Chapter 57 JC Virus Neurologic Infection
Progressive multifocal leukoencephalopathy (PML) was initially described by Astrom and colleagues in 1958,1 as a complication of chronic leukemia and Hodgkin’s disease. It has since been recognized in an increasing number of conditions associated with impairment in human cellular immunity. It became more prevalent with the more aggressive use of therapeutic immunosuppressive agents in the 1960s and 1970s, but was still regarded as a rare disease until the AIDS epidemic, with its resulting large pool of immune-compromised patients. PML is an AIDS-defining illness and one of the common opportunistic infections of the brain in this population, occurring in up to 8% of patients at autopsy.2–5 Before the availability of highly active antiretroviral therapy (HAART), it was reported as the presenting manifestation of HIV infection in 10–55% of case series.6–8 While there are no clear figures on the incidence and prevalence of PML following the introduction of HAART, the disease continues to occur, although probably at a reduced rate. The incidence and prevalence in resource-poor settings, such as sub-Saharan Africa, is unknown. PML is rare in pediatric AIDS but does occur.9
In 1971, Padgett and colleagues isolated the viral agent etiologically responsible for PML from the brain of an infected patient, and named it JC virus (JCV), from the initials of the patient.10 JCV is a polyomavirus. It is similar to the other human polyomavirus, BK, and to simian immunodeficiency virus 40 (SV40). JCV is ubiquitous in humans and is usually acquired by adolescence, and over 70% of the adult population generate a humoral response.11,12 The precise mode of transmission is not established, although it is suspected to be via the respiratory tract.1
The most common initial clinical findings reported in AIDS patients with PML include painless progressive monoparetic or hemiparetic limb weakness and/or sensory loss, gait difficulty, and visual disturbance (Table 57-1). Subtle or overt alteration of mental status may accompany other manifestations; occasionally the initial presentation may be of encephalopathy without focal findings. Prior to HAART, the course was generally rapidly progressive over weeks, leading to coma with vegetative signs and death, but the prognosis has improved considerably with effective antiretroviral therapy (ART).
Table 57-1 Neurological Manifestations of PML
| Focal deficit (monoparesis or hemiparesis) Gait disturbances (ataxia) Visual field deficits Cognitive disturbances Speech difficulties (aphasia and dysarthria) Sensory disturbances Seizure Cranial nerve deficits |
DIAGNOSIS
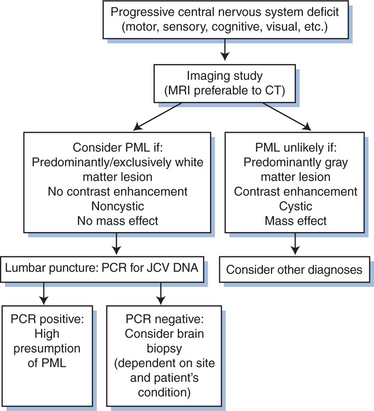
Diagnosis of PML is primarily based on clinical findings and a compatible magnetic resonance imaging (MRI) picture (Figs 57-1 and 57-2). The diagnosis is confirmed by establishing the presence of JCV and ruling out other opportunistic infections.
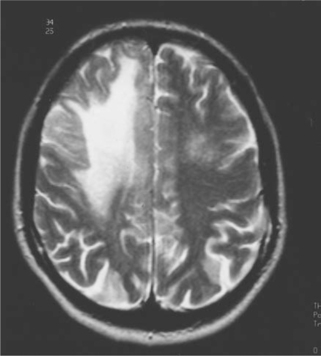
The clinical presentation is dependent on the location of active infection. Because this may involve any area of the brain, brain stem, and/or cerebellum, PML must be considered in any patient presenting with central nervous system disease. Abnormalities on computed tomography (CT) are typically hypodense white matter lesions without mass effect. Only rarely do these lesions enhance with contrast. MRI is more sensitive than CT and will typically show greater involvement.14–16 Lesions on MRI are primarily but not exclusively of white matter, and appear as single or multiple large or small bright areas on T2-weighted images, again generally without contrast enhancement or mass effect (Fig. 57-2). T1-weighted images are invariably of low density. However, it is not always possible to exclude other lesions, including lymphoma and HIV encephalitis, on MRI. Immune restoration syndrome, which may give contrast-enhancing white matter lesions with or without the presence of PML, may also create confusion in the radiological diagnosis.13
Further, while MRI is of paramount importance as a diagnostic tool, there is no evidence that the number or size of the lesions found has any prognostic significance.14 Magnetic resonance spectroscopy is an additional diagnostic tool.15 Typically, spectroscopy shows an elevated choline to creatine ratio, but this is not a specific finding.
