Cause
Common causes in older adults:
Hypertension
Diabetes mellitus
Renal vascular disease
Chronic urinary obstruction
Systemic vasculitis
Multiple myeloma
Glomerulonephritis
Nephrotic syndrome
Multifactorial etiology (e.g., renal vascular disease with chronic urinary obstruction)
GFR | |
|---|---|
Category | eGFR, ml/min/1.73 m2 |
G1 | ≥90 |
G2 | 60–89 |
G3a | 45–59 |
G3b | 30–44 |
G4 | 15–29 |
G5 | <15 |
Albuminuria | |
|---|---|
Category | ACR, mg/g |
A1 | <30 |
A2 | 30–300 |
A3 | >300 |
As CKD progresses , patients may develop kidney failure defined as an eGFR <15 ml/min/1.73 m2 or the need to initiate renal replacement therapy (RRT; hemodialysis or peritoneal dialysis) or kidney transplant [10]. End-stage renal disease (ESRD) is a related administrative term based on the payment for health care by the Medicare ESRD Program . ESRD is used to identify those receiving RRT or who have received a kidney transplant, regardless of eGFR level [11]. In Sect. 25.7 below, we describe the treatment of advanced kidney disease in older populations including dialysis, kidney transplant, and conservative management.
In contrast to CKD and kidney failure, which are considered chronic conditions, AKI is a sudden worsening in kidney function. The term AKI has replaced the diagnosis of acute renal failure to reflect that even small changes in kidney function may impact long-term kidney function and to emphasize the broad spectrum of kidney injury [12]. Current classification of AKI includes three stages based on both serum creatinine and urine output (UOP) (Table 25.2) [13]. In Sect. 25.5 below, we describe risk factors that predispose older adults to AKI and the impact of AKI on CKD progression.
Table 25.2
Stages for acute kidney injury based on increase in serum creatinine from baseline or level of urine output (UOP)
Stage | Serum creatinine increase from baseline | UOP |
|---|---|---|
1 | 1.5 to 1.9-fold, or Increase ≥0.3 mg/dL | <0.5 mL/kg per hour for at least 6 h |
2 | 2 to 2.9-fold | <0.5 mL/kg per hour for at least 12 h |
3 | 3-fold or greater, or Increase to ≥4.0 mg/dL | <0.3 mL/kg per hour for 24 h, or No UOP (anuria) for at least 12 h |
25.3.2 Burden of Kidney Disease Among Older Adults
The overall prevalence of CKD has been reported to be 13.1 % in the adult US population. However, the prevalence of kidney disease increases markedly with age [1]. Nearly half of those with CKD are 70 years of age or older, and there is a graded increase in the prevalence of CKD at older ages. Among US adults, the prevalence of CKD , defined as an eGFR <60 ml/min/1.73 m2 was reported to be 0.9, 7.5, 26.5, and 51.1 % among those aged <60, 60–69, 70–79, and ≥80 years old. A similar, but less dramatic, increase in the prevalence of albuminuria, defined as an ACR >30 mg/g, of 6.8, 14.2, 21.3, and 32.7 % at ages 60–69, 70–79 and ≥80 years, respectively, has been reported.
An increase in the prevalence of CKD over the past 2 decades has also been reported in the general US population, especially among older adults [14, 15]. For example, the prevalence of decreased eGFR (<60 ml/min/1.73 m2) in the US population ≥80 years was examined during three time periods: 1988–1994, 1999–2004, and 2005–2010. The prevalence of decreased eGFR was 40.5, 49.9, and 51.2 % during these time periods. A disproportionate increase in the prevalence of more severe CKD (eGFR <45 ml/min/1.73 m2) was found from 14.3 % to 18.6 % and 21.7 % in 1988–1994, 1999–2004, and 2005–2010, respectively. These findings were not completely explained by an increase in the prevalence of diabetes and hypertension in the older population during this time. Assuming that the prevalence of CKD remains stable in this age group, with the aging of the US population, the number of US adults ≥80 years old with eGFR <60 ml/min/1.73 m2 is estimated to increase from 4.6 million in 2005–2010 to 9.9 million and 15.8 million in 2030 and 2050, respectively (Fig. 25.1) [15].
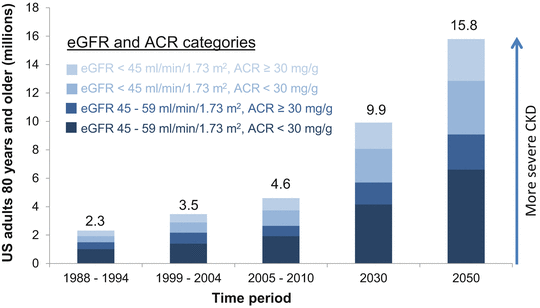

Fig. 25.1
The number of US adults ≥80 years old with CKD has doubled in the past 2 decades and will continue to increase with the aging of the populations . There has been a disproportionate increase in the prevalence of more severe CKD. eGFR estimated glomerular filtration rate, ACR albumin-to-creatinine ratio
While the prevalence of CKD defined as an eGFR <60 ml/min/1.73 m2 is highest at older age, older adults are much less likely to progress to kidney failure. The very old with CKD may be 10–20 times more likely to die before progressing to kidney failure. The competing risk of death has been examined by determining at what eGFR level is the risk of requiring RRT greater than the risk of death for different age groups. For example, among younger adults the risk of kidney failure requiring RRT is greater than the risk of death at an eGFR level of 45 ml/min/1.73 m2 and below [3]. For adults 65–84 years old, the risk of kidney failure requiring RRT is only greater than the risk of death at an eGFR of 15 ml/min/1.73 m2 and below. For those 85 years and older, the risk of death has been shown to exceeded the risk of kidney failure requiring RRT at any eGFR level.
In addition to the competing risk of death before reaching kidney failure, there are other possible explanations for the age difference in risk of kidney failure including a slower decline in kidney function among older adults. Additionally older adults may be less like to be offered or chose treatment with dialysis or transplantation in the face of kidney failure. For example, when kidney failure is categorized as treated (eGFR <15 ml/min/1.73 m2 and dialysis or kidney transplant) or untreated (eGFR <15 ml/min/1.73 m2, but no dialysis or kidney transplant), overall kidney failure is more common at older ages. However, at younger ages, treated kidney failure is more common than untreated kidney failure [16]. At older ages untreated kidney failure is much more common.
Although only a small proportion of older adults with CKD progress to kidney failure and receive RRT, the absolute number of older adults with ESRD (i.e., requiring RRT or kidney transplant regardless of eGFR) has increased over the past 20 years. Through 2010, the fastest growing group with ESRD was those 70 years and older [17]. Several factors may be contributing to the increased incidence of ESRD among older adults. This may be due in part to the increase in CKD prevalence among older adults, the aging US population, as well as an increase in the use of dialysis among older adults.
A similar pattern of graded increase in the incidence of AKI at older ages has been shown. Among hospitalized adults, the incidence of AKI among those 85 years and older is approximately 40 cases compared to 20 cases per 1000 discharges among those <65 years old [12]. The incidence of AKIs has been reported to have increased over the last 2 decades and has been explained by an increase in AKI risk factors, the aging population, as well as improvements in recognition of AKI.
25.4 Disease-Oriented Versus Patient-Centered Approach
25.4.1 Disease-Oriented Approach
The disease-oriented model of care is the prevailing clinical paradigm for the diagnosis and treatment of chronic conditions . This approach emphasizes the prevention, diagnosis, and treatment of individual disease processes [2, 18]. In the disease-oriented approach a direct causal relationship between clinical signs and symptoms and specific disease pathology is assumed. Treatments target the underlying pathophysiology and symptoms are thought to be best treated by interventions that impact the disease course, rather than as a target for intervention themselves. Treatment priorities are often determined by the availability of clinical trial evidence.
There are several strengths to this approach when applied to individual chronic conditions. The development and dissemination of CKD clinical practice guidelines have standardized CKD terminology and improved recognition and treatment of CKD. The disease-oriented approach provides a systemic framework for evidence-based management . Additionally, this approach is well suited for applying quality performance measurement and outcome tracking. Below, we describe the disease-oriented approach to CKD. Next, we describe limitations when applied specifically to older adults with CKD.
Existing CKD practice guidelines follow the disease-oriented model that assumes a direct and linear relationship between underlying kidney pathology with CKD progression, the development of concurrent CKD complications, kidney failure and ultimately death from CKD. Management strategies target the underlying risk factors for CKD and disease-specific biomarkers are used to track the progression of CKD. Clinical trials to prevent CKD progression are considered to provide the highest quality of evidence and are used to make recommendation for CKD treatment. Outcomes of interest are specific to CKD (e.g., kidney failure, mortality).
There are three main categories for CKD management : (1) slowing the progression of CKD to prevent kidney failure, (2) recognition and treatment of concurrent CKD complications, and (3) preparation for RRT [10]. Slowing the progression of CKD is considered a key goal. Approaches to slowing the progression include blood pressure (BP) control for all patients with CKD. For those with albuminuria, renin-angiotensin-aldosterone system (RAAS) interruption with angiotensin-converting enzyme inhibitors (ACE-Is ) or angiotensin receptor blockers (ARBs ) are recommended. Currently recommended BP goals for CKD patients are ≤140/90 for those with an ACR <30 mg/g and ≤130/80 for those with diabetes or ACR ≥30 mg/g. However, these recommendations are subject to change given findings from a recent clinical trial showing better outcomes among older adults who acheive lower BP targets. [19]. Guidelines also provide recommendations for protein intake, glycemic control, salt intake, and physical activity to prevent CKD progression.
The second category for CKD management is the recognition and treatment of concurrent CKD complications including anemia, metabolic bone disease, acidosis, and cardiovascular disease. Guidelines provide specific drug and lifestyle recommendations to manage these complications. In CKD, anemia is related to reduced erythropoietin and defined as <13.0 g/dL for men and <12.0 g/dL for women. Guidelines encourage evaluation for other causes of anemia and when erythropoietin stimulation agents are used, increasing hemoglobin concentrations to levels above 11.5 g/dL should be avoided. CKD metabolic bone disease includes abnormalities of calcium, phosphate, and parathyroid hormone (PTH) and is associated with increased risk of fractures. Current recommendations include dietary phosphate restriction or using oral binder to maintain serum phosphate within the normal range. Targets for treatment of hyperparathyroidism are more controversial. While clinical trials provide evidence that treatment to reduce PTH improves biomarkers of metabolic bone disease, the impact of these intermediate outcomes on clinically important outcomes such as fractures is limited. Guidelines also recommend treatment with oral bicarbonate supplementation for patients with serum bicarbonate levels <22 mmol/L with the goal to maintain bicarbonate within the normal range.
Lastly, guidelines provide recommendations for referral to nephrologists and preparation and time of RRT . Referral to nephrology is recommended, even if dialysis or transplantation is not a consideration in the presence of: AKI, eGFR <30 ml/min/1.73 m2, significant albuminuria (ACR >300 mg/g), progression of CKD, urinary red cell casts, hypertension refractory to treatment with four medications, persistent elevated serum potassium, recurrent nephrolithiasis, and hereditary kidney disease. Planning for RRT is based on the risk for progression to kidney failure. Recent studies have shown that the trajectory of CKD progression is often nonlinear and difficult to predict for older adults. Timing of RRT initiation is determined by the presence of kidney failure symptoms including serositis, acid–base or electrolyte abnormalities, pruritis, inability to control volume status or BP, progressive deterioration in nutritional status or cognitive impairment due to uremia. Recent studies have shown a trend towards initiation of RRT at higher levels of eGFR; however, evidence suggests no benefit or an increased risk for mortality among those with earlier initiation of dialysis in the course of CKD progression.
25.4.1.1 Limitations of the Disease-Oriented Approach
Despite the acceptance of the disease-oriented approach, there are several limitations of this approach when applied to older adults. Here, we describe four characteristics of older populations that may limit the relevance of the disease-oriented approach to CKD management [2]. These include (1) limited life expectancy, (2) a high burden of multimorbidity, and (3) heterogeneity in health goals and treatment preferences, and (4) exclusion from clinical trials.
Limited life expectancy is a key factor to consider for any disease-specific treatment plan for older adults. Both patients and providers recognize that there is a reduction in the years remaining in life expectancy at older ages and this has been shown in CKD. For example, a 70-year-old man with an eGFR 30–44 ml/min/1.73 m2 and ≥2+ dipstick proteinuria may expect on average to live 5 more years. In contrast, an 85-year-old with the same level of kidney function may live on average 2.6 additional years [20]. However, reports of average survival do not capture the remarkable heterogeneity in life expectancy and complexity estimating survival in older adults. One approach to determine the heterogeneity in life expectancy is to calculate not only the median survival, but also the interquartile range (IQR ) for survival defined as the 25th percentile to 75th percentile. The IQR for survival is 2.3–8.6 years for the 70-year-old man described above and 1.2–4.5 years for the 85 year old. This means that the highest 75th percentile of surviving 85 year olds may expect to live 4.5 year or longer. This suggests that many 85 year olds will live as long as or longer than the average 70 year old. Similar findings have been shown among older ESRD patients. The median survival for an 80-year-old incident ESRD patient is 1.3 years, however the interquartile range is 5 months to 3 years. Therefore, applying uniform recommendations to all older adults, some of whom may expect to live many more years and benefit from preventive treatments and others who are nearing end of life, is not appropriate.
Among older adults, CKD almost universally occurs in individuals with other chronic medical conditions. While multimorbidity , defined as the presence of two or more chronic conditions is common among older adults with CKD, existing clinical practice guidelines follow a “single disease” framework and do not account for the presence of other chronic conditions. As described above, the disease-oriented approach relies on CKD biomarkers (i.e., eGFR, ACR) to guide treatment decisions and focuses on preventing CKD-related outcomes. However, for older adults with multimorbidity, the application of multiple “single-disease” guidelines may lead to treatment recommendations that are complex and often contradictory or of limited benefit.
In addition to having multiple chronic conditions such as hypertension and diabetes, older adults with CKD have been shown to be at risk for co-occurring geriatric conditions. In the CKD population, the risk for mortality, hospitalizations, and emergency department (ED) visits increases at higher number of these problems. For example, among older adults with eGFR <60 more than two-thirds have 2 or more of 6 geriatric conditions (cognitive impairment, depressive symptoms, exhaustion, impaired mobility, falls, and polypharmacy) [21]. Compared to those with none of these problems, those with three or more experience twice the risk of dying, being hospitalized or requiring an ED visit. This “geriatric” multimorbidity is not considered in the disease-oriented clinical practice guidelines that only focus on CKD.
A third characteristic of older populations that may limit the relevance of the disease-oriented approach is heterogeneity in health goals and treatment preferences reported by older adults [2]. While CKD clinical practice guidelines prioritize the reduction of mortality and prevention of CKD related outcomes such as kidney failure, older adults often frame their health goals in terms of their overall health and maintaining functional independence. Universal health outcomes such as quality of life and functional independence may be viewed as more important than disease-specific outcomes. While a shift in health goals and preferences has been shown among older adults, it is important to recognize the variability in goals and preferences between older adults. The narrow focus on outcomes that are defined by the underlying disease pathology in the disease-oriented model often fails to address what is most important to an individual patient. Disease-oriented clinical practice guidelines lack the flexibility to allow providers to adapt the goals and treatment plans to the individual patient’s needs.
Lastly, older adults with complex multimorbidity or limited life expectancy are often excluded from clinical trials . This is often done because the magnitude of treatment effects for a given intervention is often larger in homogenous populations (i.e., smaller variability results in larger treatment effect) [18]. Exclusion of older adults limits the generalizability of individual studies to older adults and the clinical practice guidelines that generate recommendations based on these studies. For example, most of the trials underpinning the guideline recommendations for the use of ACE-Is and ARBs have been conducted in high risk populations and did not enroll participants older than 70. Because ACE-Is and ARBs may be most effective in those at highest risk for progression (e.g., among those with albuminuria), findings from these studies of a number needed to treat (NNT ) to prevent one case of ESRD ranging from 9 to 25 may not be generalizable to older adults. In fact, one recent simulation study using a real-world cohort of older adults with CKD showed large differences in the NNT based on the estimated baseline risk for ESRD. For older adults with the lowest risk of ESRD, they reported an NNT to prevent one case of ESRD to be 2500 [22].
25.4.2 Individualized, Patient-Centered Approach
There is an increasing awareness that a “one size fits all” approach to CKD management may not be appropriate. For example, the most recent CKD guidelines have added suggestions to tailor BP targets. However, approaches for how to individualize goals are not provided. Given the limitations of disease-oriented models of care in older populations, geriatricians often favor a more individualized patient-centered approach. The patient-centered approach embraces the complexity and acknowledges the importance of patient health goals and preferences for developing treatment plans. The patient-centered approach recognizes that existing evidence may not be relevant for individual patients. Symptoms are considered important targets for intervention, regardless of the underlying cause.
One approach to implementing a patient-centered approach to CKD is to include geriatric assessment as part of the clinical evaluation of CKD patients. Routine geriatric assessment could be used to identify contextual information (e.g., cognitive impairment, poor social support, markers of frailty, and limited life expectancy) to guide clinical care. It has been suggested that the recognition of geriatric conditions including functional impairment, frailty, mobility impairment, cognitive impairment, and depressive symptoms could be used to signal for the provider to consider a transition from the traditional disease-oriented approach to CKD care to a more individualized, patient-centered approach. For example, recognition of mild cognitive impairment and low social support may be used to tailor management goals such as glucose control in a patient with CKD and diabetes to reduce the risk for hypoglycemia. Recognition of these problems may also facilitate a shared decision-making approach to discussions about RRT. In these discussions, providers can address prognostic markers associated with poor survival on dialysis (e.g., non-ambulatory status, frailty) to help patients make an informed decision regarding dialysis versus conservative management. Eliciting goals of both the individual patient and family and caregivers can be used to prioritize outcomes beyond those reported in the CKD guidelines. In this approach, the CKD-specific diagnosis and management is not abandoned completely and may be incorporated into individualized treatment plans, depending on the extent to which disease-based recommendations are aligned with the preferences and goals of the patient. In Table 25.3, we highlight several components of geriatric assessment, their implications for CKD, and how these might be used to facilitate a patient-centered approach to CKD management.
Table 25.3
Geriatric assessmenta facilitates individualized, patient-centered approach to the management of CKD in older adults
Assessment | Relevance to CKD | Examples of how geriatric assessment facilitates a patient-centered approach |
|---|---|---|
Functional status | Functional impairment increases at lower levels of kidney function. At dialysis initiation 50 % of older adults are dependent in ADLs | Use a shared decision-making approach that considers prognosis Anticipate and plan for increased functional assistance after dialysis initiation |
Cognition | The prevalence and incidence of cognitive impairment increases at lower eGFR. Cognitive impairment is common among older adults with kidney failure | Simplify CKD self-management tasks
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|