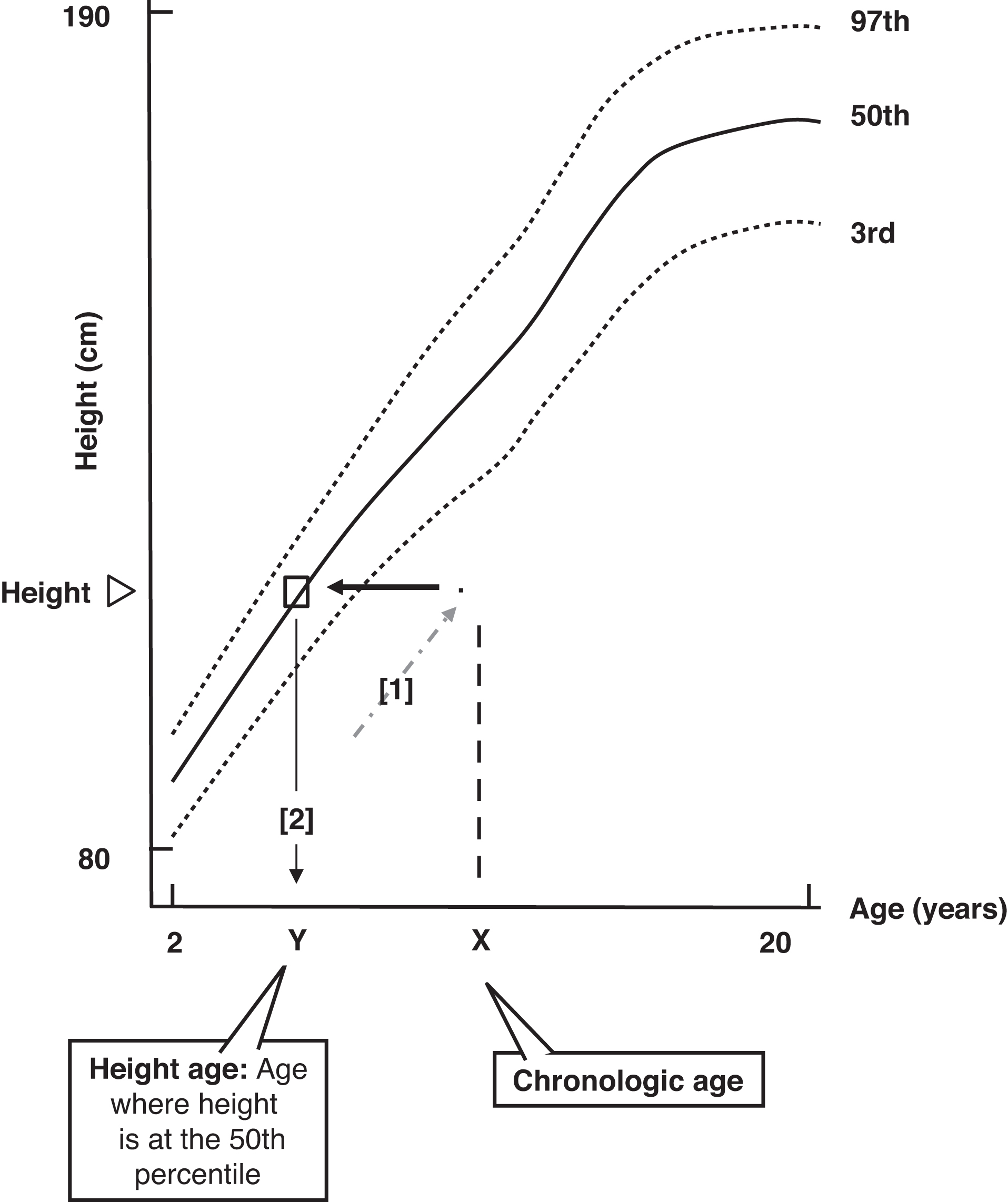
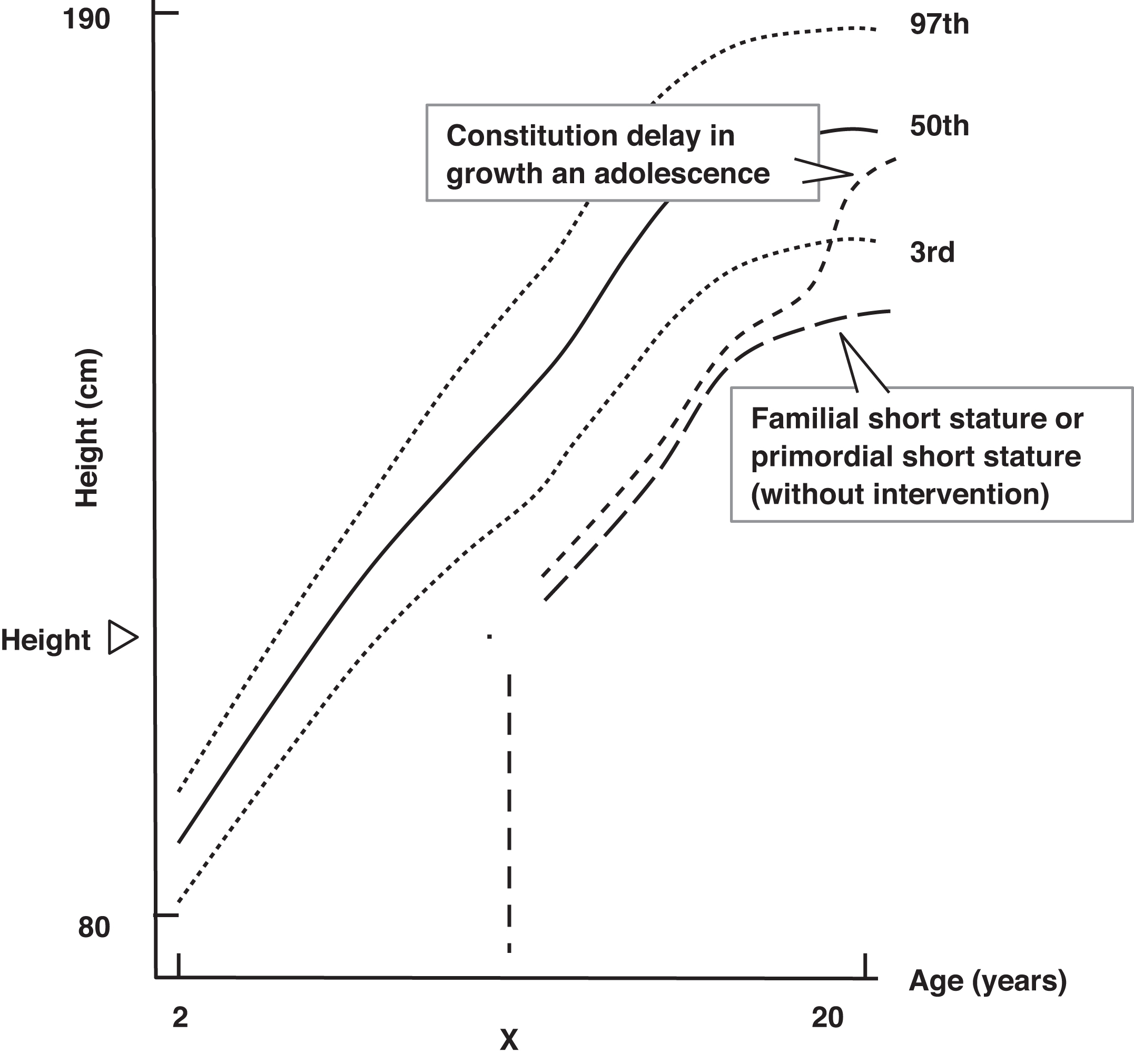
Bone age represents the child’s biologic potential: in other words, there is more growth potential if a 10-year old has a bone age of 7 years than if the child’s bone age is equal to their chronologic age (CA) of 10 years. Height age (HA) is the age at which the child’s present height represents the 50th percentile. In familial short stature, the bone age is approximately equal to the CA, and both are greater than the HA ( Fig. 14.3 ). This child is likely to be short once growth ceases ( Fig. 14.4 ).
Familial short stature is a diagnosis but not a disease. Familial short stature is not the consequence of the effects of any one genetic locus . It does not require therapy, and no laboratory studies are absolutely required. In general, in children with short stature and a normal growth velocity, a general laboratory evaluation is optional because chronic disease is unlikely based on the normal growth velocity, and the yield of such screening tests is very low ( Table 14.1 ). Because radiological assessment of bone age is almost always diagnostically helpful, bone age determination is required in essentially all children evaluated for short stature. In suspected familial short stature, the bone age is close to the chronological age.
| Information desired | Tests |
|---|---|
| Screen for chronic disease | CBC; consider ESR or CRP measurement |
| Fluid and electrolyte balance | Electrolytes (Na + , K + , Cl − , HCO 3 − ) |
| Exclude diabetes mellitus | Fasting plasma glucose, 2-h PG, OGTT, or HbA1c |
| Renal function evaluation | BUN, creatinine, urinalysis |
| Hepatic synthetic ability and nutritional assessment | Total protein, albumin |
| Hepatic detoxifying function | Total bilirubin |
| Hepatocyte membrane and biliary tract integrity | Alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP) |
| Celiac disease | IgA tissue transglutaminase autoantibody testing |
| Bone and vitamin D metabolism | Calcium, phosphate, alkaline phosphatase (ALP) |
| Thyroid function | TSH, free thyroxine |
When children grow slowly for no apparent reason and they are biochemically sufficient in terms of growth hormone (GH) and insulin-like growth factor-I (IGF-I) concentrations, the term “idiopathic short stature” (ISS) can be applied. Children with ISS can display greater height deficits by the age of 5 than children with either familial short stature or CDGM . This would be therapeutically important because ISS is an indication for GH therapy whereas familial short stature and constitutional delay in growth and adolescence are not indications for GH therapy.
Constitutional delay in growth and adolescence
Constitutional delay in growth and adolescence is also termed “delayed maturation” . This condition is very common, accounting for “late bloomers” who were short during childhood but grew to normal height with puberty. In delayed maturation, the biologic/maturational “clock” (involving the control of growth and adolescence in the hypothalamus) is “ticking” more slowly than normal, and puberty is delayed. When puberty is delayed, a child grows at the normal prepubertal rate of 5–6 cm/year, whereas the heights of their peers accelerate to 10–12 cm/year with normal puberty, and quite quickly, the children affected with delayed maturation appear to be much shorter and younger than their peers. At the same time, as their peers advance through puberty and either masculinize (boys) or feminize (girls), the affected child’s appearance remains prepubertal and immature compared with his or her peers. This can be concerning to the parent and affected child alike.
In delayed maturation, the bone age and HA are usually approximately equal and are both delayed compared with the CA ( Fig. 14.3 ). This indicates that the child has more growth potential than is evident by their CA and they will catch up during puberty, although puberty will be delayed ( Fig. 14.4 ).
Usually, there is a family history of delayed puberty, with the father being short in high school yet growing to a normal height in his late teens or even his early 20s. As well, the father may not have begun to shave until his late teenage years or early adult years.
The child’s ultimate height in delayed maturation is appropriate for the child’s family history (e.g., these children achieve their genetic potential). Delayed maturation is more common in boys than in girls. On the other hand, precocious puberty is more common in girls than in boys; however, precocious puberty is far less common than delayed maturation. Early in childhood, precocious puberty leads to relatively tall stature and advanced bone age. However, because the growth plates fuse prematurely, adult height in children with precocious puberty may be ultimately below normal.
Delayed maturation (constitutional delay in growth and adolescence), like familial short stature, is a diagnosis but not a disease. Of interest, one research article could find no difference in the mid-parental heights of children otherwise classified as having familial short stature versus delayed maturation . Other than a bone age radiograph, no biochemical testing is absolutely required. However, general laboratory testing can be performed in search of occult disease if there is any suggestion that short stature may be due to chronic disease. It is not intended that every test listed in Table 14.1 must be ordered: the careful physician can judge which assembly of tests is warranted. Some boys with delayed maturation suffer psychological stress from (1) being shorter than their peers, and (2) displaying less pubertal maturation than their peers .
Delayed maturation may be treated by using low-dose testosterone injections (25–50 mg of testosterone enanthate in oil every 3–4 weeks) or oxandrolone (0.1 mg/kg; dose range, 2.5–20 mg/day), a weak oral androgen. These androgens can accelerate growth temporarily and may advance biologic maturation sufficiently such that endogenous puberty will be triggered. The keys to the diagnosis of delayed maturation include (1) sexual delay if the child is of pubertal age, (2) delayed bone age approximately equal to HA, (3) normal growth velocity, and (4) family history of delayed maturation.
Primordial short stature
Primordial can be defined as “existing at or from the beginning of time (e.g., from the time of birth). In primordial short stature, a prenatal insult has caused short stature. This “insult” could be exogenous (e.g., maternal malnutrition) or endogenous including genetic disorders . The infant’s stunted size is recognized at birth as “intrauterine growth retardation” (IUGR) with the infant described as being “SGA” (e.g., small for gestational age: smaller than normal for infants of similar gestational age and sex). The causes of SGA include fetal disorders (e.g., a TORCH infection), maternal disease (e.g., chronic kidney disease, hypertension, long-standing poorly controlled type 1 diabetes with arterial vascular disease), and fetoplacental insufficiency of various causes. Approximately 10% of children that were SGA at birth do not exhibit catch-up growth between ages 2 and 4 years . Similar to familial short stature, bone age and CA are similar, with a retarded HA suggesting that short stature will be present with the cessation of growth ( Figs. 14.3 and 14.4 ).
An example of primordial short stature is fetal alcohol syndrome where ethanol toxicity due to maternal alcoholism is manifested in many clinical findings, including short stature . Typically, children with fetal alcohol syndrome display congenital heart disease, delayed development, small palpebral fissures (the opening of the eyes formed by the upper and lower eyelids), amblyopia (lazy eye), and hirsutism (increased body hair usually located laterally along the arms and legs). Lesser degrees of alcohol toxicity cause “fetal alcohol effects.”
In summary , for children with normal growth velocities, if the bone age and the HA are near equivalent and delayed compared with the chronological age, delayed maturation is likely, with a good prognosis for ultimate adult height ( Fig. 14.4 ). If bone age and CA are nearly equal, and HA is retarded, ultimate short stature is likely secondary to familial short stature or SGA without catch-up growth (primordial short stature) and with cessation of growth, short stature will be present ( Fig. 14.4 ). This latter entity, SGA without catch-up growth, is an FDA-approved indication for GH treatment.
Short stature with low growth velocity
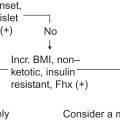
The laboratorian is most likely to become involved in cases of short stature associated with a low growth velocity (e.g., less than ~4–5 cm/year). There are four major considerations in the child with short stature and low growth velocity: (1) chronic disease, (2) Turner syndrome, (3) psychosocial short stature (including deprivation, abuse, and neglect), and (4) endocrinopathies causing short stature. Excluding Turner syndrome, all other causes of short stature with a low growth velocity can display a delayed bone age. The greatest delay in bone age is seen with hypothyroidism.
Chronic disease
Any form of severe chronic illness involving the central nervous, cardiovascular, pulmonary, renal, or gastrointestinal systems can cause low-velocity growth failure . In cases of low growth velocity, occult disease should be sought by history, physical examination, and a general laboratory evaluation ( Table 14.1 ).
Because hypothyroidism may be difficult to diagnose clinically while in its early stages, thyroid-stimulating hormone (TSH) and free thyroxine (FT4) measurements should be included in the general laboratory testing for short stature and low growth velocity . Evidence of malabsorption (e.g., a history of diarrhea, low ß-carotene, low xylose absorption, etc.) or a positive IgA tissue transglutaminase autoantibody test (e.g., celiac disease) should trigger referral to a pediatric gastroenterologist.
Biochemical markers of malnutrition include anemia, hypoalbuminemia, and low concentrations of transthyretin (e.g., thyroxine-binding prealbumin, erroneously often referred to as “prealbumin”). If inflammatory bowel disease is a consideration (e.g., Crohn disease or ulcerative colitis), an erythrocyte sedimentation rate or C-reactive protein (CRP) concentration should be measured as an index of inflammation. Other tests of inflammation include the serum protein electrophoresis in search of a polyclonal hypergammaglobulinemia as a marker of chronic inflammation. When recurrent pneumonia and steatorrhea are noted, cystic fibrosis should be excluded by pilocarpine-iontophoresis sweat chloride testing . Measurements of blood urea nitrogen (BUN), creatinine, urinalysis, and serum electrolytes (including serum CO 2 ) examines renal function (e.g., rule/out: renal tubular acidosis). The findings on physical examination in rickets include widened metaphyses, bowed limbs (especially legs due to weight bearing), Hutchinson groove (an indentation of the rib cage where the diaphragm attaches to the rib cage), and rachitic rosary (a hyperplastic costochondral junction at the front of the rib cage). In rickets, serum calcium is low normal, phosphate is low, and alkaline phosphatase is elevated (see Chapter 10 regarding calcium and phosphate biology).
In cases of short stature possibly due to chronic disease, the clinician is dependent on the history and physical examination to guide the choices of the appropriate radiological and laboratory evaluations. In order to treat short stature in chronic disease, the chronic disease must be treated optimally. Bone age in children with chronic disease may be delayed in comparison with CA, whereas HA may be even more retarded.
Turner syndrome
If chronic disease diagnostically appears unlikely based upon the ongoing clinical and laboratory workup, the diagnosis of Turner syndrome should be considered in girls with low growth velocities. Turner syndrome results from a complete deletion of a single X chromosome (45,X karyotype) or a partial or complete deletion of the short arm of the X chromosome [e.g., 46,X,Xp- or a long-arm isochromosome X, 46,X,i(Xq), or a ring chromosome, 46,X,rX]. Haploinsufficiency of the SHOX gene (e.g., only one copy of the SHOX gene versus normally two copies of the SHOX gene in females) is the cause of short stature in Turner syndrome .
The SHOX (Short Stature Homeobox) gene, encoding a 292 amino acid, 32 kDa transcription factor, is located in the pseudo-autosomal regions of the X and Y chromosomes (Xp22.33 and Yp11) so that two copies of SHOX are present in the normal state. Mutations in one copy of SHOX causes Leri–Weill dyschondrosteosis , whereas mutations in both copies of SHOX cause Langer mesomelic dysplasia . Some women with type I Mayer–Rokitansky–Kuster–Hauser syndrome display a SHOX gene duplication .
In Turner syndrome, mosaic karyotypes (mixtures of two or more karyotypically unique cell lines derived from a single zygote), such as 45,X/46,XX, 45,X/46,X,Xp-, 45,X/46,X,i(Xq), 45,X/46,X,rX, and 45,X/46,XX/47,XXX are commonly observed. X chromosome abnormalities in Turner syndrome are distributed as follows: 50%, 45,X; 25%, 46,X,Xp-, 46,X,rX, and 46,X,i(Xq); and 25% are mosaics. One in 2500 girls is affected with Turner syndrome, and half of these girls are short at birth. With sensitive techniques, some researchers report chromosomal mosaicism in 70% of girls with Turner syndrome. It has even been postulated that all girls born with Turner syndrome have some degree of mosaicism .
Besides short stature, girls with Turner syndrome may display a webbed neck, edema of the hands and feet at birth, left-sided congenital heart disease (e.g., bicuspid aortic valve and/or coarctation of the aorta), multiple cutaneous lentigines (freckle-like brown skin spots that are not related to sun exposure), increased carrying angle of the arms (e.g., when the arms are placed at the side, they are abducted away from the torso), short fourth and/or fifth metacarpals and/or metatarsals, widely spaced nipples, hyperconvex nails, a shield-like chest, horseshoe kidneys (kidneys that are connected at the lower poles forming a horseshoe shape) and other renal anomalies. Streak gonads (nonfunctional gonads that are predominantly composed of fibrosis tissue) that result from premature depletion of oocytes are very common in girls with Turner syndrome. As a result of the X-chromosome deletion, girls with Turner syndrome are usually infertile. At the normal time of puberty, although axillary and pubic hair develops from the normal effects of rising adrenal androgen concentrations (e.g., “adrenarchy”), breast development and menstruation are usually absent because of female sex hormone deficiency. Rare births have been reported to women with Turner syndrome; however, their offspring suffer a high frequency of chromosomal abnormalities . Such “fertile” women with Turner syndrome are commonly chromosomal mosaics (e.g., 45,X/46,XX).
During late childhood prior to the expected onset of puberty (e.g., roughly between ages 10 and 12), follicle-stimulating hormone (FSH) can be measured as an index of gonadal insufficiency in cases of suspected Turner syndrome. If the FSH is elevated into the postmenopausal range, the gonads are likely abnormal (e.g., streak gonads), and a formal karyotype should be obtained when the diagnosis of Turner syndrome has not been established. Even without an elevated FSH, if there is a substantial clinical suspicion of Turner syndrome, a karyotype should be obtained.
Girls with Turner syndrome can be treated with oxandrolone (0.1 mg/kg; dose range, 2.5–20 mg/day) and/or GH to improve their adult height. Turner syndrome is an FDA-approved indication for the use of GH (you need to review the FDA-approved indications for each commercial GH product). Administering GH without the coadministration of oxandrolone in girls with Turner syndrome is the standard of care. GH treatment does not normalize the height of girls with Turner syndrome . Estrogen is used at the time of puberty to induce feminization. Although women with Turner syndrome are commonly infertile because of their streak gonads, once breast development is advanced, hormone replacement should include cycled estrogen and progesterone to prevent uterine hyperplasia that results from unopposed estrogen administration. Because Hashimoto thyroiditis is common in girls with Turner syndrome, these girls should be screened for the presence of thyroid thyroperoxidase autoantibodies and, if negative, thyroglobulin autoantibodies . If such autoantibodies are positive, thyroid function should be periodically assessed.
Psychosocial short stature
Psychosocial short stature results when a child receives inadequate calories or is placed in such a stressful psychological environment (e.g., deprivation, neglect or abuse is present) that they suffer from lack of emotional nurturing, acquired hypothyroidism, and/or acquired growth hormone deficiency (GHD). GHD in psychosocial short stature is transient, not permanent, and is caused directly by the psychosocial situation. Symptoms suggestive of psychosocial short stature include hyperphagia, abnormal eating habits (e.g., hoarding or scavenging food) , polydipsia (increased drinking of fluids), disturbed behavior, global developmental lag, enuresis (bed wetting), and/or encopresis (defecation in one’s cloths).
The diagnosis of psychosocial short stature is dependent upon the history being compatible with poor caloric intake and/or an abusive or neglectful environment. Psychosocial short stature is manifested in infants as failure to thrive, where the greatest decrement is in weight, with height and head circumference (brain growth) being somewhat spared. Brain growth is the most protected growth parameter in infancy.
With failure to thrive, placement of the infant in a new environment (e.g., foster care) with loving support and adequate calories for periods as short as 2 weeks can result in marked weight gain, confirming that the diagnosis is indeed deprivation/neglect/abuse. In older children, longer periods of time in a new environment may be required to observe weight gain, and even longer periods of time are required to reveal increased height.
Other than the laboratory demonstration of malnutrition (e.g., anemia, low albumin, low β-carotene, low transthyretin) in the absence of underlying disease, the greatest aid to the clinician includes a skeletal survey to exclude trauma from the parent or caregiver, as well as social worker assessment of the child, their family, and the family environment.
Endocrinopathies
Following exclusion of other causes of short stature with a low growth velocity, the diagnosis of an endocrine disorder should be considered .
Hypothyroidism
The first endocrine condition to exclude is hypothyroidism . Thyroid hormone is required for normal growth and development in children. Thyroid hormone is also required for normal secretion of IGF-I in response to GH. Hypothyroidism can induce a transient form of GHD that is remedied by thyroid hormone replacement. As well, hypothyroidism can produce resistance to the effects of GH in generating IGF-I.
Hypothyroidism is evaluated by performing thyroid function tests that were part of the general screen for children with low growth velocities. If a high TSH concentration and low free thyroxine (FT4) concentration are recognized, primary hypothyroidism is confirmed. Tri-iodothyronine (T3) or free tri-iodothyronine (FT3) testing does not have a routine role in the evaluation of patients for possible hypothyroidism.
The most common cause of primary hypothyroidism is Hashimoto thyroiditis . Therefore, a request for thyroperoxidase autoantibodies (and, if negative, thyroglobulin autoantibodies) should be made to diagnose Hashimoto thyroiditis. Hashimoto thyroiditis may be accompanied by other organ-specific autoimmune diseases such as type 1 diabetes, adrenalitis, and/or chronic lymphocytic gastritis, which produces pernicious anemia. However, Hashimoto thyroiditis most often occurs as an isolated finding.
Hashimoto thyroiditis patients usually initially display goiter. However, if the gland is destroyed by autoimmune thyroiditis, the gland can become small and/or firm. If a goiter is never detected, atrophic thyroiditis should be considered. In this disorder, antagonistic autoantibodies bind to the TSH receptor and block TSH binding to the receptor, leading to thyroid gland hypofunction and atrophy .
In most developed nations, congenital hypothyroidism is usually diagnosed by newborn screening programs . Other causes of primary hypothyroidism include surgical removal of the thyroid gland (e.g., as therapy for hyperthyroidism or thyroid cancer), irradiation, 131 I ablation of the thyroid gland as treatment for hyperthyroidism or thyroid cancer, inborn errors of thyroid hormone biosynthesis, iodine deficiency or excess, lingual thyroid, a maldescended thyroid gland or ingestion of dietary goitrogens. Predilection to autoimmune thyroid disease is frequently inherited as an autosomal dominant trait that is more often penetrant in women than in men. Despite this apparent Mendelian inheritance, no single gene or set of genes explains this familial proclivity. Inborn errors in thyroid hormone biosynthesis (e.g., iodide pump deficiency, thyroperoxidase deficiency, coupling enzyme deficiency, etc.) are inherited as autosomal recessive traits.
If the child is hypothyroid, and the TSH is normal or low in the face of a low free thyroxine concentration, central hypothyroidism is diagnosed . This results from hypothalamic thyrotropin-releasing hormone (TRH) deficiency (tertiary hypothyroidism) or pituitary TSH deficiency (secondary hypothyroidism). In ~10% of cases of central hypothyroidism, TSH concentrations may even be mildly elevated. In such circumstances, the TSH lacks full biologic activity (but is immunoreactive and is detected in the immunoassay) producing central hypothyroidism . Pituitary disease or TRH deficiency can lead to decreased TSH bioactivity. For full bioactivity, TSH must be glycosylated and sialylated appropriately.
In cases of central hypothyroidism, a search for the central nervous system (CNS) lesions must be sought by computed axial tomography (CT) or magnetic resonance imaging (MRI). Partial or pan-hypopituitarism must also be excluded. Isolated TRH or TSH deficiencies are very rare conditions. Treatment of the CNS lesion (e.g., craniopharyngioma, adenoma, hamartoma, etc.) must be addressed. TSH deficiency caused by destructive lesions or tumors is usually accompanied by multiple other pituitary hormone deficiencies. These deficiencies can include GHD (also contributing to short stature), gonadotropin deficiency (causing delayed or absent puberty), adrenocorticotropic hormone (ACTH) deficiency (causing potentially life-threatening glucocorticoid deficiency), and antidiuretic hormone (ADH; vasopressin) deficiency (producing diabetes insipidus). If the pituitary is intact, but the pituitary stalk has been damaged or transected, prolactin levels may be elevated. Prolactin secretion by the anterior pituitary is normally under the suppressive influence of dopamine [a.k.a.—“prolactin release inhibiting hormone” (PRIH)] that is secreted by the hypothalamus. There are a number of rare genetic causes of hypopituitarism that have been described that cause TSH deficiency (see below).
Growth hormone deficiency
Once hypothyroidism has been ruled out, the possibility of GHD must be explored. An older name for GH is “somatotropin.” GHD in children is manifested as short stature with a low growth velocity. Other findings in GH-deficient children include increased adipose tissue, immature facial appearance, and possible hypoglycemia. Recall that GH is one of the four antiinsulin hormones that include cortisol, epinephrine, and glucagon.
In adults, GHD is evidenced by decreased lean body mass, increased fat mass, decreased strength, decreased bone mineral density, increased cholesterol levels, premature coronary artery disease, and decreased quality of life (e.g., reduced “enjoyment of life”) . There are studies reporting improved quality of life in adults treated with GH . Despite many benefits of the treatment of adult GHD, evidence of improved cardiovascular outcomes and reduced mortality are lacking .
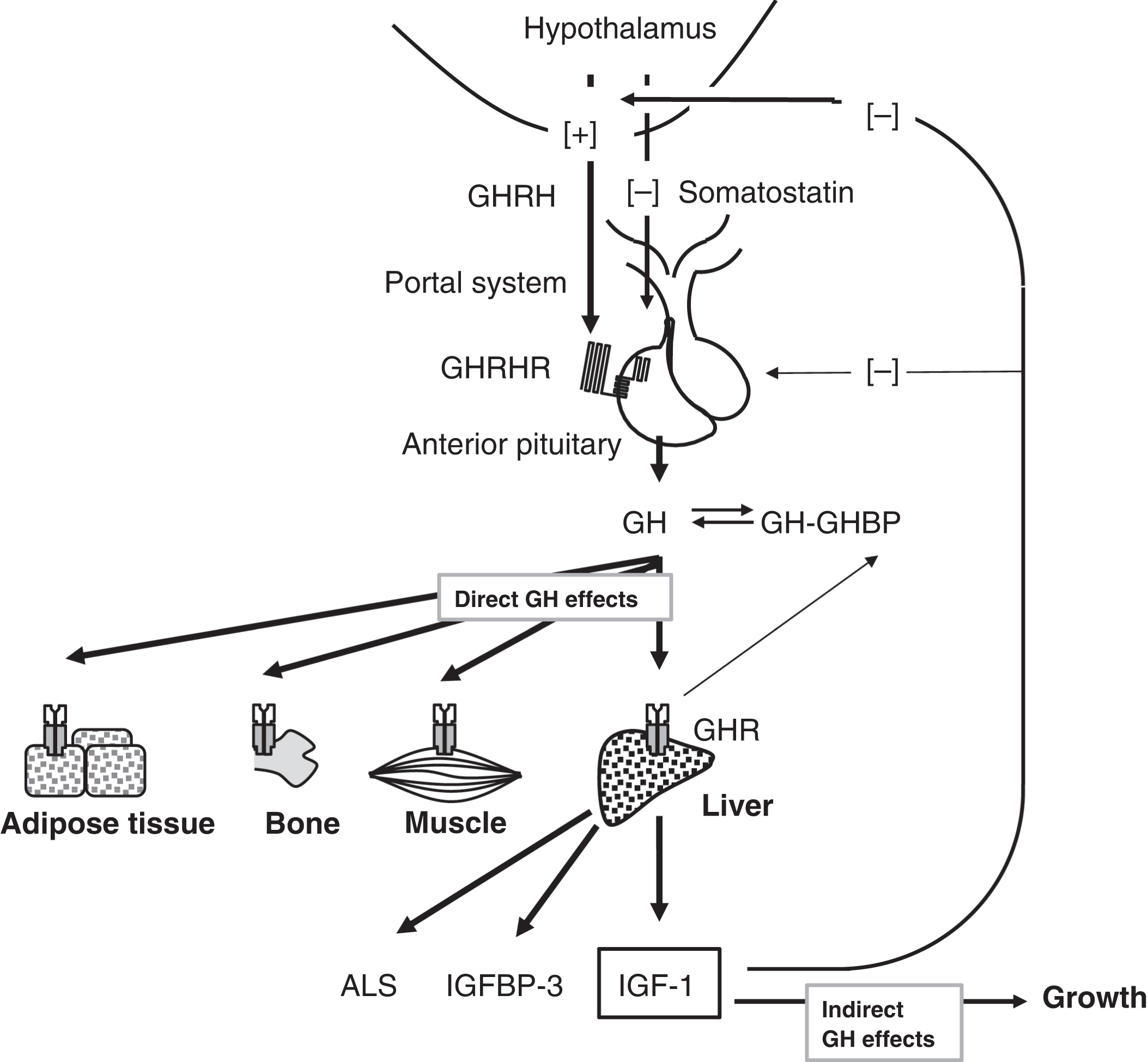
The hypothalamus controls pituitary GH secretion. GH is the only anterior pituitary hormone for which the hypothalamus secretes a stimulatory hormone [GH-releasing hormone (GHRH)] and a suppressive hormone [somatotropin release–inhibiting hormone (SRIH; somatostatin)] ( Fig. 14.5 ). Somatostatin illustrates the principle that a variety of CNS hormones are also found in the gastrointestinal tract. Somatostatin is produced in the δ cells of the pancreatic islets and suppresses both insulin and glucagon secretion. GHRH binds to its receptor on pituitary somatotrophs, activates a G s system, increases cyclic AMP production, and releases GH .
The native form of GH contains 191 amino acids with a predicted molecular mass of 22,125 Da. GH has two intramolecular disulfide bridges. In addition to the complete 22-kDa GH form representing 85%–90% of circulating GH, 5%–10% of circulating GH is 20 kDa in mass and lacks amino acids 32–46. This minor form of GH results from alternative splicing of the GH mRNA transcript. In the circulation, GH can exist as aggregates and oligomers . Big GH is a dimer of GH monomers, whereas big-big GH represents GH plus its binding protein (GHBP).
GHBP is the extracellular portion of the GH receptor (GHR) . GHBP is produced when the extracellular portion of the GHR is cleaved by what is now known as ADAM metallopeptidase domain 17 (ADAM17; chromosome 2p25.1) . “ADAM” stands for “a disintegrin and metalloproteinase.”
To manifest its biological activities, GH binds to tissue GHRs. The 620-amino acid GHR includes a 246-amino acid extracellular domain, a 24-amino acid transmembrane domain, and a 350-amino acid cytoplasmic domain . In its basal state, GHRs exist as cell-surface dimers and bind to Janus-associated kinase (JAK) 2 (a type of adaptor tyrosine kinase) molecules ( Fig. 14.6 ). With GH attachment to the GHR, the conformation of the GHR is perturbed, and JAK2 achieves tyrosine kinase activity. JAK2 undergoes autophosphorylation, and JAK2 also phosphorylates the GHR.
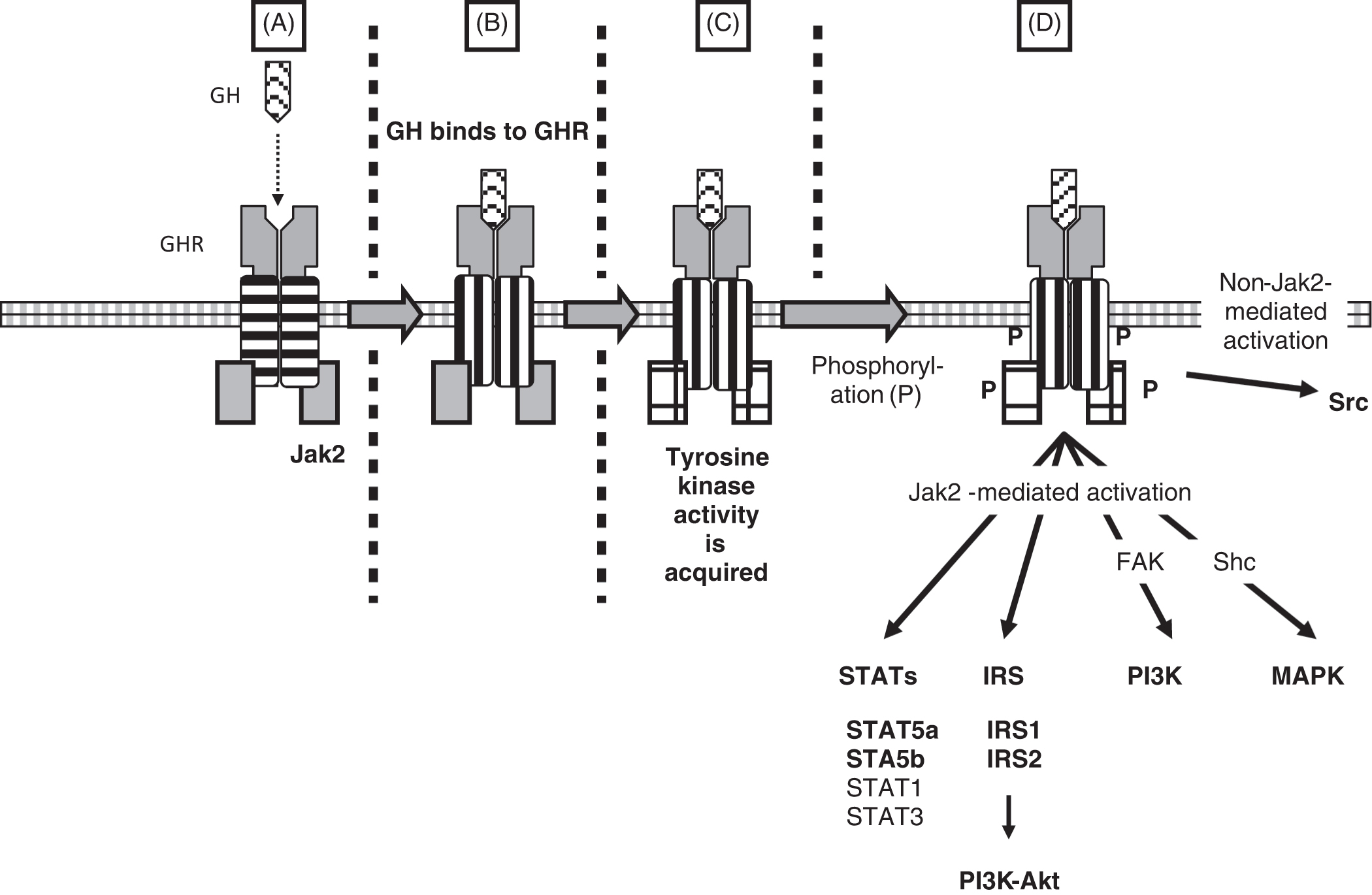
JAK2 activates several intracellular pathways via substrate phosphorylation. These pathways include signal transducers and activators of transcription (STATs), insulin receptor substrate (IRS-1 and IRS-2), phosphatidylinositol 3-kinase [PI3K via FAK [a.k.a. PTK2 (protein tyrosine kinase 2); chromosome 8q24.3], and mitogen-activated protein kinase (MAPK via Shc). The most important STATs are STAT5a and STAT5b. Indeed, a mutation in STAT5b has been reported to cause short stature . FAK is also known as PTK2 (protein tyrosine kinase 2; chromosome 8q24.3). Shc1 is SHC adaptor protein 1 (chromosome 1q21.3). Intracellular signaling exclusive of JAK2 may occur via the tyrosine kinase Src (SRC proto-oncogene, nonreceptor tyrosine kinase; Src is the Rous sarcoma virus proto-oncogene; chromosome 20q11.23).
The effects of GH are both direct and indirect ( Fig. 14.5 ). The direct actions of GH are to (1) reduce insulin sensitivity, (2) raise free fatty acid (FFA) concentrations, and (3) stimulate production of (1) IGF-I, (2) the 28.5-kDa IGF-binding protein (IGFBP)-3 (the major plasma binding protein for IGF-I), and (3) the 88-kDa acid-labile subunit (ALS). The indirect effects of GH are growth stimulation mediated by IGF-I. An older name for IGF-I is somatomedin-C. Deficiency of ALS has been reported .
Insulin resistance induced by GH decreases glucose utilization to raise plasma glucose concentrations. Elevated FFAs, by providing an alternative fuel source to glucose, spare glucose from peripheral utilization allowing plasma glucose levels to rise. An occasional consequence of GHD is hypoglycemia, especially in newborns and young infants .
As noted above, the growth-promoting effects of GH are direct, as well as indirect via IGF-I action on tissues. Target cells for GH are essentially all cells in the body because of the widespread expression of the GHR. Although the circulating IGF-I levels reflect predominantly hepatic IGF-I secretion in response to GH, it is the local GH actions and subsequent local IGF-I production and secretion acting in paracrine and autocrine fashions that stimulates somatic growth. In GHD children, GH treatment produces more growth than IGF-I treatment alone. In addition to its effects on growth, when pathologically elevated in concentration, IGF-I can lower blood glucose concentrations causing hypoglycemia .
Growth hormone testing
Randomly obtained measurements of GH are frequently low (e.g., ~2–3 ng/mL) and do not necessarily indicate GHD. Normally, children have seven to eight pulses of GH secretion per day, most of which occur during the early hours of sleep. Sampling during sleep requires hospitalization for insertion of an indwelling intravenous catheter and multiple measurements of GH (e.g., approximately every 20 min), which will cost more than performing a standardized outpatient GH stimulation test. Indeed, GH is best measured after stimulation following an overnight fast as will be described below. Serial blood sampling to observe spontaneous elevations in GH is not recommended diagnostically by the 2016 Pediatric Endocrine Society guidelines. Random measurements of GH in search of GHD are definitely not recommended.
To exclude GHD, peak GH concentrations after stimulation should exceed 7–10 ng/mL depending upon the GH assay employed (see below). If such peak levels are not achieved with adequate stimulation, GH is deficient.
Many pediatric endocrinologists perform an exercise tolerance test as an initial screen for GHD. Children are vigorously exercised for ~20 min (e.g., by having the child run up and down stairs), and then a GH level is drawn. Besides the +20-min sample, some endocrinologists will draw a +40-min specimen. Alternatively, clonidine or l -dopa are administered, and a GH level is subsequently obtained. If clonidine is used, GH is measured 90 min later.
Administration of low-dose sex hormones (testosterone or estrogens in boys or estrogen in girls) and/or propranolol prior to screening and definitive testing decreases the number of falsely abnormal (false-positive) results. However, sex steroid or propranolol priming is not universally endorsed . The Pediatric Endocrine Society recommends the use of β-estradiol or low-dose testosterone ( Table 14.2 ).
| Agent | Sex of patient | Dose(s) |
|---|---|---|
| β-Estradiol | Male or female | Body weight <20 kg: 1 mg each evening x2 prior to testing. |
| Body weight=>20 kg: 2 mg each evening x2 prior to testing. | ||
| Testosterone | Male | 50–100 mg intramuscular 1 week before testing. |
Another approach to screening for GHD is the measurement of IGF-I and/or IGFBP-3. Their concentrations are stable during the day and therefore random measurements can be obtained. GH is not the only regulator of IGF-I and IGFBP-3 concentrations. As noted elsewhere in this chapter, decreased IGF-I can result from hypothyroidism, chronic disease, chronic inflammation, malnutrition, or sex steroid deficiency. Therefore a low IGF-I concentration is not synonymous with GHD.
If the result of the initial GH screening (either exercise or pharmacologic GH stimulation, or IGF-I and/or IGFBP-3 measurements) is normal, no further testing is required. However, if the screening GH concentration is <7–10 ng/mL (depending upon the GH level that the laboratory and clinician have defined as abnormal), formal (a.k.a. “definitive” or “provocative”) GH testing should be pursued. During formal GH testing, multiple GH levels are measured following the administration of a stimulus, requiring an indwelling catheter to avoid multiple venipunctures.
Two GH-stimulatory tests should be performed when provocative GH testing is carried out. Alternatively, the Growth Hormone Research Society states that a single stimulation test is adequate if the child has (1) recognized CNS pathology, (2) a history of CNS irradiation, (3) multiple pituitary hormone deficiencies, or (4) a genetic defect . Of the general population of children, 95% will show a normal response to at least one of the two stimuli. However, only 80% of the general population will display a normal GH response to a single stimulus; therefore there is the need to provide two stimuli that can be administered on the same day sequentially.
The various stimulants include insulin-induced hypoglycemia, arginine infusion, or administration of stimulatory drugs such as glucagon, l -dopa, or clonidine ( Table 14.3 ). Combinations of stimulatory tests are usually administered. Arginine HCl can be infused intravenously over 30 min (0.5 g/kg to a maximum of 30 g) followed 30 min later by bolus insulin injection (~0.1 unit/kg) to induce acute hypoglycemia [the arginine-insulin tolerance test (AITT)]. Care must be taken during testing; for example, arginine overdose can produce fatal acidosis . If the subject is insulin resistant, a higher dose of IV insulin should be administered (e.g., 0.15 units/kg).