Routine testing for antibodies to JCV is not of value in establishing the diagnosis of PML. Immunoglobulin G antibody identification by hemagglutination inhibition is positive in most healthy adults worldwide. The titer does not rise with active disease, and there is generally a lack of immunoglobulin M antibody in either serum or cerebrospinal fluid (CSF), indicating PML results from reactivation of a latent infection.12 Routine CSF analysis is generally unhelpful. Although some elevation of cell count, protein, and immunoglobulin may be found,3 it is not clear whether this occurs more often than in HIV-infected patients without PML.16
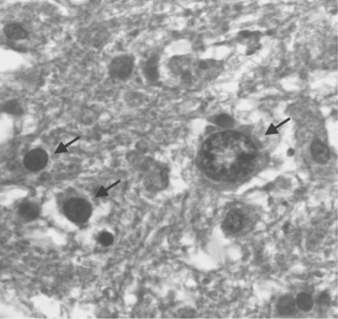
Before the advent of CSF JCV polymerase chain reaction (PCR) analysis the definitive diagnosis was dependent on pathologic examination of brain tissue. The typical appearance on biopsy is of multiple areas of demyelination at the corticomedullary junction, extending in severe cases into large areas of white matter (Fig. 57-3). In advanced disease, gray matter may also be involved. The oligodendrocytes have large, deeply staining nuclei, many with inclusions and with large numbers of virions (Fig. 57-4). Reactive astrocytosis is generally present, and there may be giant astrocytes similar to those found in glioblastoma multiforme.17 Neurons are not infected. HIV-infected patients have a higher incidence of very large, confluent lesions, with prominent necrosis and a higher incidence of marked perivascular inflammatory infiltrates, compared to patients with other causes of immune compromise.18
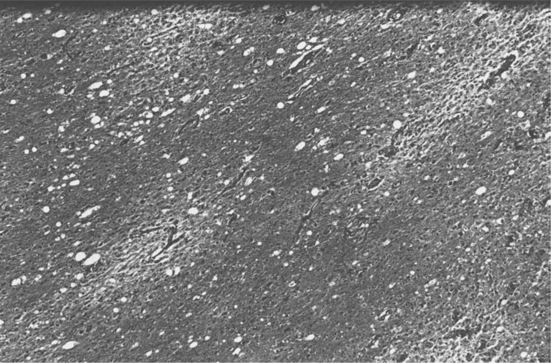
Figure 57-3 Multiple foci of demyelination unaccompanied by significant inflammatory infiltrate (×45).
Stereotactic brain biopsy offers less morbidity than open biopsy and is generally the preferred procedure,19,20 but may not always give adequate tissue for definitive microscopic diagnosis. The use of in situ hybridization techniques21 and immunocytochemistry increases the diagnostic potential of stereotactic biopsy, and these are useful adjunctive diagnostic tools.22,23 Utilizing PCR, JCV DNA has been identified in the brains of HIV-infected patients with and without PML, and in uninfected control brains. This presumably reflects indolent infection of brain tissue, and suggests that PCR of brain biopsy tissue is too sensitive for specific diagnosis of active disease.24–26
In an effort to spare the patient the need for brain biopsy, much work has been done on analysis of easily accessible body fluids to provide a reliable method for diagnosis. It is clear that the kidney may act as a viral reservoir in healthy populations; in different series, 20–80% of urine samples from healthy adults have yielded isolates of JCV.27,28 PCR in urine is thus unsuitable for diagnosis of disease. The same is true of evaluation of peripheral blood. Fifteen percent of samples from healthy subjects without immune compromise will yield positive JCV PCR.29 In addition, Tornatore et al detected JCV DNA in lymphocytes of 89% of patients with PML and in 38% of asymptomatic HIV-positive patients.30 Dubois et al found JCV DNA in lymphocytes of over 40% of HIV-infected patients without PML.31
Much greater specificity has been achieved through the evaluation of CSF. Weber et al identified JCV DNA in CSF from each of three HIV-infected patients with PML but in none of 30 HIV-infected patients without PML.32 Later, the same group reported CSF analysis from 28 patients with PML and 82 HIV-infected patients without PML. JCV DNA was detected in 82% of the PML patients and none of the controls.33 De Luca and colleagues evaluated CSF from 19 patients with clinical PML and 83 patients with advanced HIV disease but no evidence of PML. Nested PCR had 74% sensitivity and 100% specificity, with no positive results in the patients without PML.34 McGuire et al, using conditions optimized to detect one viral copy in 50 mg CSF, reported both sensitivity and specificity of 92%.35 Thus it is now accepted, even for clinical trials, that a firm diagnosis of PML can be reached based on typical clinical and radiological appearance along with a positive JCV DNA analysis in CSF. Brain biopsy confirmation would then only be required if the CSF evaluation was negative, if the clinical picture was atypical, or if there was concern about a concomitant condition, such as lymphoma. Current treatment trials are based on this algorithm. It is generally accepted that greater than 90% of patients with PML will have positive JCV PCR in the CSF, and this does not occur in normal subjects or HIV-infected patients without PML. However, a number of studies have demonstrated that all CSF samples will not be positive, so in cases with a high clinical suspicion and an initially negative PCR, repeat CSF evaluation may be helpful. A further complication in interpreting the CSF results is the observation in a number of trials that treatment with HAART may be associated with disappearance of JCV from the CSF.36,37 The JCV viral load in the CSF also appears to be of prognostic value, the higher the number of copies, the worse the prognosis.38,39
PCR for JCV is available commercially through the laboratories listed in Table 57-2.
Table 57-2 Laboratories offering JCV PCR
| Molecular Microbiology Mayo Medical Laboratories Mayo Clinic 200 First Street SW Rochester, MN 55905 (Tel. 1-800-533-1710) |
| Diagnostic Virology Laboratory University of Colorado Health Sciences Center 4200 East 9th Avenue Room MS 1632 Denver, CO 80220 (Tel. 303-372-8182) |
| Virolab, Inc 1204 10th Street Berkeley, CA 94710 (Tel. 510-524-6201) |
THERAPY
Before the advent of ART, the prognosis for HIV-infected patients who develop PML was grave. Median survival from diagnosis has generally been from 2.6 to 4 months.18,37,40 Wiley and colleagues have theorized that the prognosis may be worse in HIV infection because JCV, in addition to its direct toxicity, may increase ingress to the brain of HIV-infected macrophages.41 However, ∼10% of HIV-infected patients had a more protracted course, with stabilization over months or years and occasionally with apparent reduction or resolution of lesions.42,43 Based on retrospective and prospective cohort studies and individual case reports, there is good evidence that treatment with HAART has resulted in improvement in the survival of patients with PML.44–48 Maximization of ART is therefore a clear goal in treatment. Despite this, a significant number of treated patients still succumbed to PML. In addition there are a number of reports of patients who developed PML after the institution of ART, perhaps related to the immune reconstitution syndrome.49–51 This inconsistent and incomplete response to ART, in addition to the emergence of ART resistance, mandate the continued search for more specific and effective therapy aimed at JCV. Such therapy would also be of great value in the 20% of PML patients who are not HIV-infected. A recent reminder of this possibility was the emergence of PML in a patient with Crohn’s disease treated with natalizumab, a humanized monoclonal antibody which binds to the adhesion molecule alpha 4 integrins, and two patients with multiple sclerosis who were being treated with a combination of natalizumab and interferon-β1a.52,53
The infrequency of PML before the AIDS pandemic precluded the development of adequately controlled studies, and therapeutic measures were based on anecdotal case reports using agents with theoretical potential efficacy. A number of such agents have been tried and some suggested benefit in single cases or small series. Despite the increase in cases, controlled studies have remained a rarity, and to date there has been no convincing evidence of improvement other than that associated with ART administration. As all patients should be treated with maximized ART, any current and future studies of specific therapy must also account for the beneficial effects of HAART. The prognostic factors for PML are listed in Table 57-3.
Table 57-3 Prognostic Factors for PML
| Favorable Factors | Unfavorable Factors |
|---|---|
Completed Studies
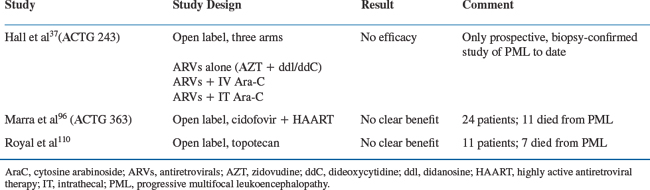
Immune compromise is a common factor in the development of PML. In the AIDS population, antiretroviral agents, by improving immune function, might be expected to have the potential to reverse or retard the progression of disease. There is strong evidence to support this, both from uncontrolled case reports and from incidence studies of populations before and after the era of HAART. In 1988, Fiala et al first reported improvement in an AIDS patient with suspected PML following the administration of zidovudine (AZT).54 In 1990, Conway et al reported a case of biopsy-proven PML in an AIDS patient with marked improvement following AZT administration.55 Britton and colleagues reported that 7/26 patients stabilized or showed improvement on ART alone.56 Not all investigators reported positive results in the pre-HAART era.57 The ACTG 243 study, primarily designed to evaluate cytosine arabinoside (Ara-C) (see further ahead), encouraged the use of moderately high-dose (900 mg/day) AZT in combination with another antiretroviral, generally didanosine (ddI) or zalcitabine (ddC), in each of its three arms. There was no control arm against which to compare the effects of ART, but the mean survival in the three arms, ∼3 months, suggests there was no significant improvement over historical survival figures.37
Several studies have now indicated improved survival in AIDS-associated PML patients treated with ART.46,58–63 Dworkin, on review of 415 cases, found improved survival with ART in general, particularly when the regimen included a protease inhibitor. Beringuer reported a median survival of over 2 years in 118 PML patients treated with HAART, with neurological improvement in many. In 101 patients, Antoniri also found marked improvement in survival with HAART treatment.64,65 Clifford, in a descriptive study of 57 HAART treated PML patients, found a median survival of greater than 46 weeks. Cinque, Miralles, De Luca, and Albrecht each reported series with considerable neurologic improvement and a more favorable outcome. Overall, these different studies reported improvement in mean survival varying from 46 weeks to 2.2 years. Thus while there remains a dearth of prospective controlled studies, the evidence is overwhelming that HAART is beneficial.
Therapeutic agents with specific anti-JC activity are listed in Table 57-4. Adenine arabinoside66,67 and iododeoxyuridine68 have been unsuccessful in altering the clinical course of PML. Isolated reports and small case studies suggested encouraging results with Ara-C, which has fairly powerful antiviral effects in cell culture studies.69 These reports suggested some patients benefited from intravenous treatment, some from intrathecal, and some from combination, and they included both HIV-infected patients and those with other reasons for immune compromise.56,70–75
Table 57-4 Therapeutic Agents with Anti-JC Activity
| Cidofovir Cytarabin Topotecan (derivative of camptothecin) Immunotherapy (IFN alpha, IFN beta, IL-2) Phenothiazine derivatives, e.g, Chlorpromazine Serotonin receptor (5-HT2A) antagonist, e.g., Mirtazepine, Cyproheptadine |
Not all investigators have reported positive effects from Ara-C.76–78 To address the question, the Neurologic AIDS Research Consortium, in conjunction with the AIDS Clinical Trials Group, designed a three-arm study (ACTG 243) to compare the efficacy of high-dose ART alone to ART with either intravenous or intrathecal Ara-C (Table 57-5). The study was terminated in June 1993 after interim analysis demonstrated no benefit in either the intravenous or the intrathecal arm, and Ara-C, which has major bone marrow toxicity, cannot currently be recommended as therapy when delivered by intravenous or intraventricular injection.37 It has recently been suggested that there may be a role for Ara-C in combination with HAART (D Clifford, personal communication), but this remains purely speculative.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree