Chapter 59 Kaposi Sarcoma
Kaposi sarcoma (KS) was one of the first opportunistic diseases described in association with the acquired immune deficiency syndrome (AIDS).1 Despite a decline in its incidence, which began before the introduction of potent antiretroviral regimens for human immunodeficiency virus-1 (HIV-1) but which has accelerated since their widespread introduction in 1996,2 KS continues to be the most frequently diagnosed AIDS-associated neoplasm and a cause of significant morbidity and occasional mortality in HIV-infected patients. The rapid decline in KS incidence that followed the introduction of potent antiretroviral regimens for HIV and the observed regression of established KS lesions after their use may be a consequence of a decrease in the production of KS-stimulatory cytokines and viral proteins associated with poorly controlled HIV infection. Alternatively or in addition, effective antiretroviral therapy might permit the development of an effective immune response against human herpesvirus 8 (HHV-8), also known as the KS-associated herpesvirus (KSHV), the virus required for development of KS. The emergence of drug-resistant HIV strains and intolerance or lack of adherence to effective antiviral regimens make it difficult to know whether the decreased incidence of KS in developed countries will continue. In addition, the incidence of KS remains high in parts of the world where effective antiretroviral therapy is not widely available and where the incidence of HIV and HHV-8 co-infection is high.3
KS most commonly presents with lesions on the skin that may be widely disseminated from the outset, although other sites may be initially involved. Although the course of KS is quite variable, in some patients KS not only disseminates in the skin but also involves the oral cavity and visceral organs, especially the lungs and gastrointestinal (GI) tract, and it is often complicated by lymphedema of the lower extremities, and, less commonly the face, trunk, upper extremities, and genitalia. Depending on its location and severity, KS can cause serious functional disability. KS lesions of the feet may be painful and limit mobility. Oral KS may cause difficulty eating and speaking. Edema may be associated with ulceration, infection, pain, and reduced mobility. GI KS may be asymptomatic but sometimes causes bleeding, pain, and obstruction. Pulmonary KS can cause respiratory insufficiency; and untreated pulmonary KS was associated in one study with a median survival of only 2.1 months.4 Even in the absence of symptomatic visceral disease or edema, KS often impairs the quality of life when it causes disfigurement, leads to social isolation, or serves as a visual reminder of an AIDS diagnosis.
Several treatment options are available for KS, and the choice is dictated by the extent of disease, the rate of disease progression, and the presence and severity of symptoms affecting function and quality of life. The choice of treatment may also be influenced by the severity of the underlying HIV infection and the presence of co-morbid opportunistic complications of HIV infection. KS usually presents multifocally, without a defined ‘primary’ lesion, so staging according to a standard T (umor)/N (ode)/M (etastasis) classification is not appropriate. In addition to tumor extent, immune status and the presence of systemic manifestations of HIV infection are relevant to the prognosis of HIV-infected patients with KS. The most commonly applied staging classification for KS, which takes these factors into account, is the TIS system proposed in 1989 by the AIDS Clinical Trials Group (ACTG) Oncology Committee5 (Table 59-1). This staging system divides patients into good-risk or poor-risk groups according to extent of tumor, immune system status measured by CD4+ T-lymphocyte count, and symptoms of systemic HIV-associated illness. An analysis of 294 consecutive patients entered into eight ACTG KS therapy trials between 1989 and 1995 showed that each of the TIS variables was significantly associated with survival.6 In a multivariate analysis, the level of CD4+ T lymphocytes and tumor extent were the only significant factors predicting survival.6 In this analysis, a CD4 T-lymphocyte count of 150/μL provided better discrimination between good- and poor-risk groups than the originally recommended cut-off point of 200 cells/μL. A more recent multivariate analysis of prognostic factors conducted after the introduction of highly active antiretroviral therapy (HAART) identified extent of KS and systemic HIV-associated symptoms as the most important predictors of survival, and established pulmonary KS as a particularly poor prognostic feature.7 Little information exists, however, about the factors predicting response to KS therapy or if TIS staging is a useful guide to the choice of therapy. Studies are underway to determine whether either the HIV-1 viral RNA level or the HHV-8 DNA level adds to the predictive value of TIS staging with respect to survival or response to treatment.
Table 59-1 AIDS-Associated Kaposi Sarcoma Staging and Prognosis
| Validation Studies | ||
|---|---|---|
| ACTG Prognostic Categoriesa | Pre-HAARTb | Post-HAARTc |
| Univariate Analyses | ||
| T: Tumor extent | Median survival: | 3-Year survival: |
| T0: Confined to skin and/or lymph nodes minimal (non-nodular) oral cavity lesions | T0: 27 mos | T0: 85% |
| P < 0.001 | P = 0.007 | |
| T1: 15 mos | T1: 69% | |
| T1: Extensive oral KS and/or symptomatic tumor-associated edema or ulceration and/or non-nodal visceral KS | I0: 40 mos | I0: 83% |
| p < 0.001 | P = 0.06 | |
| I1: 13 mos | I1: 71% | |
| I: Immune status | S0: 22 mos | S0: 83% |
| I0: CD4 count ≥200/μL | P = 0.04 | P = 0.003 |
| I1: CD4 count <200/μL | S1: 16 mos | S1: 63% |
| Multivariate Analyses | ||
| S: HIV-related systemic illness | T0I0: Not reachedd | T0S0: 88% |
| S0: KPSe ≥70; no B symptoms;f no AIDS-defining illnesses or thrush | T0I0: 35 mos | T1S0: 80% |
| P < 0.001 | P = 0.0001 | |
| TanyI1: 13 mos | T0S1: 81% | |
| S1: KPS < 70; B symptoms; any AIDS-defining illness or thrush | T1S1: 53% | |
a Adapted from Krown SE, Metroka C, Wernz JC, Kaposi’s sarcoma, in the acquired immune deficiency syndrome: a proposal for uniform evaluation, response and staging criteria. J Clin Oncol 7: 1201, 1989.
b Adapted from Krown WE, Testa MA, Huang J. AIDS-related Kaposi sarcoma: propective validation of the AIDS clinical trial group staging classification. J Clin Oncol 15: 3085, 1997.
c Adapted from Nasti G, Talamini R, Antinori A, et al. AIDS-related Kaposi sarcoma evaluation of potential new prognostic factors and assessment of the AIDS clinical trial group staging system in the HAART era – the Italian Cooperative Group on AIDS and Tumors and the Italian Cohort of Patients Naive From Antiretrovirals. J Clin Oncol 21: 2876, 2003.
d For this analysis, I0 was defined as a CD4 lymphocyte count of ≥150 cells/μL and I1 was defined as a CD4 lymphocyte count of 150 cells/μL, which gave better discrimination between the good- and poor-risk groups than the previous cut-off point of 200 cells/μL.
e KPS, Karnofsky performance status.
f B symptoms are unexplained night sweats or fever, > 10% unexplained weight loss, or unexplained diarrhea lasting 2 weeks or more.
DIAGNOSIS
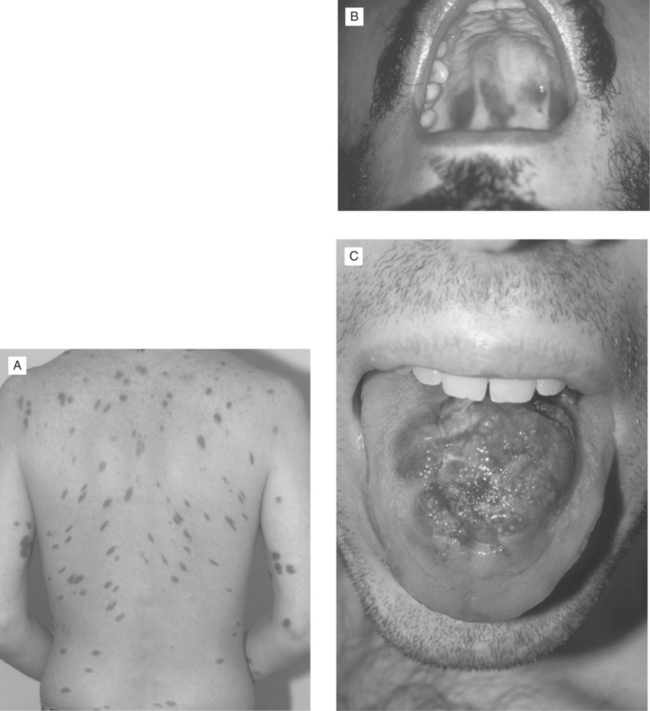
Although a presumptive diagnosis of KS may be based on visual identification of typical red or violaceous skin or oral lesions in a patient at risk (Fig. 59-1), biopsy of at least one lesion is important for establishing the diagnosis and distinguishing KS from other pigmented skin lesions such as bacillary angiomatosis.8 A diagnosis of GI or pulmonary KS may be more difficult to establish. KS may occur throughout the GI tract and sometimes occurs in the absence of cutaneous or oral disease.9 Although estimates of the frequency of GI KS have ranged from 40% at the time of KS diagnosis to 80% in autopsy series,10,11 it is frequently asymptomatic, and there is no evidence that the presence of asymptomatic GI lesions adversely affects prognosis or response to treatment. Therefore, it is currently recommended that patients be evaluated for the presence of GI KS only when symptoms (e.g., pain, occult or gross bleeding, dysphagia, obstructive signs) are present. Endoscopy is generally required for diagnosis, as KS lesions are often submucosal and not easily visualized on contrast radiographs or scans, which are nondiagnostic. Upper or lower GI endoscopy (or both) permits direct visualization of the red lesions in the esophagus, stomach, duodenum, or colon; digital rectal examination often discloses lesions in the anorectal area. Although endoscopic biopsy of typical KS lesions may confirm the diagnosis pathologically, superficial biopsies sometimes yield only normal mucosa, especially when the lesions are not ulcerated. In the presence of pathologically confirmed KS elsewhere in the body, however, the visual identification of typical lesions in the GI tract can be considered presumptive evidence of GI KS.
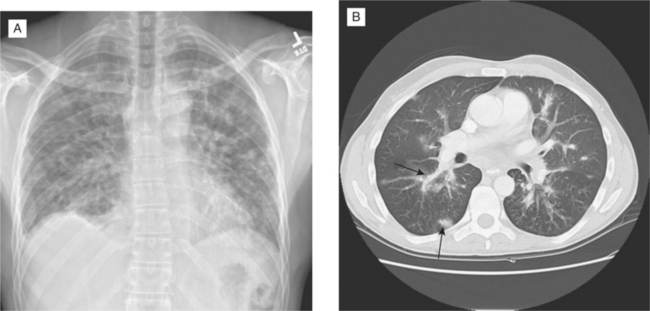
Pulmonary KS is generally seen as a late complication of KS, but on rare occasions the lung is the initial or sole site of KS. KS may present as intrathoracic adenopathy or involve the pleural surfaces, the lung parenchyma, and the bronchial tree. Pulmonary KS usually, but not invariably, causes symptoms that may include dyspnea, cough, or hemoptysis, although gross bleeding is uncommon. The radiographic picture may include enlarged hilar and mediastinal lymph nodes, pleural effusions, diffuse interstitial or alveolar infiltrates, poorly defined nodules, or some combination of these features (Fig. 59-2A), although some patients with endobronchial lesions have normal plain films despite prominent respiratory symptoms.12 Bronchoscopy is the diagnostic procedure of choice because it allows direct visualization of red endobronchial lesions (when present), and coexisting infectious diseases can be diagnosed or excluded. When endobronchial lesions are not present, however, a definitive diagnosis of KS may not be possible, as transbronchial biopsies often do not yield diagnostic tissue.13 Gallium (or combined gallium–thallium) scanning has been advocated as a technique to distinguish pulmonary KS from other neoplastic or infectious conditions.14 KS has been reported to be gallium-negative and thallium-positive, whereas infections or other inflammatory diseases of the lung typically show the opposite pattern. In practice, however, thallium scanning is not widely used and false-negative gallium scans have been documented in patients with various opportunistic infections. A negative gallium scan alone is therefore not sufficient to exclude infectious causes of an abnormal radiograph, particularly in a febrile patient. In an afebrile patient with an established KS diagnosis, however, a negative gallium scan together with a negative infection workup (including bronchoscopy) and compatible radiographs may be accepted as presumptive evidence of pulmonary KS. Rarely, open lung biopsy or computed tomography (CT)-guided needle biopsy is required to establish a diagnosis of pulmonary KS, particularly when the differential diagnosis suggests other neoplasms or infections. CT scans offer better definition of pulmonary KS involvement than plain radiographs12 and are helpful for following the response to treatment, especially in patients with poorly defined lesions on routine films. Typically, KS nodules show a peribronchovascular distribution on CT and are larger than one centimeter in diameter (Fig. 59-2B).15
THERAPY OF AIDS-ASSOCIATED KS
Management of HIV Infection
A successful KS management strategy includes optimal antiretroviral therapy for the stage of HIV infection and prophylaxis for, and prompt recognition and treatment of, opportunistic infections. There are several reasons to believe that HIV suppression is a critical component of KS management. The observation that KS sometimes regresses after initiation of potent antiretroviral regimens suggests that in some cases a decrease in immunosuppression, pathologic immune activation, and/or HIV replication may be sufficient to control KS. Although it is attractive to speculate that the development of anti-KSHV immune reconstitution accounts for KS regression, in one study a sustained increase in anti-KSHV-specific CD8+ T-lymphocyte cell responses required more than 12 months of antiretroviral therapy,16 whereas KS regression often occurs much earlier, so other mechanisms may be involved. KS spindle cell proliferation in vitro is stimulated by inflammatory cytokines,17,18 whose production is increased in the setting of acute opportunistic infections and active HIV replication. It has also been suggested that the HIV Tat protein may stimulate KS lesion development and growth. Tat has been shown to act synergistically with growth factors to increase KS cell proliferation in vitro19,20 and to accelerate formation of KS-like tumors in transgenic mice that express the KSHV G protein-coupled receptor (GPCR), a virally encoded chemokine receptor that is believed to play an important role in the development of KS.21 Additionally, direct angiogenesis-inhibitory effects of certain protease inhibitors have been described in experimental models.22 Although there is no evidence that potent antiretroviral regimens that do not include an HIV-1 protease inhibitor are less effective than protease inhibitor-containing regimens in their ability to prevent KS development,23 there is conflicting and largely anecdotal data on the relative efficacy of protease inhibitor-based and non-nucleoside reverse transcriptase inhibitor-based antiretroviral regimens in controlling established KS.24,25
The foregoing observations support optimization of HIV suppression as part of the management strategy for all patients presenting with KS. In the setting of a relatively low tumor burden and the absence of highly symptomatic or life-threatening KS, a trial of antiretroviral therapy can be considered before specific anti-KS therapy is instituted. It should be emphasized, however, that successful control of HIV replication does not invariably lead to KS regression, and specific KS therapy may need to be administered. Although there is only anecdotal evidence that antiretroviral therapy alone leads to regression of advanced KS in the absence of concomitant chemotherapy,26 there is clearer evidence that potent antiretroviral therapy prolongs the time to treatment failure for patients receiving local or systemic KS therapy.27,28 Additionally, in a small proportion of patients, KS lesions may initially develop or progress more rapidly after starting effective potent antiretroviral therapy; this is considered a manifestation of the immune reconstitution inflammatory syndrome.29–31
Local Therapy
Several studies that investigated topical application of a9-cis-retinoic acid (alitretinoin; Panretin) gel32,33 provided the basis for US Food and Drug Administration (FDA) approval of this agent for KS treatment. In one vehicle-controlled trial in which cutaneous lesions were treated two to four times daily, 35% of patients who received a 12-week course of 0.1% 9-cis-retinoic acid gel and 18% of patients treated with the vehicle gel showed an objective response (P = 0.002). The responses were partial in all but one patient. With longer treatment, additional responses were observed with 9-cis-retinoic acid gel. Application-site reactions (erythema, pain, pruritus, flaking, desquamation, crusting, swelling) were common but were rarely severe.33
Before the introduction of 9-cis-retinoic acid gel, the most commonly used local approaches were liquid nitrogencryotherapy34 and intralesional injections of vinblastine.35 There are few published studies on response rates or the characteristics of lesions best suited to these types of local therapy. In one study35 a single intralesional injection of vinblastine was administered to 33 lesions in 11 patients. The size of the treated lesions was not specified. Of the 33 injected lesions, 20 (61%) showed complete clinical response, and nine others (27%) showed partial regression. Raised (papular or nodular) lesions became macular after treatment, but nearly all the lesions healed with postinflammatory hyperpigmentation. Of 12 regressed lesions observed 4–7 months after treatment, five (42%) had relapsed, and three of four biopsy specimens from persistently ‘regressed’ lesions showed histologic evidence of residual KS. Transient (≤2 min) pain was associated with injection and was followed, after 6–48 h, by more severe aching pain that was generally relieved by non-narcotic analgesics. In another study34 liquid nitrogen therapy was given to 61 lesions in 20 evaluable subjects. Each lesion received an average of three treatments (range one to eight) at 2- to 3-week intervals to allow for healing of local blisters. The average area of the treated lesions was relatively small, 68 mm2 (range 10–230 mm2). Based on the area of the residual tumor, 80% of the lesions showed a complete response, and 7% showed partial regression. An independent evaluation of pre- and post-treatment color slides for 44 lesions, however, was interpreted as complete absence of residual KS in 50% and partial response in 27% of lesions. Overall cosmetic results were scored as complete (normal skin) in 20.5% and partial (>50% improvement) in 50%. At a 6-month follow-up in 13 of the 20 subjects, only one showed progressive KS in a treated area. In addition to local blistering and crusting, no significant side effects were reported other than short-lived (≤1 h) local pain relieved by acetaminophen.
Several other locally injected agents have also been tested in small clinical trials, including recombinant interferon-alpha (IFN-α),36 recombinant granulocyte/macrophage colony-stimulating factor (GM-CSF),37 recombinant platelet factor 4,38 and human chorionic gonadotropin.39 Although all of these agents have been shown to induce local KS regression, there are no convincing data to support their superiority over the more commonly used local agents. Local injections of vinblastine and the sclerosing agent Sotradecol40 have also been reported to induce regression of oral KS lesions. Local treatments have the advantage of inducing few systemic side effects and can often be completed quickly, yielding acceptable cosmetic results in some patients. The benefits of local treatment are confined to the treated lesions, however, and there is no evidence that local lesion control inhibits the development of new lesions elsewhere, so systemic therapy is often preferable for long-term disease control. Lesions may recur at treated sites, and local side effects may include pain, eschar formation, and hyper- or hypopigmentation.
Radiation Therapy
Radiation therapy (RT) is most often used to treat KS of the skin and oral cavity and less frequently to treat visceral KS. A review of the literature on RT for KS reveals no uniform ‘standard’ approach.
In a prospectively randomized trial, Stelzer and Griffin41 compared treatment of individual KS skin lesions with 6 MeV electrons given as 800 cGy in one fraction (a frequently recommended regimen42), 2000 cGy in 10 fractions over 2 weeks, or 4000 cGy in 20 fractions over 4 weeks to the palpable tumor with a 2 cm margin. Complete resolution of the palpable lesion was significantly better for the fractionated (2000 or 4000 cGy) regimens (79% and 83%) than for the single-dose (800 cGy) regimen (50%); and complete resolution of residual pigmentation was significantly better for the 4000 cGy regimen (43%) than for the 2000 or 800 cGy regimen (8% for each). The 4000 cGy regimen also led to a significantly longer median duration of lesion control (43 weeks) than 2000 cGy (26 weeks) or 800 cGy (13 weeks). Acute toxicity was somewhat higher in the 4000 cGy lesions, but this was limited to mild erythema, dry desquamation, local alopecia, and hyperpigmentation. These data suggest that the type of RT should be individualized based on the intent of treatment and the overall health status of the patient. If long-term cosmesis is the primary objective, a 4000 cGy regimen over a protracted course is optimal. However, for lesions of lesser cosmetic importance or for treating extremely ill or symptomatic patients who have limited mobility or a short overall life expectancy, a more rapid fractionation regimen may produce acceptable local results without the need for repeated treatment visits over many weeks. In addition, the 4000 cGy regimen was subsequently reported in one patient to be associated with a radiation recall reaction when bleomycin was administered, whereas there was no reaction in other lesions in the same patient that had been treated with 800 or 2000 cGy.43 Thus for patients in whom a future need for systemic chemotherapy is anticipated, the more rapid fractionation regimens may be associated with a lower risk of recall reactions.
RT of more extensive KS, such as diffusely involved extremities, with or without edema, usually requires larger photon fields. Severe local reactions (skin erythema, pain, desquamation of the skin on the soles of the feet) were observed in five of seven patients who received 2000 cGy to the feet over 2 weeks.44 Berson et al42 observed a lower incidence of high-grade local reactions with a single 800 cGy fraction to treat KS of the foot. Stelzer (personal communication) suggested, however, that lower doses per fraction and a planned rest period may allow delivery of higher total doses without severe acute reactions and may avoid later radiation-induced edema from subcutaneous fibrosis. Although KS-associated edema is often reduced with RT, its resolution is rarely complete.42,44
Although oral KS has also been treated successfully with RT, patients with HIV infection have sometimes been noted to have unusual radiation sensitivity of normal tissues. Oral radiation using 4 MeV photons at 180 cGy daily for 9 days (total 1620 cGy), was reported by Chak et al44 to be associated with severe mucositis, mouth dryness, and altered sense of taste, which was decreased but not eliminated by lowering the total dose to 1400 cGy. Berson et al42 also reported a high incidence of severe mucositis when the oropharynx was treated with high-dose fractions (180–400 cGy) to total doses of 2000–2400 cGy. The severity was decreased by using 150 cGy fractions to a total dose of 1500 cGy.42 Stelzer (personal communication) has advocated using 150 cGy fractions 5 days a week for 10 doses to the oral cavity, followed by a 1-week scheduled break in therapy to reduce the risk of mucositis. Patients may then be given as many as five additional fractions, depending on tolerance. Tumor shrinkage is rapid with oral RT and can induce rapid relief of symptoms from bulky lesions. Systemic therapy is also effective for many patients with oral KS, so the decision to choose local RT or systemic therapy may depend on whether other indications for systemic KS treatment coexist with the oral disease.
Although chemotherapy is a more common approach to symptomatic lung or GI tract involvement, RT has also been used to treat selected patients with visceral KS. Berson et al42 reported responses in 88% of patients treated with involved field photons to GI lesions located mainly in the anorectum, and they described relief of obstructive symptoms in two patients with upper GI lesions. Rapid subjective improvement has also been reported42,45 in patients with pulmonary KS who received whole-lung radiation, generally in 150 cGy fractions to total doses of 900–1500 cGy. Meyer45 reported a significant reduction in hemoptysis and need for supplemental oxygen, but only 28% of patients with radiographic abnormalities showed a 50% or more reduction in measurable lesions.
IFN Therapy
IFNs have the potential to influence many of the complex processes involved in the growth of KS17,46 through their antiviral effects and multiple effects on cell growth and function. Recombinant IFNs-α2a (Roferon-A) and α2b (Intron-A) were approved for the treatment of certain patients with AIDS-associated KS on the basis of studies performed before the introduction of antiretroviral drugs. The approved doses were therefore based on the results of studies of IFN as a single agent in which extremely high doses (e.g., 36 million units daily, or 30 million units/m2 three times a week) were required to achieve KS regression. The use of such doses was often complicated by fatigue, malaise, anorexia, and hepatotoxicity. In those early studies the overall tumor response rates were ∼30%. Responses were usually observed only in patients with CD4+ T-lymphocyte counts of 200/μL or more who had no history of opportunistic infection and who lacked other signs and symptoms of advanced HIV infection. In patients with these ‘good risk’ features, regression of extensive cutaneous, oral, or GI KS was sometimes observed.47 Median response durations were 6–12 months for partial responders and up to 2 years among complete responders.
Subsequently, IFN was generally administered in combination with antiretroviral agents and at lower doses. Although combined IFN and chemotherapy regimens have been poorly tolerated and have not yielded superior therapeutic results,48–50 improved results have been described when IFN-α was combined, at lower doses than those used for monotherapy, with nucleoside reverse transcriptase inhibitors. Several phase I studies that combined IFN-α with zidovudine demonstrated KS response rates exceeding 40% in patients treated with IFN-α doses ranging from 4 million to 18 million IU/day.51–53 These high response rates were confirmed in a phase II trial of the combination, which used a daily IFN dose of 18 million IU and a zidovudine dose of 100 mg every 4 h.54 The IFN-α–zidovudine combination induced KS regression in 25–30% of patients with CD4+ T-lymphocyte counts less than 200/mL,51,54 whereas fewer than 10% of such patients responded to high-dose IFN-α monotherapy.48 Although the dose-limiting neutropenia frequently seen with the combination51–54 could be prevented or reversed by administration of GM-CSF,55,56 later trials evaluated IFN in combination with less myelosuppressive antiretroviral drugs. In one such trial (ACTG 206) patients were randomly assigned to receive IFN-α at a dose of either 1 million or 10 million IU/day together with standard doses of didanosine. Similar rates of objective tumor regression were observed in both dosage groups, but the lower dose was significantly better tolerated.57 More recently, a phase I study conducted by the AIDS Malignancy Consortium (AMC 004) evaluated the safety and maximum tolerated dose of IFN-α2b in combination with protease inhibitor-based combination antiretroviral therapy.58 Dose-limiting toxicities were neutropenia and malaise. The maximum tolerated IFN dose was 5 million IU/day. The median CD4+ T-lymphocyte count increased from 260/mL at baseline to a maximum on-study value of 359/μL. In six patients with paired baseline and on-study values, the median HIV RNA level decreased from 20 179 copies/mL to a minimum on-study value of 309 copies/mL. Of 13 patients whose KS response could be evaluated, five (38%) showed objective tumor regression. Responses occurred in both HAART-experienced and HAART-naive subjects.58
Chemotherapy
Single agents with reported activity include etoposide,59–64 vinblastine,65 vincristine,66 bleomycin,57–69 and doxorubicin,70–72 each of which has been studied alone or as part of combination regimens in multiple clinical trials. In addition, single clinical trials have indicated anti-KS activity for teniposide,73 vinorelbine,74 and epirubicin.75 Despite their demonstrated activity, with reported objective response rates as high as 76%, and averaging over 40%, disease control by these agents has often been limited by their toxicities, the most common of which are alopecia, mucositis, and neutropenia with etoposide and doxorubicin; neutropenia with vinblastine; peripheral neuropathy from vincristine; and fever and cutaneous and cumulative pulmonary toxicities from bleomycin. High cumulative doses of doxorubicin are also associated with cardiac toxicity. The reported response rates and response durations for these agents are difficult to interpret or compare, as patient characteristics and response definitions varied from study to study, the use of antiretroviral therapy and infection prophylaxis was inconsistent, the methods of disease documentation and response definitions were often ambiguous and inconsistently applied, and with rare exception the studies were not controlled.
Before the introduction of liposomal anthracyclines and paclitaxel, combination chemotherapy was generally considered to induce higher response rates than single-agent therapy but at the expense of increased toxicity, which often limited long-term use. Nonetheless, by the early 1990s combination therapy was considered the standard of care. The ABV regimen71 combining doxorubicin (Adriamycin), bleomycin, and vincristine, was most commonly used in the United States. Several variations of the ABV regimen were used, with and without concomitant antiretroviral therapy and hematopoietic growth factor support,71,72,76–80 but the most commonly used regimen was doxorubicin 20 mg/m2, bleomycin 10 U/m2, and vincristine 1 mg, administered every 2 weeks. Other frequently used combinations included bleomycin and vincristine,81,82 which was more widely used than ABV as a standard regimen outside the United States, and vinblastine alternating with vincristine on a weekly schedule.83 Several more-intensive chemotherapy regimens were tested,84,85 but they generally induced unacceptable toxicity without a corresponding increase in therapeutic activity or response duration. The results of trials with chemotherapy combinations have been extensively reviewed elsewhere;46,86,87 their use has largely been supplanted by the liposomal anthracyclines and paclitaxel. Although high response rates, averaging in excess of 60%, were reported for these regimens, the same caveats described for interpretation of the reported results for single-agent therapy apply to the combination regimens. Notably, much lower response rates were observed for combination chemotherapy when these regimens were tested in randomized, controlled trials in comparison with liposomal anthracyclines in which more stringent response criteria were applied (discussed later). In addition, all of these regimens were tested before the introduction of HAART; their activity in combination with current antiretroviral regimens is unknown.
The first chemotherapeutic agents approved by the FDA specifically for treatment of advanced AIDS-related KS were Doxil and DaunoXome.87,88–99 The liposomal formulations prolong the circulating half-life of the anthracyclines (hours vs minutes for the unencapsulated drugs), increase drug concentrations in tumor tissue, and modify their toxicities.98,100,101 Neutropenia is frequently induced by these agents,90,91,102,103 but alopecia, nausea, and vomiting, which are common after administration of free doxorubicin, are uncommon with the liposomal agents.102,104,105 Anthracycline-induced cardiac toxicity has been observed rarely after administration of high cumulative doses to patients with KS,98,106,107 but maximum safe cumulative doses have not been defined. Doxil (but not DaunoXome) treatment has sometimes been associated with the hand-foot syndrome (palmar-plantar erythrodysesthesia).108 This reaction, consisting of painful erythema and desquamation of the palms and soles, is generally associated with chemotherapeutic agents administered by continuous infusion. It is believed that the markedly increased serum half-life (∼48 h) associated with the PEG-coated liposome used to formulate Doxil simulates a continuous drug infusion, whereas the uncoated liposome used to formulate DaunoXome has a t½ of only ∼4 h. Both Doxil and DaunoXome administration, on occasion, have been complicated by acute infusional reactions characterized by back or chest pain, a sensation of choking, and intense flushing.109 The latter reaction usually occurs within minutes of starting treatment and generally subsides quickly after stopping the drug infusion.
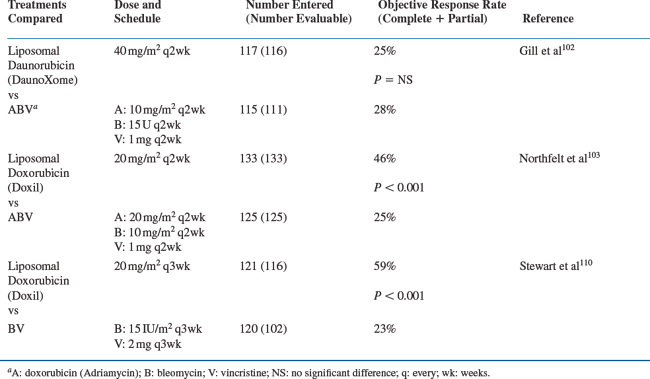
Doxil was approved in 1995 for treatment of advanced KS after failure or intolerance of combination chemotherapy. Tumor response rates of 27% and 48%, respectively, were documented depending on whether a global disease assessment was used as the response criterion or the response was based on changes in selected indicator lesions. The median response durations were 2.4 and 2.3 months, respectively, from the time a partial response was recorded by these two assessment methods. Significantly, the lesions of some patients whose tumors had progressed on regimens containing conventional (unencapsulated) doxorubicin responded subsequently to Doxil. In practice, however, Doxil is most often used as first-line chemotherapy. The recommended dose and treatment schedule for Doxil is 20 mg/m2 as a slow (30–60 min) intravenous infusion every 3 weeks. DaunoXome was approved in 1996 for first-line chemotherapy of advanced KS based on a randomized comparison with a standard ABV regimen102 (described later). The labeled response rate is 23%, lasting a median of 3.7 months from the time a partial response was documented. The approved dose is 40 mg/m2 every 2 weeks. Escalation to 60 mg/m2 has sometimes induced responses in patients who did not respond or who relapsed after treatment at the lower dose. The 60 mg/m2 dose has also proven effective for patients with pulmonary KS.99 Objective response rates reported in the literature for these agents have varied widely. Some early reports in relatively small numbers of patients suggested that response rates might be as high as 70–90% even in previously treated patients.88–92,95–98 Larger multicenter trials that applied strict response criteria, however, have documented response rates between 25% and 59%.94,102,103,110 Although strictly defined response rates may be somewhat lower than originally believed, many patients experience palliation of KS-associated symptoms without achieving 50% tumor regression. At the time these studies were conducted, standard evaluation criteria for KS did not specifically address clinical benefits of treatment (e.g., pain relief, increased mobility associated with reduced edema). A joint National Cancer Institute (NCI)/FDA/AMC initiative has attempted to address this gap. Revised evaluation criteria have been developed that quantitatively evaluate changes in KS-associated signs and symptoms that affect patient function and quality of life.111 These clinical benefit assessments are now being tested prospectively. Additionally, a double-blind, randomized trial of Doxil and DaunoXome, with the primary aim of documenting clinical benefit in symptomatic KS patients, demonstrated clinical benefit (relief of KS-associated symptoms) in 80% of Doxil-treated patients and 63% of DaunoXome-treated patients, which were higher than the objective tumor response rates of 55% and 32%, respectively.112
Several prospectively randomized studies performed in the early 1990s, before the introduction of potent combination antiretroviral regimens, compared liposomal anthracyclines with conventional combination chemotherapy (Table 59-2). One study compared Doxil 20 mg/m2 with ABV every 2 weeks. A significantly higher response rate was observed with Doxil (46%) than with ABV (25%).103 A comparison of DaunoXome 40 mg/m2 with ABV every 2 weeks showed comparable response rates, which were 25% and 28%, respectively.102 A third study compared Doxil (20 mg/m2) with the combination of bleomycin (15 U/m2) and vincristine (2 mg).110 Each regimen was given every 3 weeks. A significantly higher response rate was observed among patients who received Doxil (59%) than among those who received bleomycin and vincristine (23%).110 In each of the three studies, patients who received the liposomal anthracycline showed a significantly lower incidence of peripheral neuropathy, nausea, and vomiting than those who received combination therapy. Doxil induced more neutropenia than bleomycin and vincristine, and more mucositis than ABV, but it was less likely than ABV to cause significant alopecia and severe neutropenia. Response rates in each of these randomized trials were considerably lower than those reported previously for both the liposomal anthracyclines (in uncontrolled trials) and for the standard combination regimens (in both single-arm and randomized studies); but as noted before, objective response rates are not necessarily the equivalent of clinical benefit.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree