Key points
- 1.
Invasive vulvar cancer is a relatively rare tumor, accounting for 4% of all female genital malignant neoplasms.
- 2.
Squamous cell carcinoma of the vulva develops by human papillomavirus- (HPV-) dependent and HPV-independent pathways.
- 3.
Sentinel lymph node biopsy represents the largest innovation in the care of vulvar cancer patients in the past decade.
- 4.
The standard of care is pelvic lymph node irradiation for patients with positive inguinal nodes.
- 5.
Recurrences may be local or distant, and more than 80% will occur in the first 2 years after therapy, demanding initial close follow-up.
Invasive vulvar cancer is a relatively rare tumor, accounting for 4% of all female genital malignant neoplasms with the American Cancer Society projecting just over 6120 cases resulting in 1350 deaths in 2020 in the United States. Except for the rare sarcomas, the peak incidence is in women between 65 and 75 years old ( Fig. 6.1 ); in some series, almost half are 70 years of age or older. Although classically a disease of elderly women, the trend in recent years is an increasing prevalence among younger women, which cannot be accounted for by immune suppression alone. Human papillomavirus (HPV) is a key age-dependent risk factor that causes preinvasive disease in the form of vulvar intraepithelial neoplasia (VIN) that is often associated with a history of tobacco use. HPV-related VIN lesions are rarer in older women, and these malignancies may be associated with chronic vulvar dystrophies, such as lichen sclerosis, although a direct association remains unproven. No race or culture is spared, and gravidity and parity appear unrelated in the pathogenesis of this neoplasm. Vulvar cancer is common in poor and elderly women in most parts of the world, and this has led to the hypothesis that inadequate personal hygiene and medical care are contributing factors in disease development.
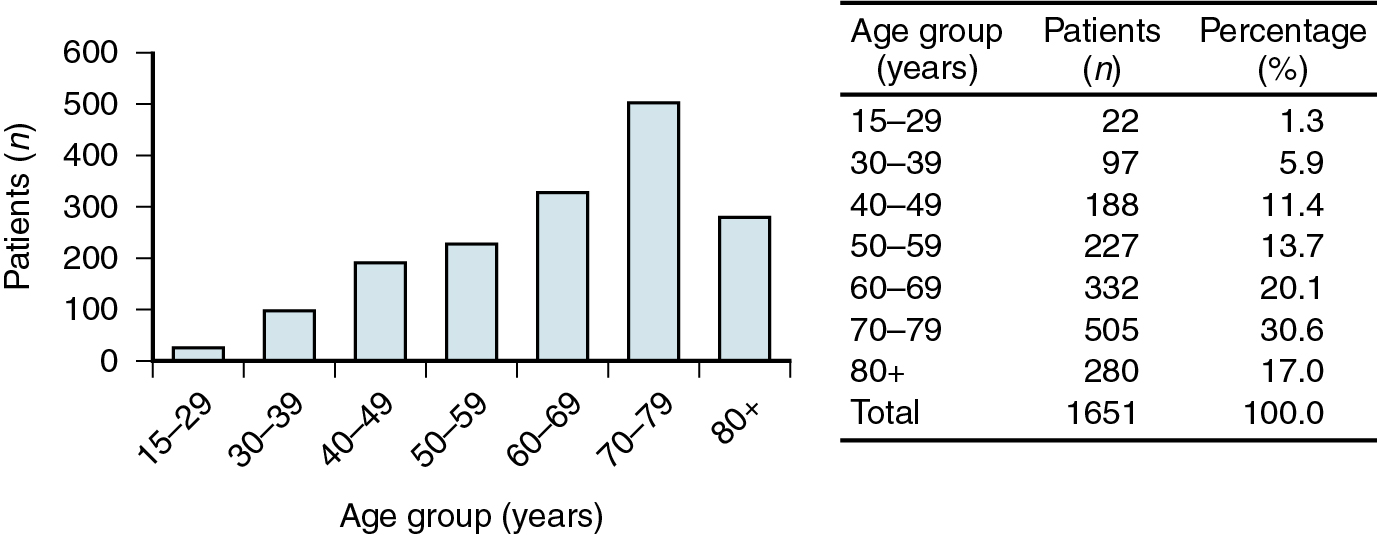
Younger patients frequently have early stromal invasion associated with diffuse VIN. Choo found 17 patients younger than age 35 with invasive carcinoma of the vulva. Of these, eight had microinvasion. Al-Ghamdi and coworkers evaluated 21 patients younger than age 40 with invasive vulvar cancer and found that most, but not all, had associated HPV. Outcomes in these populations were excellent. Lanneau and colleagues have corroborated these results in 56 women younger than age 45 years with squamous cell carcinoma (SCC) of the vulva. They concluded that vulvar cancer in this population is associated with early-stage disease, HPV, VIN, and smoking.
SCC of the vulva develops by HPV-dependent and HPV-independent pathways. The association between HPV and both preinvasive and invasive urogenital lesions has been well described. Susceptibility of the cervical, vaginal, and vulvar epithelium is referred to as the “field effect,” which is more pronounced in tobacco users and in patients who are immunocompromised, such as those with HIV/AIDS or organ transplants. The association of condyloma acuminatum with vulvar carcinoma is well known, but no cause-and-effect relationship has been confirmed yet. HPV infection has been reported to be attributed to vulvar tumorigenesis from 30% to 69% of cases, with Faber et al. reporting almost 40% of vulvar cancers resulting from HPV infection in a meta-analysis of over 5000 vulvar cancers, with the most common high-risk HPV strains being HPV 16, 33, and 18. Although the incidence of vulvar cancer has remained relatively stable over the past several decades, the incidence of VIN has increased, especially that associated with HPV. Hoang and coworkers contrast these multicentric HPV related precursors with VIN of the differentiated type that occurs in postmenopausal women, often in a background of lichen sclerosis or chronic inflammatory dermatoses associated with a high number of somatic mutations, particularly in TP53. Sznurkowski pointed out the need to better understand the relationship between vulvar cancer and HPV for not only causation but also for developing potential therapeutics. Increasing adoption of routine HPV prophylactic vaccination can reduce the burden of HPV-related vulvar dysplasia and subsequent cancer development.
Many previously reported associated features seen in patients with vulvar cancer, such as diabetes, obesity, hypertension, and arteriosclerosis, may just reflect the increased incidence of these diseases associated with aging.
Invasive squamous cell carcinoma
Histology
The overwhelming majority of vulvar cancer is squamous in origin. Table 6.1 depicts the incidence of vulvar neoplasia from several studies in the literature. The following discussion focuses mainly on SCC because of its preponderance, but as a generalization, the other lesions can be treated similarly, except as noted.
Clinical presentation and diagnosis
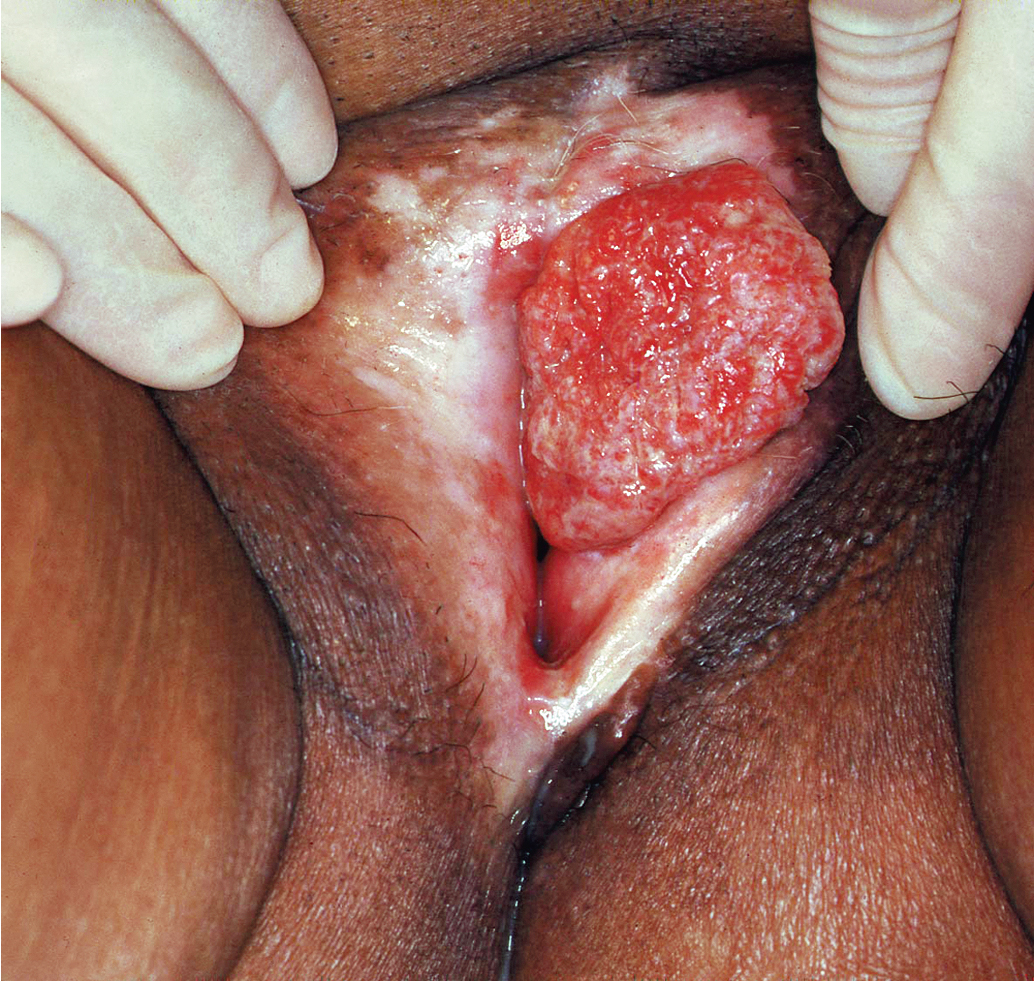
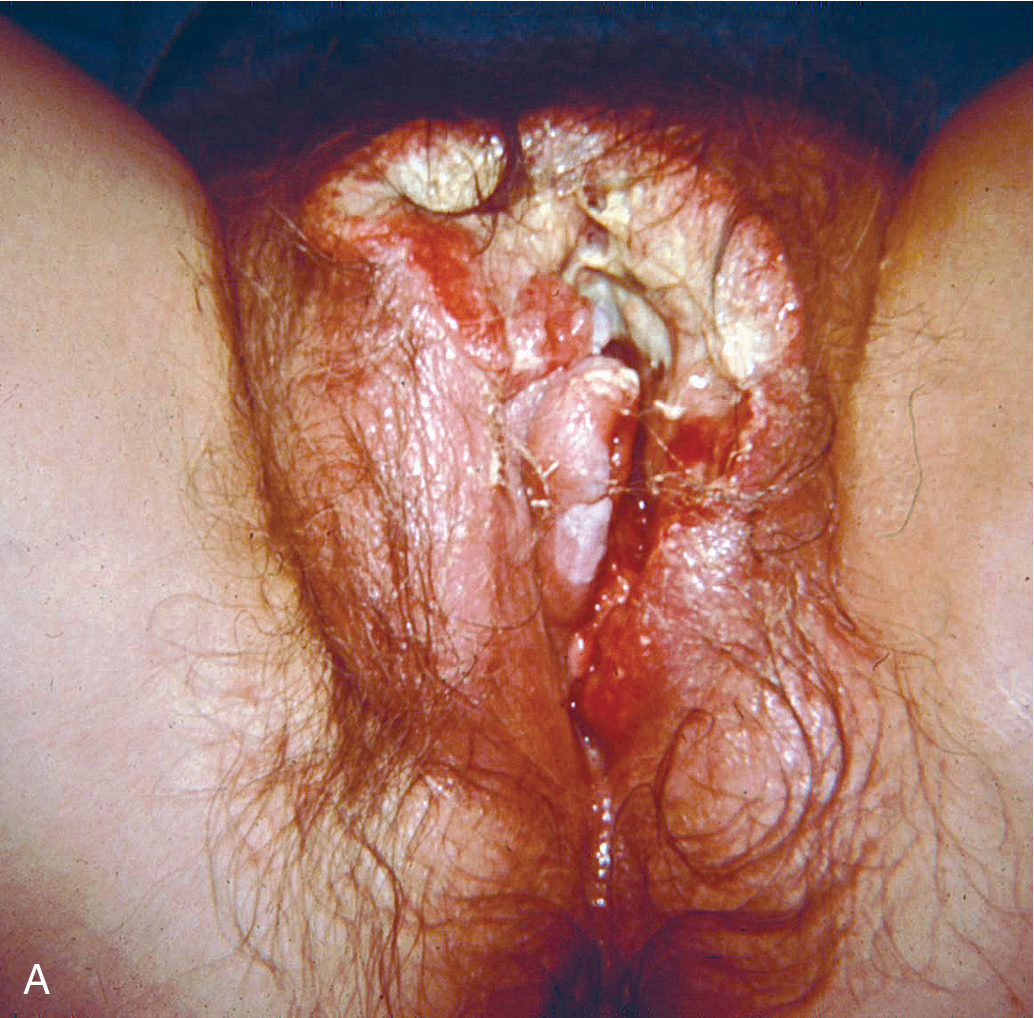
Squamous cell vulvar cancer usually arises from an area of intraepithelial neoplasia that develops into a small nodule that can ulcerate ( Figs. 6.2 and 6.3 ). Alternatively, small, warty, or cauliflower-like growths evolve, and these may be confused with condyloma acuminatum. Long-term pruritus or a lump or mass on the vulva is present in more than 50% of patients with invasive vulvar cancer ( Table 6.2 ). Biopsy must be performed of all suspicious lesions of the vulva, including lumps, ulcers, and pigmented areas, irrespective of symptoms ( Table 6.3 ).
| Sign or Symptom | Incidence (%) |
|---|---|
| Pruritus | 45.0 |
| Mass | 45.0 |
| Pain | 23.0 |
| Bleeding | 14.0 |
| Ulceration | 14.0 |
| Dysuria | 10.0 |
| Discharge | 8.0 |
| Groin mass | 2.5 |
|
A significant delay in diagnosis and appropriate treatment is common. Several series of carcinoma of the vulva report delays of 2 to 16 months after onset of symptoms before medical attention is sought. Further delay occurs when medical treatment of vulvar lesions continues without biopsy for definitive diagnosis.
Fortunately, vulvar cancer is often indolent, extends slowly, and metastasizes fairly late. Hence, there is an opportunity for preventing the development of advanced stages of this disease through patient and physician education.
Location and spread pattern
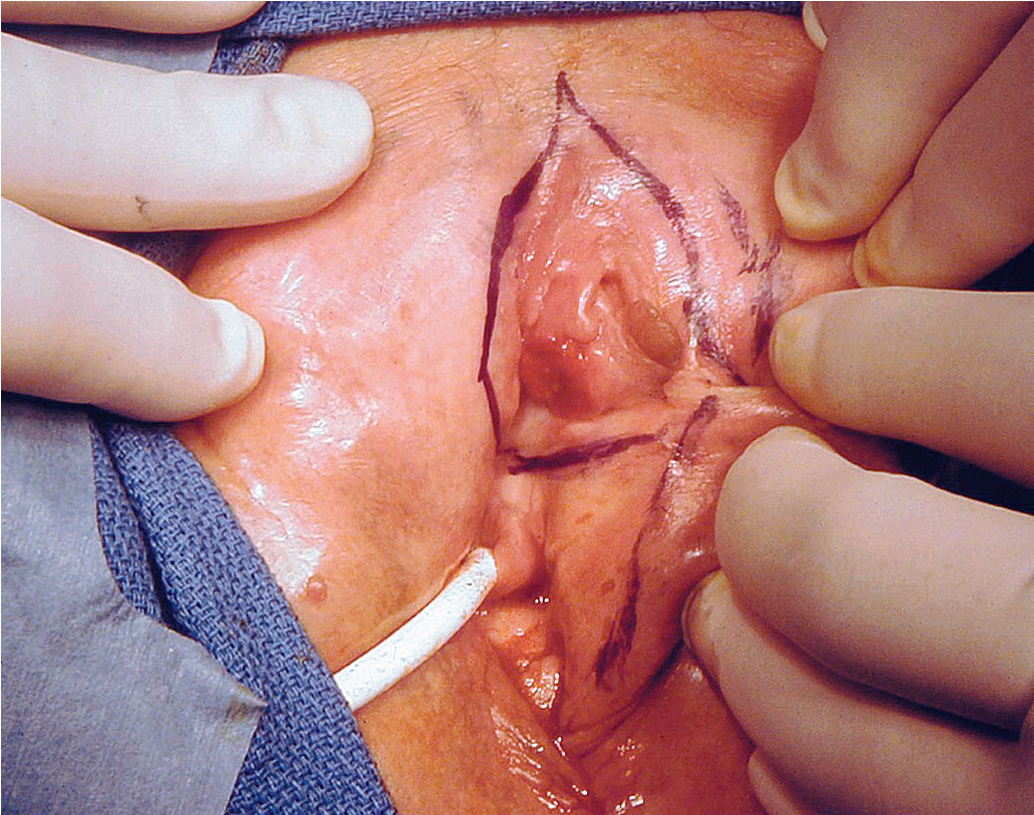
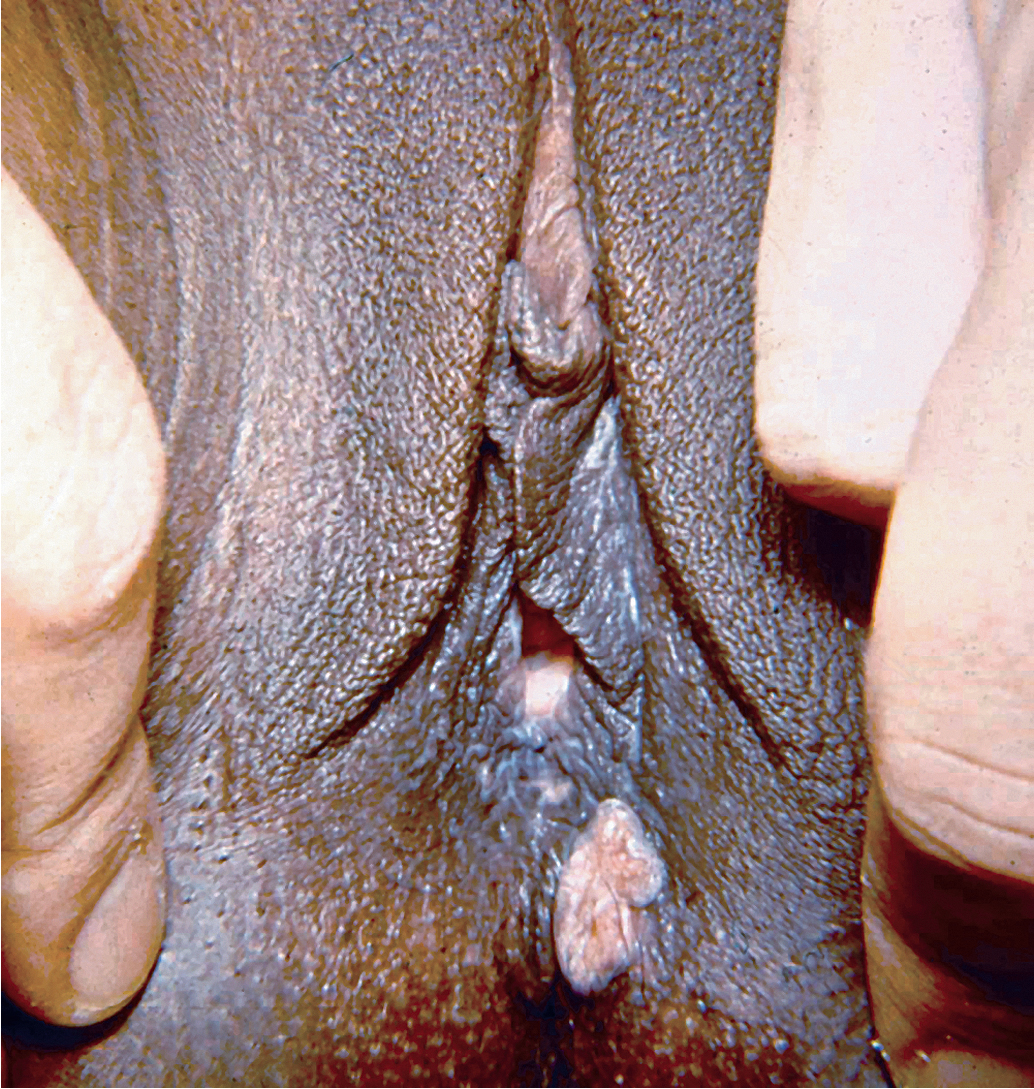
Primary disease can appear anywhere on the vulva. Approximately 70% arise primarily on the labia. Disease more commonly occurs on the labia majora; however, it may appear on the labia minora, clitoris, or perineum. The disease is usually localized and well demarcated, although it can occasionally be so extensive that the primary location cannot be determined ( Fig. 6.4 ). Multifocal growth pattern in invasive SCC of the vulva is uncommon, except for so-called “kissing lesions” that can occur.
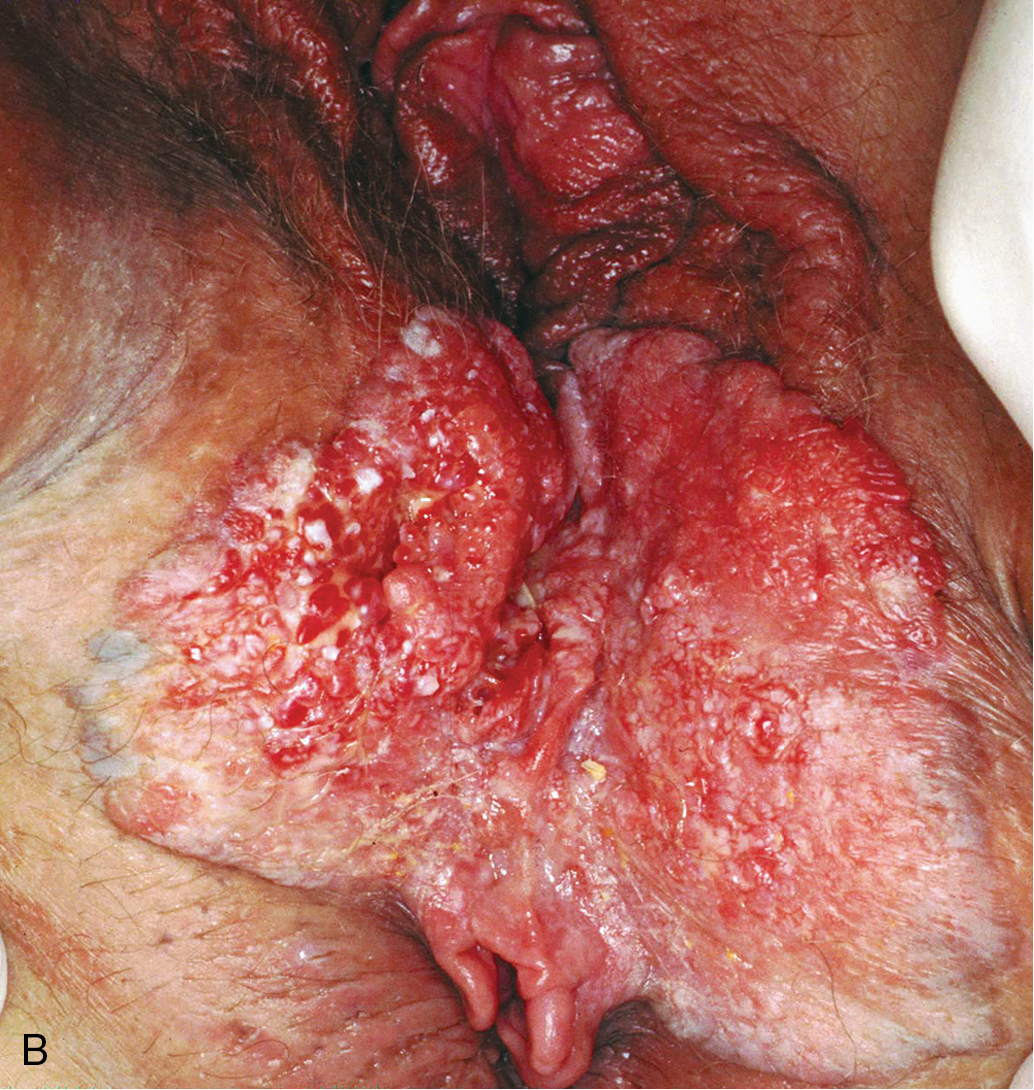
Verrucous carcinoma of the vulva ( Fig. 6.5 ) is a special and unusual variant of SCC that is locally invasive but non-metastasizing. The lesion, which may involve the cervix, vagina, and the vulva, presents as a warty, fungating, ulcerated mass with a bulky, elevated appearance reminiscent of a benign HPV lesion. Identification of this variant is important because the biologic behavior of the disease influences therapy. Condyloma may initially be diagnosed on microscopic examination, but distinction from ordinary condylomata is aided by the absence of fibrovascular cores within the proliferating papillary masses of tumor. There is usually a uniform lack of malignant features histologically. Adequate material, including underlying stroma for pathologic evaluation, is necessary to differentiate verrucous carcinoma from condyloma. Tumor may invade deeply into the underlying tissue, often requiring extensive surgery, and has a propensity to recur locally. Woodruff reported no nodal metastases in 27 patients, including a literature review, who were treated with radical vulvectomy and inguinal lymphadenectomy. As a result, a more conservative approach is advocated with wide local excision and tumor-free margins as the therapeutic aim. Lymphadenectomy is of questionable value except when nodes are obviously involved. Historically, it has been thought that radiotherapy is contraindicated because of its ineffectiveness, and reports have indicated that it can incite more aggressive behavior by this tumor.
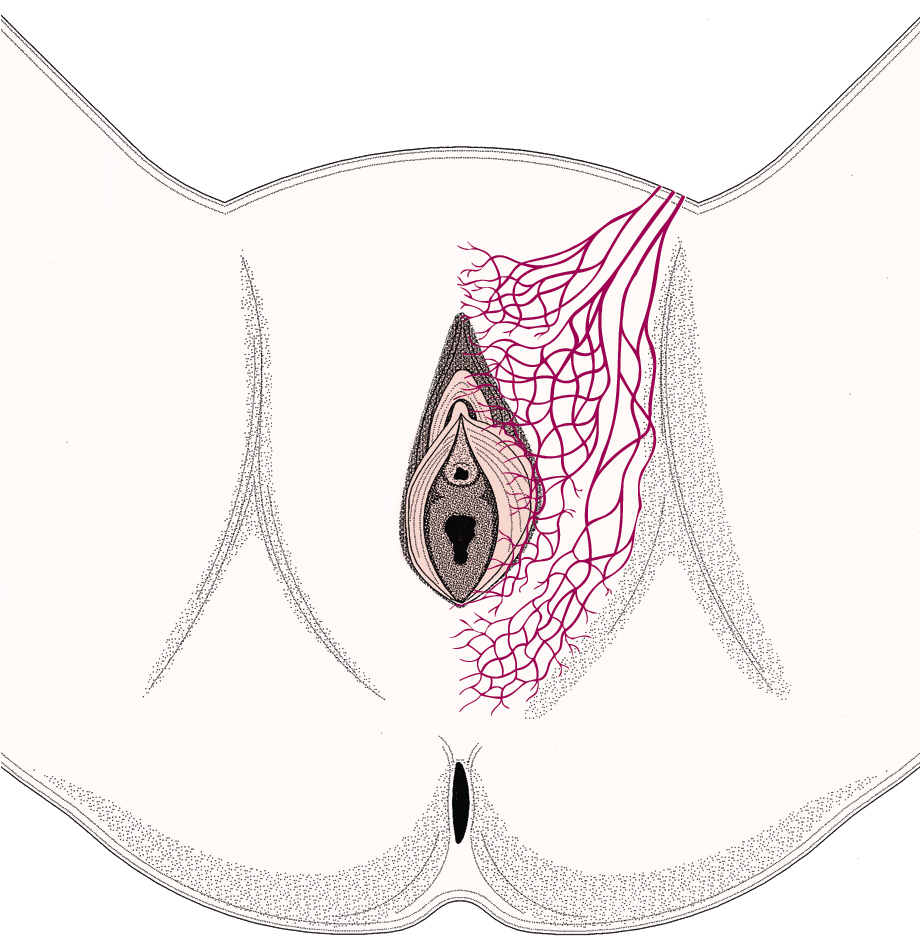
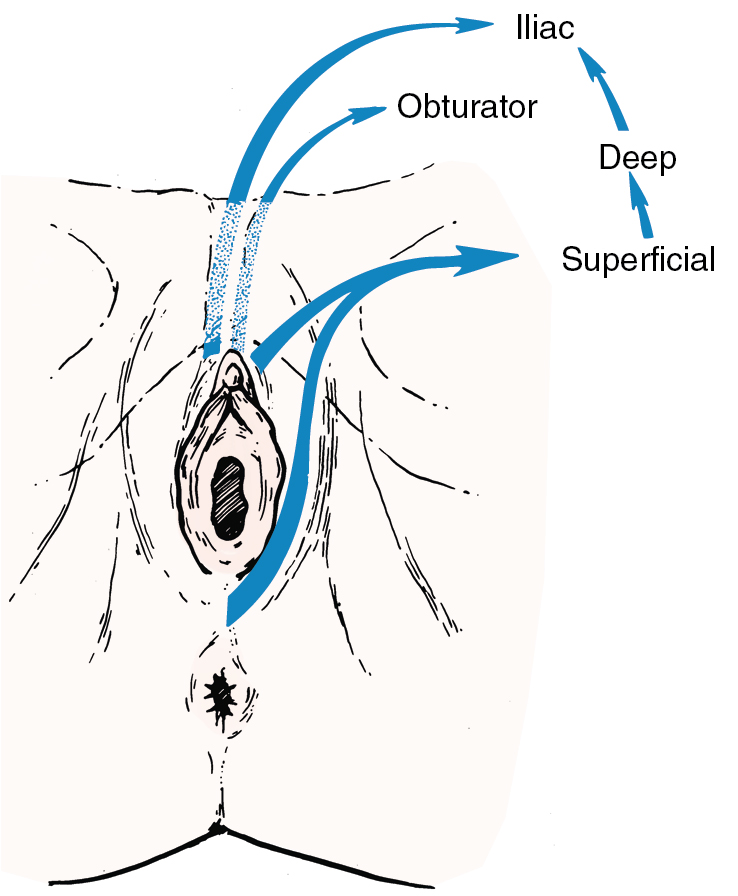
Fundamental to the understanding of therapy for invasive cancer of the vulva is thorough knowledge of the lymphatic drainage of this region. In general, the four histologic types of invasive cancer primarily metastasize via the lymphatic route ( Fig. 6.6 ). Lymphatic drainage of the external genitalia begins with minute papillae that connect to a multilayered meshwork of fine vessels that extend over the entire labium minus, the prepuce of the clitoris, the fourchette, and the vaginal mucosa up to the level of the hymenal ring ( Fig. 6.7 ). Ultimately, drainage proceeds to the superficial inguinal nodes, followed by the deeper inguino-femoral nodes. Drainage is usually limited initially to the medial upper quadrant of the femoral node group. The nodes are medial to the great saphenous vein above the cribriform fascia and in turn may drain secondarily to the deep femoral group. The next echelon of nodes is the pelvic/iliac nodes.
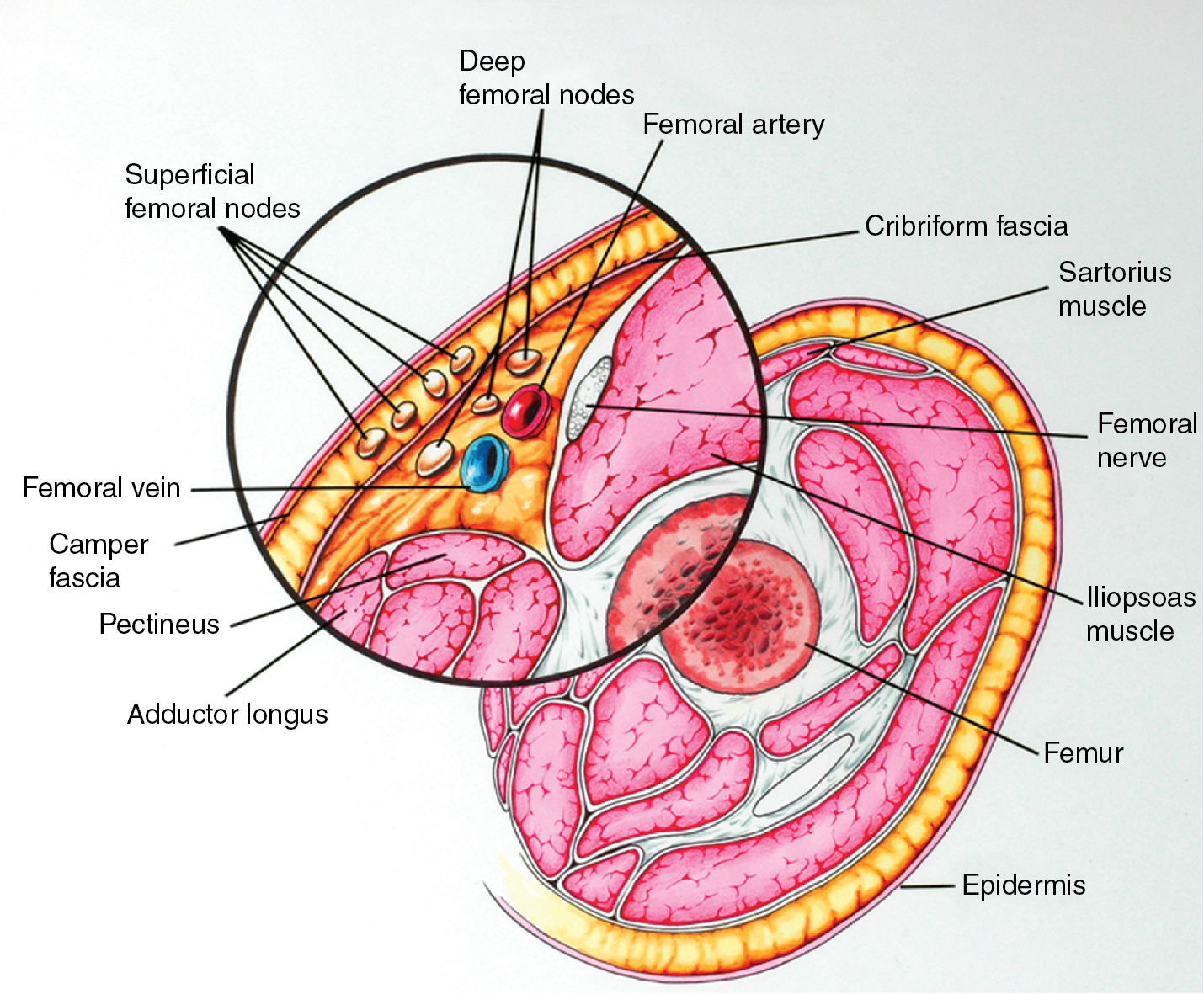
The inguinofemoral lymph nodes are contained in the femoral triangle, for which the boundaries include the inguinal ligament superiorly, the medial border of the adductor longus muscle medially, and the medial border of the sartorius muscle laterally. From lateral to medial, the contents of the femoral triangle are the femoral nerve and its terminal branches, the femoral artery, the femoral vein and its proximal tributaries (i.e., the great saphenous vein and deep femoral veins), and the inguinal lymph nodes and lymphatics.
The superficial inguinal lymph nodes are located immediately beneath the integument and Camper fascia, with an average of 8 to 10 in number. Most authors agree that these nodes are the primary drainage for the vulva, thereby containing the sentinel lymph node (SLN) ( Fig. 6.8 ). The deep femoral nodes, which are by classic teaching located beneath the cribriform fascia, are the secondary node recipients and are involved before drainage into the deep pelvic nodes. The Cloquet node , the last node of the deep femoral group, is located just beneath the Poupart ligament. The multilayered meshwork of lymphatics on the vulva itself is always limited to an area medial to the genitocrural fold ( Fig. 6.9 ). Lymphatic drainage of the vulva occurs via a progressive systematic mechanism, and therapy can be planned according to where in the lymphatic chain the tumor is present. Very posterior lesions that approach and involve the anus may not have the same lymphatic drainage as a vulvar cancer involving the labia or clitoris. Lymphatics drain to the mesorectal (inferior rectal) lymph nodes, followed by the inguino-femoral nodes, and finally to the pelvic nodal chain.
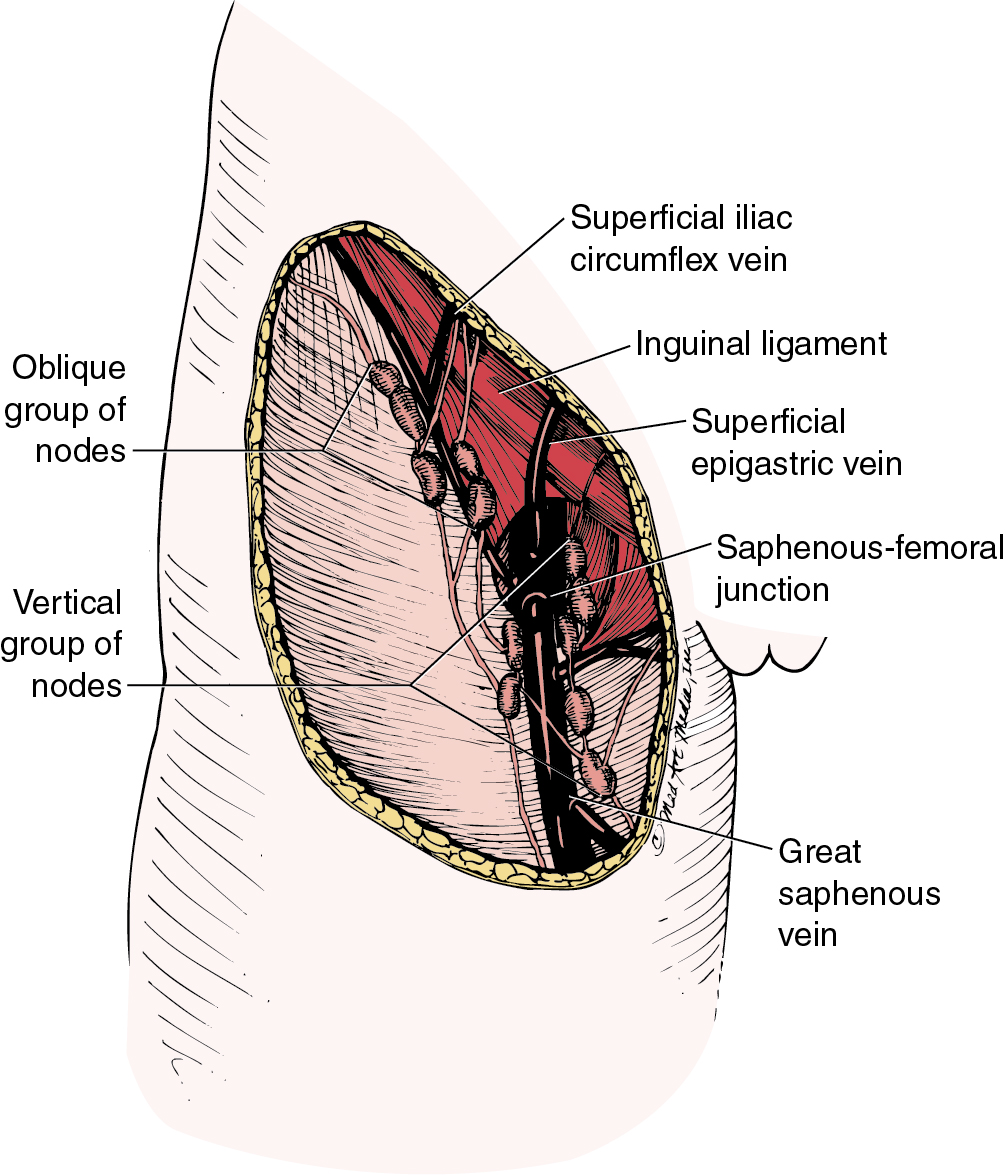
Borgno and colleagues examined 100 inguinal lymphadenectomy specimens at autopsy and demonstrated that the deep femoral nodes are always situated within the openings in the fascia at the fossa ovalis, and no lymph nodes are distal to the lower margin of the fossa ovalis under the fascia cribrosa. The clinical implication is that a carefully performed deep femoral lymphadenectomy does not require removal of the fascia lata (cribriform fascia) because no lymph nodes are to be found between the femoral vein and artery lateral to the artery or distal to the lower margin of the fossa ovalis beneath the cribriform fascia. They also found that the node of Cloquet or Rosenmüller, which is the uppermost node among the deep femoral lymph nodes, was absent in 54% of dissections. Borgno and colleagues also demonstrated that during surgery, when traction is persistently applied to the lymphovascular fat tissue above the cribriform fascia, all inguinal nodes can be removed. No nodal tissue was found when the cribriform fascia and the fat beneath were submitted separately for pathologic review. Hudson and colleagues confirmed this finding in cadaveric dissections, and Micheletti and coworkers found supporting evidence with embryologic studies.
Although lymphatics draining from the clitoris directly to the deep pelvic lymph nodes are described, their clinical significance appears to be minimal. It is unusual to find a case in which metastasis is present in the pelvic lymph nodes without metastatic disease in the inguinal lymph nodes even when the clitoris is involved. Curry and associates noted clitoral involvement in 58 patients; none had positive deep pelvic nodes without concomitant inguinal node involvement. Similar results were observed in a study by Ericksson and coworkers of 38 patients with carcinomas of the clitoris; they found also that the deep inguinal or femoral nodes were never positive in the absence of positive superficial inguinal nodes.
The incidence of positive inguinal and pelvic nodes varies considerably, as noted in Table 6.4 . Unfortunately, most of these studies were unstaged, although, in general, the larger the tumor, the greater the propensity for inguinal and pelvic node metastases. Morley noted a 21% incidence of lymph node involvement with a T1 lesion (<2 cm in diameter) versus 45% with a T2 lesion (>2 cm but limited to the vulva). Malfetano and colleagues reported that the incidence of inguinal node metastases in patients with stage III and stage IV lesions was 53% and 90%, respectively. Clinical evaluation of the groin is somewhat more accurate than tumor size. Homesley found that approximately 24% of patients with clinically nonsuspicious nodes had positive nodes when dissected, and approximately 75% of patients with suspicious (palpable, fixed, or ulcerated) groins had positive nodes. Table 6.5 demonstrates the association between inguinal lymph node involvement and tumor size, depth of invasion, and clinical examination. Of note, these data did not evaluate laterality or unifocal versus multifocal lesions.
| Series | Cases (n) | Positive Groin or Pelvic Nodes (%) | Positive Pelvic Nodes (%) |
|---|---|---|---|
| 374 | 37.0 | — | |
| 191 | 30.0 | 4.7 | |
| 122 | 50.0 | 10.0 | |
| 150 | 24.0 | — | |
| 277 | 29.2 | — | |
| 1553 | 31.0 | — |
| Feature | N | Positive Groin Nodes | % |
|---|---|---|---|
| Tumor Diameter | |||
| ≤2.0 cm | 190 | 36 | 19 |
| >2.0 cm | 390 | 163 | 43 |
| Invasion | |||
| ≤5 mm | 272 | 57 | 21 |
| >5 mm | 286 | 137 | 48 |
| Clinical Examination | |||
| N0-1 | 477 | 114 | 23 |
| N2-3 | 111 | 89 | 80 |
The application of SLN dissection has been integrated into the formal staging for vulvar cancer to decrease morbidity associated with a full inguino-femoral lymphadenectomy. The distribution of sentinel nodes has been evaluated and found that 100% of sentinel nodes are located over or medial to the femoral vessels. Rob and colleagues reported that in almost 60 vulvar cancer patients with tumors less than 4 cm, sentinel nodes were located superficially in almost 85% of cases, while the remainder were found deep to the cribriform fascia.
Staging
Many staging systems have been applied to invasive cancer of the vulva. Significant discrepancy exists between clinical and surgical-pathologic evaluation of lymph node status, specifically regarding clinical impression of lymph node positivity. Iversen demonstrated that overdiagnosis (clinically suspicious but pathologically negative nodes) occurred in 40 of 258 patients (15%). Of the 100 patients with metastasis to the inguinal lymph nodes, lymph node involvement was clinically unsuspected in 36 patients. Patients with “micrometastasis” (lymph node involvement not suspected clinically but positive microscopically) had a significantly better survival rate than did those with gross metastasis. These repeated findings suggest that staging be based on surgical-pathologic evaluation instead of clinical evaluation alone. The Federation of International Gynecologists and Obstetricians’ (FIGO) staging of vulvar cancer dates to 1969. In 1988, FIGO replaced the clinical staging system with a surgical-pathologic, or tumor–node–metastasis (TNM) classification system. The most recent 2009 FIGO staging for carcinoma of the vulva ( Table 6.6 ) further characterizes the number and type of lymph node metastases. Previous studies noted decreased survival among individuals with bilateral lymph node metastasis; thus, laterality was incorporated into the staging system. However, recent studies have demonstrated that the number of lymph node metastases and extracapsular spread are predictive of survival regardless of laterality. Some reports in the literature use older staging systems, and thus data must be evaluated within the context of the staging system used.
| Stage | Description |
|---|---|
| I | Tumor confined to the vulva |
| IA | Lesions ≤2 cm in size confined to the vulva or perineum and with stromal invasion ≤1.0 mm a ; no nodal metastasis |
| IB | Lesions >2 cm in size confined to the vulva or perineum with stromal invasion greater than 1.0 mm a ; no nodal metastasis |
| II | Tumor of any size with extension to adjacent perineal structures ( 1 ⁄ 3 lower urethra, 1 ⁄ 3 lower vagina, anus); no nodal metastasis |
| III | Tumor of any size with or without extension to adjacent perineal structures ( 1 ⁄ 3 lower urethra, 1 ⁄ 3 lower vagina, anus) with positive inguinofemoral lymph nodes |
| IIIA |
|
| |
| IIIB |
|
| |
| IIIC | With positive nodes with extracapsular spread |
| IV | Tumor invades other regional ( 2 ⁄ 3 upper urethra, 2 ⁄ 3 upper vagina) or distant structures |
| IVA | Tumor invades any of the following: |
| |
| |
| IVB | Any distant metastasis, including pelvic lymph nodes |
a The depth of invasion is defined as the measurement of the tumor from the epithelial–stromal junction of the adjacent most superficial dermal papilla to the deepest point of invasion.
Donaldson and colleagues demonstrated that lesion size predicted incidence of lymph node metastasis (19% metastasis for lesion <3 cm and 72% for lesion >3 cm). Likewise, tumor grade correlated with node metastasis (one-third of well-differentiated tumors had metastasis compared with 75% for poorly differentiated lesions). Of 38 patients, 11 (29%) had node involvement if invasion of the primary lesion was 5 mm or smaller compared with 17 of 28 (61%) if invasion was larger than 5 mm. If the tumor did not involve lymphatic or vascular spaces, only 2 of 33 (6%) had positive nodes, but 26 of 33 patients (79%) with lymphatic or vascular space involvement had metastasis to the regional lymph nodes. None of 25 patients with lesions invading less than 5 mm and without lymphatic or vascular space involvement had lymph node metastasis.
Depth of tumor invasion is related to risk of nodal metastasis as well. In superficially invasive disease (< 1 mm), the risk of nodal spread is low, roughly 1% ( ). As invasion deepens, so does the risk of nodal spread. Gonzales et al. reported at 2 mm depth of invasion, the nodal spread risk increases to 7% to 8%, at 3 mm invasion the nodal metastasis is 12% to 17%, and at 5 mm depth of invasion, nodal spread approaches 17%. These data are important for staging recommendations, as minimally invasive disease of 1 mm or less, or stage 1A, are not recommended to undergo surgical lymph node dissection (LND) given the low risk of nodal spread (< 1%). Given the increased risk of nodal spread to 8% for stage IB patients (with depth of invasion of more than 1 mm), lymphadenectomy is recommended.
Tabbaa and associates reported the largest cohort study to evaluate the effect and prognostic performance of the 2009 FIGO staging. They reported FIGO stage I, II, III, and IV 5-year overall survival (OS) rates of 84%, 75%, 48%, and 9%, respectively. The 5-year OS rate was 85% for patients without LN metastasis, and for patients with three or more lymph node metastases, the 5-year OS rate was 30%. They surmised that bilateral nodal disease does not appear to impact cause-specific survival, justifying its omission from the 2009 staging system, and that separating node-positive stage III from node-negative stage II cases appears justified.
Molecular markers and tumor etiology
Meaningful interpretation of the molecular landscape in which vulvar SCC develops remains a challenge. Immunohistochemistry (IHC) has been utilized to investigate molecular pathological markers and oncologic outcomes; however, results have been conflicting or not particularly impactful for patient management. There are two clear subtypes of vulvar SCC based on etiology: HPV-dependent and HPV-independent. Recent molecular data from the AGO-CaRE-1 trial have suggested three molecular subtypes of vulvar SCC, with differences in prognosis. Woelber et al. found that while HPV-independent cancers were often associated with the vulvar dermatoses of lichen sclerosis and harbored TP53 mutations, HPV-dependent cancers were shown to over-express p16, which was suggested as a surrogate marker for HPV-induced malignant transformation. Of the 648 tumor samples analyzed, they found that p16 IHC was positive in 30.2%, and HPV DNA was detected in 74.8% of the p16+/p53-negative tumors, with HPV 16 as the most common subtype (85.1%). p53 IHC was positive in 31.3%. They found a large third molecular group, however, in which neither p16 nor p53 over-expression was noted. Additionally, they found that these molecular subgroups had different prognostic implications. Patients with p16+ cancers were younger (63 vs. 70 years for p16- cancers), had lower rates of lymph node involvement (29% vs. 39.7%), and had lower recurrence rates. Oncologic outcomes were impacted as well, as the p16+ group had significantly improved 2-year disease-free survival (DFS) (65.5% vs. 47%, P < .001) and 2-year OS (82.7% vs. 70.4%, P = .003) compared to p53+ group. A third molecular subtype of vulvar SCC was identified in the AGO-CARE-1 translational data. This subgroup was found to be both p16 and p53 negative on IHC. This subgroup was found to have an intermediate prognosis with a 2-year DFS of 53% and 2-year OS of 72.6%. More studies are needed to investigate this third subgroup of vulvar SCC. The authors conclude that there is a clear positive prognostic impact on oncologic outcomes based on HPV presence, and while p53/p16 IHC biomarker data may not guide treatment at this point, it can offer prognostic information and guide more (in p53+ tumors) or less intense surveillance practices.
Sentinel lymph node biopsy
Sentinel lymph node biopsy (SLNB) represents the most significant innovation in the care of patients with vulvar cancer in the past decade. The use of the SLN technique in the evaluation of vulvar cancer was first described in the late 1970s based initially on the experience in penile cancers, followed by the successes reported in breast cancer and melanoma. The past 20 years have witnessed a myriad of reports featuring SLN techniques in the management of gynecologic cancers, including vulvar cancer, that have provided experienced clinicians with a standard of care option for surgical lymph node management in appropriately selected patients. The concept of SLNs depends on reliable prediction of a tracer substance identifying the primary nodal drainage of the tumor. The assumption is that if the SLN is negative for malignancy, then the other nodes will be negative, thus precluding the need for full nodal dissection and thereby significantly reducing the procedural related short-term infection, wound breakdown, and long-term edema associated with inguinal femoral lymph node dissection (IFLND).
The modern SLN concept requires a team comprised of the surgeon, the radiologist, and the pathologist. A 0.2 to 0.5 cc injection of radiocolloid ( 99m Tc-SC filtered or unfiltered) is administered peri-lesionally, subcutaneously, and intradermally, and the images can be viewed online in the operating room (or hard copy films can be prepared). The surgeon prepares 2.5 mL of 1% lymphazurin (isosulfan blue) and using a 25-gauge needle, the dye is injected into the dermis at the junction of the tumor and normal vulvar skin. Gentle massage at the injection site helps with dye uptake. The dye is visible in the field for 30 to 45 minutes following injection, and the groin incision should be made a minimum of 5 minutes following injection of dye. The surgeon reports the location of the sentinel, whether it was hot or cold by the Geiger counter, whether it was blue or not blue, consistency (i.e., firm vs. soft), and size. Midline lesions require bilateral groin dissection, and therefore the primary lesion must be injected at the two points closest to the respective groin.
Only approximately 30% of early-stage vulvar cancers have positive nodes with IFLND, thus exposing many women to ostensibly an unnecessary morbid procedure wherein historically up to two-thirds of patients experience lymphedema. More recently, Carlson and colleagues published the results of the Lymphedema and Gynecologic (LEG) study (Gynecologic Oncology Group [GOG] 244) and reported the incidence of lymphedema in vulvar cancer patients at 43%. Therefore, reducing the radicality of groin lymphadenectomy by SLNB merits implementation as a reliable alternative to full nodal dissection that requires balancing the slightly higher groin recurrence rates of SLNB versus the significantly greater morbidity of IFLND.
Concerns raised in adopting SLNB as the standard of care include, first, the reliability of SLN detection. How feasible is this technique in terms of how often one detects an SLN, and which technique should be used in terms of injection site and tracer selection? Second, what is the reliability of a negative SLN to predict that all the other inguinal nodes are likewise negative for malignancy? In other words, what is the potential for a false-negative result, whereby an SLNB does not demonstrate metastases, but additional groin dissection discovers lymphatic spread? Finally, what is the groin recurrence rate? This question is critical because untreated groin metastases will result in groin recurrences that are most often fatal. It is important to recall that the gold standard for the rate of groin relapse in a node-negative groin is historically 0% to 2% based on Homesley et al.’s GOG experience and others. The data for detection rate, sensitivity, or false-negative results can be considered from the perspective of each groin, viewed as a separate entity, or from the patient’s perspective, whereby the outcomes of SLNB are considered for both groins as a unit.
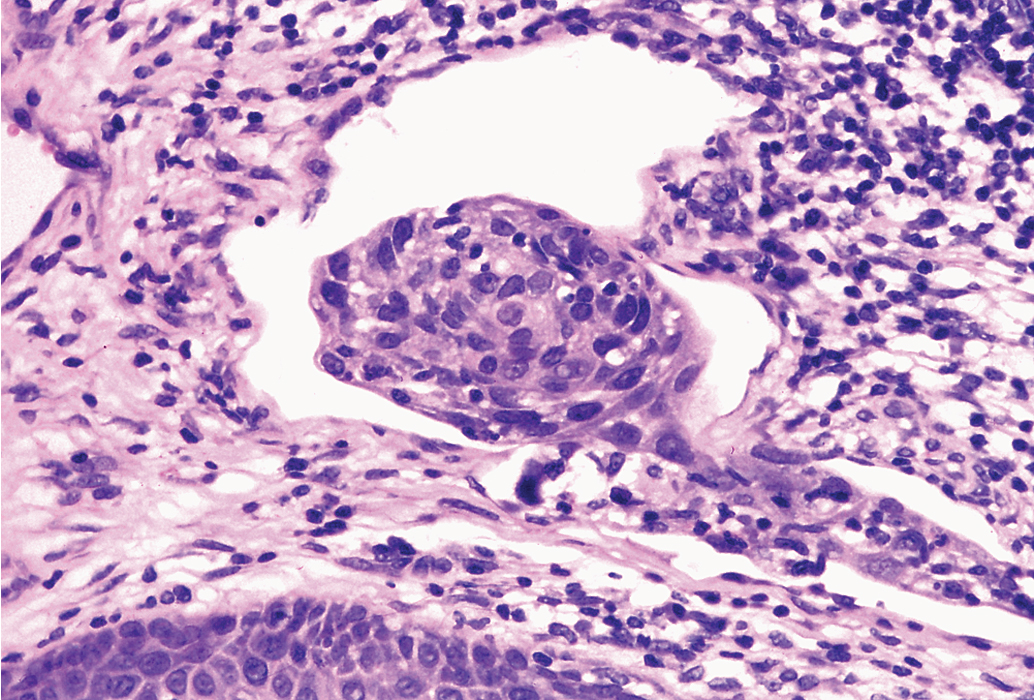
Another important concept is the role of nodal ultrastaging with very fine sectioning and use of IHC as an adjunct to conventional staining, in which the SLN is subjected to far greater scrutiny in terms of pathologic sectioning than nodes removed via traditional IFLND. Ultrastaging is comprised of bisecting the sentinel node on the long axis and then preparing 2 to 3 mm slices that can then be stained with hematoxylin & eosin and IHC for cytokeratin AE1:AE3. Isolated tumor cells (ITC) are single cells or clusters less than 0.2 mm, and micrometastases are denoted when the tumor is greater than 0.2 mm but less than 2 mm.
Nodal yield from traditional IFLND is much greater in number, and these nodes are not usually subjected to these labor-intensive techniques because of time and resource constraints. Also, a statistical concept known as the false-negative predictive value (FNPV), defined as 1- Negative predictive value, needs to be understood to properly consider the burgeoning SLNB literature in the management of vulvar cancer.
Several small studies were instrumental in establishing the feasibility of SLNB, with the original lymphatic mapping data being published by Levenback and colleagues in 1994. Recent studies have continued to demonstrate improved outcomes for SLNB over time. Initial mapping studies of SLNB in vulvar cancer evaluated 21 patients with isosulfan blue dye only, and an SLN was identified in only 86% of patients and 66% of groins. Addition of lymphoscintigraphy (LSG) with radiolabeled technetium significantly improved the SLN detection rate. A collective review of the literature in 2002 noted a detection rate of 92% with the combined technique.
One of the first large prospective multicenter observational trials to assess SLNB was the GROINSS-V I study reported by Van der Zee and associates that included 403 assessable patients using the combined technique. Eligible patients had T1 or T2 lesions smaller than 4 cm, invasion greater than 1 mm, and clinically nonsuspicious inguinofemoral lymph nodes. SLNB was performed, and if the SLN result was negative, then an IFLN was not performed; only when SLN metastases were identified was an IFLND performed either at the time of surgery based on frozen section or subsequently based on final pathologic analysis of the SLN. A total of 623 groins in 403 patients were evaluated. The authors selected 276 selected patients with unifocal vulvar disease and a negative SLN to describe in detail, and the 3-year survival rate was 97%, with a mean follow-up of 35 months. Adjuvant radiotherapy was administered to the groin and pelvis when more than one lymph node demonstrated metastasis or extracapsular nodal tumor growth was identified. Those who underwent SLNB had an FNPV of 2.9% with a false-negative rate of 6%. The groin recurrence rate for those with negative SLNs was 2.3% (6 of 259 patients). Patients with unifocal lesions had only a 2% recurrence rate compared with 12% for those with multifocal lesions on the vulva. Lymphedema was noted in 2% for SLNB versus 25% in those with positive SLN who underwent full IFLND. Wound breakdown and recurrent cellulitis occurred in 12% and 0.4% versus 34% and 16% for SLNB versus completion IFLND procedures, respectively. Ultrastaging with three sections per mm was performed only on SLNs that were negative on routine histopathologic examination. Ultrastaging significantly increased detection of nodal metastases. Routine pathologic examination detected 95 (58%) of the 163 groins with metastatic SLNs, and ultrastaging detected the remaining 68 (42%) groins demonstrating metastatic SLNs.
A subset analysis of the GROINSS-V I study conducted by Oonk and colleagues demonstrated that the risk of non-SLN metastases increased with increasing size of SLN metastasis (4.2% with ITC and up to 62.5% with SLN metastases >10 mm). Also, the risk of non-SLN metastases was higher when the SLN was found to be positive with routine pathology rather than with ultrastaging. The 5-year disease-specific survival rates were 97% for women with ITC in the SLN, 88% for those with SLN metastases smaller than 2 mm, 70% for those with metastases measuring 2 to 5 mm, and 69% for those with metastases larger than 5 mm. No cutoff provided complete assurance that the non-SLNs would be free of metastases, thus given the high risk of mortality if such nodes are missed, additional groin treatment remains the current recommendation for all patients with SLN involvement.
GOG 173 reported by Levenback and associates included 452 women who underwent SLNB followed by IFLND irrespective of SLNB findings. Eligibility criteria included SCC confined clinically to the vulva with at least 1 mm of invasion and tumor size up to 6 cm in greatest diameter. Patients with groin nodes that were clinically suggestive of cancer were excluded. Every woman underwent intraoperative lymphatic mapping using isosulfan blue dye; however, 2 years into the study, the protocol was amended to require preoperative LSG and intraoperative radiolocalization, which previously had been optional. Nodes were subjected to ultrastaging by serially sectioning them into 3-mm blocks with at least two sections 40 microns apart per block. The use of frozen section was discouraged. If routine hematoxylin and eosin staining was negative for metastatic disease on the first slide, IHC cytokeratin staining was performed on the second slide. A total of 772 groin dissections were performed (bilateral = 320 and unilateral = 132) in 452 women, of whom 418 exhibited at least one SLN. Incidence of node positivity among women with at least one SLN identified was 32%. Of the 132 women with positive nodes, 11 had false-negative findings on SLNB (8.3%; 90% confidence interval [CI], 4.7% to 13.4%); thus, the sensitivity was 92%. The negative predictive value (NPV), was 96.3%. Importantly, this study showed that the false-negative rate for SLNB increased significantly when tumor size was 4 cm or larger (7.4% vs. 2% for tumors <4 cm). They also demonstrated a favorable FNPV of 3.7%, which was the primary statistical endpoint of this trial. This FNPV is comparable to that observed in breast cancer, but salvage rates for that disease are more favorable. This study highlighted the role of pathologic analysis as 23% of the positive nodes were missed on conventional staining and required IHC to detect cancer. SLN ultrastaging is also important via step sectioning, which significantly increases the chance of finding metastases, according to Puig-Tintore et al.
Coleman et al. published a subset analysis of patients from GOG 173 that examined the importance of the location of the primary tumor relative to the midline and the role of LSG, which was optional in this study. A total of 234 patients were eligible, with 64 having lateral lesions (>2 cm from midline) and thereby undergoing only unilateral IFLND after SLNB; the 105 women with midline and 65 with near-midline lesions (<2 cm distance from midline) underwent bilateral completion IFLND. Bilateral groin drainage using LSG was discovered in 22% of lateral, 58% of near-midline, and 70% of midline tumors. At mapping, no SLNs were found in contralateral groins among patients with near-midline and midline tumors who had unilateral-only LSGs, yet groin metastases were found in four of 32 patients with midline tumors undergoing contralateral dissection; none were found in 27 patients with near-midline tumors. They concluded that the likelihood of detectable bilateral drainage using preoperative LSG decreases as a function of distance from midline. Patients with near-midline primary tumors exhibiting unilateral drainage on LSG may safely undergo unilateral SLN. Further validation of this concept is indicated.
A Cochrane review of SLNB in vulvar cancer management was reported by Lawrie et al. that assessed the diagnostic test accuracy of various techniques using traceable agents for SLNB to diagnose groin lymph node metastasis in women with FIGO stage IB or higher vulvar cancer. A total of 34 studies evaluating 1614 women and approximately 2396 groins were included in the analysis. Use of blue dye only provided a sensitivity of 0.94 (68 women; 95% CI 0.69 to 0.99); for mixed tracers, sensitivity was 0.91 (679 women; 95% CI 0.71 to 0.98); and for technetium only, it was 0.93 (149 women; 95% CI 0.89 to 0.96). Sensitivity for combined tests was 0.95 (390 women; 95% CI 0.89 to 0.97). NPVs for all index tests exceeded 95%. Mean detection rate for blue dye alone was 82%, compared with 95%, 96%, and 98% for mixed tests, technetium only, and combined tests, respectively. It was estimated that for 100 women hypothetically undergoing SLNB assuming groin metastases prevalence of 30% for the combined or technetium only tests, one and two women with groin metastases might be “missed,” and for mixed tests, three women with groin metastases would occur as false-negative results. This review concluded that there are only minor differences in diagnostic test accuracy between the technetium and combined tests. Combined testing may reduce the number of false-negative results compared with technetium alone. It must be recognized that there is a learning curve to successful SLNB, and combined tests may further increase detection for less experienced surgeons. Blue dye alone may be associated with more false negatives (FNs) compared with tests that include technetium. They further concluded that SLNB would reduce the need for full IFLND by 70% in patients with early vulvar cancer, but long-term survival data are needed.
A systematic review was presented by Meads et al. to assess the accuracy of SLNB with technetium 99 and/or blue dye–enhanced LSG. A total of 29 studies that included 1779 women were analyzed. Most studies used technetium-99 combined with blue dye. Pooling when appropriate revealed mean SLN detection rates of 94% for technetium, 69% for blue dye alone, and 98% for both. SLNB revealed a pooled sensitivity of 95% (95% CI 92% to 98%) with an NPV of 97.9% in studies using technetium with blue dye, ultrastaging, and IHC with inguinal lymphadenectomy as reference. Less morbidity was reported for those undergoing SLNB. The authors concluded that SLNB should be done in carefully selected patients with technetium and blue dye with ultrastaging and IHC. They further concluded that patients must make an informed choice between the slightly higher groin recurrence rates of SLNB versus the higher morbidity with full lymphadenectomy.
Another systematic review by Hussanzade et al. included 49 studies and reported a false-negative rate by patient and groin perspectives of 8%. Pooled NPVs were 97% for patient- and groin-based analyses. They pointed out that those with palpable groin nodes had lower detection rates and sensitivity with SLNB. They concluded that SLNB with radioactive tracer plus blue dye was accurate in appropriately selected patients with exclusion of those with clinically suspicious inguinal nodes. They also raised the concern of an increased FN rate for midline tumors.
Slomovitz and colleagues performed a review that declared SLNB as the standard of care for early-stage vulvar cancer secondary to lymphedema rates of 30% to 70% after traditional IFLND combined with the robust prospective data from GOG 173 and the GROINSS-V I studies that confirmed the feasibility and utility of SLNB with similar false-negative rates of 6% for tumors smaller than 4 cm. Important differences between these studies include the fact that GROINSS-V I had smaller tumors (T1 or T2 <4 cm) versus GOG 173, in which T1 and T2 tumors measuring 2 to 6 cm were included. Mapping techniques also differed because GROINSS-V I required both dye and tracer and a minimum threshold of 10 cases for skill verification versus blue dye only, which was initially required with no skill verification in GOG 173. The authors concluded that the groin relapse rate attributable to a false-negative SLNB for primary vulvar tumors smaller than 4 cm was less than 3%.
Covens and associates convened a working group panel that performed an additional systematic review with meta-analysis. They reported a per groin detection rate for SLNB using radiocolloid tracer and blue dye of 87% and a false-negative rate of 6.4%. They recommended SLNB for women with unifocal tumors smaller than 4 cm with clinically nonsuspicious groin nodes, provided that conditions of specific infrastructure and SLN experience and volume are met. The groin recurrence rates are 6.6% for superficial IFLND and 1.4% for complete IFLND versus 3.4% for SLNB. Expert panel recommendations from this paper included using radiocolloid with blue dye and not using blue dye alone secondary to low detection rates. Four quadrant intradermal injections into normal tissue at the margins of the tumor were recommended. Near-infrared tracers (e.g., indocyanine green [ICG]) and the role of frozen sections require further study. Radiocolloids can be injected 30 minutes to 24 hours before surgery, depending on the size of the radiocolloid and manufacturer directions, and blue dye should be injected in the same location as the radiocolloid after induction of anesthesia. A node that has more than five times the background radioactivity should be used to identify an SLN. Pathologically, the panel advocated for SLN ultrastaging by serially sectioning into 3-mm blocks and at least two sections 40 μm apart from each block. If routine hematoxylin and eosin staining is negative for metastatic disease on the first slide, IHC cytokeratin staining should be performed subsequently. Finally, the panel endorsed omission of IFLND in the contralateral side to a positive node when the SLN has been negative in that contralateral side, although they acknowledged that data are limited.
Some issues with SLNB remain less clear, but data are emerging. What is the risk of contralateral non-SLN metastasis in patients with unilaterally positive SLNs? Woelber and colleagues addressed this question in 140 patients, 124 with bilateral and 16 with unilateral SLN dissection. A median number of two SLNs with a range of one to seven per groin were dissected. Of 33 patients with unilaterally positive SLNs, 28 (85%) underwent complete bilateral IFLND despite a contralateral negative SLN, and none demonstrated contralateral non-SLN metastasis. Of the remaining five patients, three received postoperative radiotherapy to the groins. One woman (3.6%), however, did develop groin recurrence in the initially SLN-negative, fully dissected groin after 19 months. These data support the omission of contralateral IFLND if negative contralateral SLN is found, but clearly, further study is needed before altering current guidelines that mostly advocate for bilateral IFLND in these circumstances because these numbers are small and possibly confounded by groin irradiation.
Another unanswered question with SLNB heretofore has been the effect on long-term survival. This issue was recently addressed by te Grootenhuis and associates, who reported the long-term follow-up from the GROINSS-V I study with a median follow-up period of 105 months. The overall local vulvar recurrence rates were 27% at 5 years and 40% at 10 years after primary treatment. These recurrence rates are high considering that these were patients with unifocal tumors smaller than 4 cm. Notably, 89% of patients underwent wide excision versus only 10% who underwent radical vulvectomy for the primary tumor. For SLNB-negative patients, recurrence rates were 25% and 36% at 5 and 10 years, respectively, but SLNB- positive patients demonstrated 33% and 46% recurrence rates at 5 and 10 years, respectively. A total of 39 (15%) SLNB-negative patients underwent IFLND secondary to a local recurrence. The isolated groin recurrence rates were 2.5% for SLNB-negative patients and 8% for SLNB-positive patients at 5 years of follow-up. The overall 10-year disease-specific survival rates were 91% for SLNB-negative patients versus 65% for SLNB-positive patients. For all patients, 10-year disease-specific survival rate decreased from 90% for patients without a local recurrence to 69% for those with a local recurrence. Klapdor and colleagues reported on a subgroup analysis of the AGO-CaRE-1 study and compared oncologic outcomes of patients with tumors less than 4 cm who underwent SLN dissection or radical lymphadenectomy with negative findings for SLN and LN metastases ( N = 556). While the full IFLND group had larger tumors (20 mm vs. 13 mm, P < .001) and deeper invasion (4 mm vs. 3 mm, P = .002), the isolated groin recurrence rates did not differ between groups (SLN 3% vs. IFLND 3.4%, P = 0.845). Multivariate analysis after controlling for tumor characteristics revealed no statistical difference in progression-free survival (PFS) (hazard ratio [HR] 0.97, 95% CI 0.51 to 1.82) or OS (HR 0.69, 95% CI 0.26 to 1.84) between SLN dissection and radical IFLND in node-negative patients with tumors less than 4 cm.
Additional questions with SLNB remain, including optimal imaging techniques such as ultrasonography, computed tomography (CT), magnetic resonance imaging, positron emission tomography/CT, and most recently, single-photon emission computed tomography (SPECT) preoperatively, that would best exclude clinically suspicious nodes that are thought to considerably increase the FN rate for SLNB. Nodes that are totally replaced by tumor may not be identified by mapping because of blockage of lymphatic channels by tumor, thereby resulting in a false-negative result. The role of prior local recurrences and prior SLNB also is poorly understood in terms of the accuracy of repeating SLNB. Furthermore, the clinical consequences of micrometastases are unclear. As SLN metastasis size increases, the probability of non-SLN metastases increases, which correlates with decreasing survival. SNLB appears to be cost-effective in separate reports (McCann et al. and Erickson et al.); nonetheless, Sutton and associates performed a model-based economic analysis that clearly showed that IFLND was most cost-effective and dominated all other strategies considering 2-year OS, but when morbidity-free outcomes were considered, then SLNB using a combined identification technique with ultrastaging and IHC was superior.
Future directions in optimizing SLNB will include development of novel tracers and dyes such as fluorescent dyes (e.g., ICG) for enhanced SNL identification. Additionally, GROINSS-V-II-GOG270 has recently resulted and investigated the role of adjuvant groin irradiation as a substitute for completion IFLND in those with positive SLNB. It is noteworthy that this trial suspended accrual in 2010 (after 54 months of inclusion) because of higher than allowable groin recurrences in the cohort of patients with positive SLNB who received adjuvant radiotherapy. At the time of suspension, 9 groin recurrences were identified in 82 such patients. Extensive analysis of these cases revealed that tumor metastases size (≥2 mm) and extranodal extension were associated with a statistically higher rate of groin recurrence. The trial was reopened for accrual after amending the eligibility criteria with only those with micrometastases (≤2 mm) remaining eligible for radiation (50 Gy); those with macrometastases (>2 mm) were to undergo IFLND. The results of this trial were recently presented at the Society of Gynecologic Oncology Annual Meeting in March 2020 by Slomovitz, van der Zee, Oonk and colleagues. Overall, this trial represents the largest prospective surgical trial in vulvar cancer to date, with 1540 eligible patients enrolled. There were 1218 patients with a negative SLN and 322 with a positive SLN. In the SLN-negative group, after a median follow-up of 24.3 months (0 to 73.7), 171 patients experienced a recurrence (14%), 14 of those who also experienced a groin recurrence (1 patient had an isolated pelvic node recurrence). Of the 36 isolated groin recurrences (2.9% at 2 years; 95% CI 2.1% to 4.1%), 10 had protocol violations (including 1 incomplete treatment of the groin, 1 primary tumor >4 cm, 3 with not all SLNs on imaging removed, and 2 with false-negative pathology). With the protocol violations excluded, there was a 2.2% (95% CI 1.4 to 3.1) isolated groin recurrence rate among negative SLN patients. Overall, the disease-specific survival was 97.9% at 2 years for the negative SLN group. The investigators conclude that omitting IFLND in patients with vulvar cancer less than 4 cm and a negative SLN is safe. There were 160 patients with a positive micrometastasis on SLN. After a median follow up of 23.9 months (range 0.3 to 68.4 months), 127 patients underwent radiotherapy, 15 patients underwent an IFLND (4 of which also had radiation), and 18 patients had no treatment for various reasons. Excluding those patients who also had an IFLND, patients with positive micrometastatic SLNs experienced an isolated groin recurrence after 2 years at a rate of 1.8% in the radiation group as opposed to 15.4% in the no adjuvant therapy group ( P = .009). The mean size of metastasis in the radiation group was 0.76 mm compared to 0.63 mm in the no radiation group ( P = .87). There were 162 patients with positive macrometastatic SLNs, of which 50 were prescribed just radiation prior to the protocol amendment, and 115 patients who underwent IFLND after the protocol amendment (ultimately 52 underwent radiation, 104 underwent IFLND, and 6 patients had no treatment). After 22.5 months median follow up (range 0 to 40.5), in patients with a positive macrometastatic LN, the isolated groin recurrence rate at 2 years was 25.0% in the radiation group compared to 8.2% in IFLND group ( P = .012). The investigators conclude that radiation to the groin in patients with SLN metastasis ≤2 mm results in very low groin recurrence rate (1.6%), the toxicity of radiation was minimal in most patients and with less frequent long-term morbidity compared to IFLND, and finally for patients with SLN metastasis greater than 2 mm, radiation with a total dose of 50 Gy is not a safe alternative to IFLND. The next question to be answered is whether chemoradiation is a safe alternative to IFLND in patients with macrometastatic SLNs. This question is being addressed in the upcoming GROINSS-V III prospective phase II trial in which radiation boost to 56 Gy with weekly cisplatin will be prescribed to this patient population.
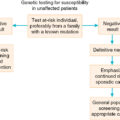
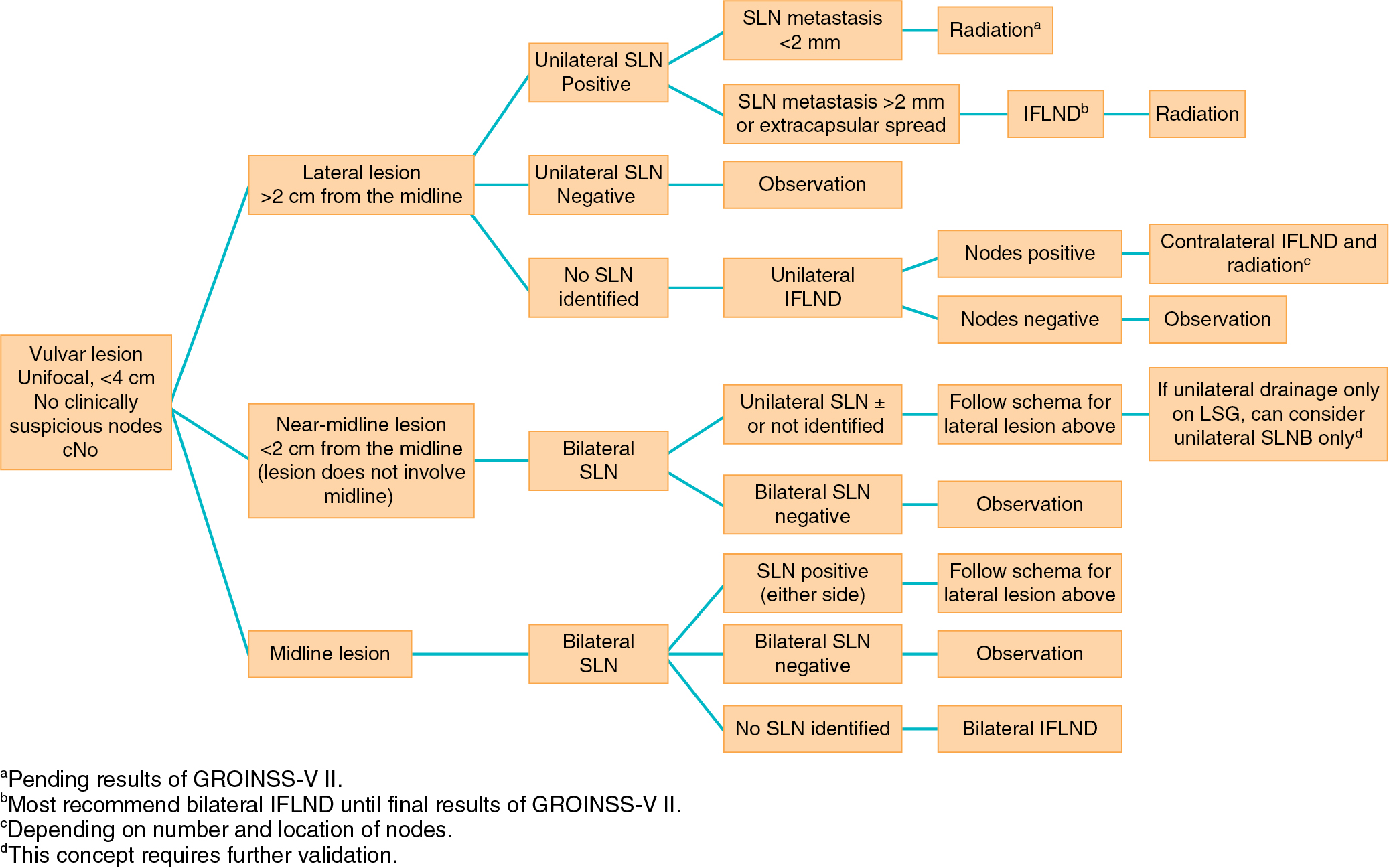
In summarizing, if an SLN is not identified and in any case in which the mapping procedure appears inadequate, SNLB should be abandoned in favor of complete IFLND. Patient selection is crucial to success. Patients should have a negative metastatic workup. Table 6.7 summarizes factors that affect the success of SNLNB. Ideal candidates have unifocal T 1 or T 2 primary tumors smaller than 4 cm in diameter and clinically nonsuspicious groin nodes. SNLB performance characteristics from select studies are summarized in Table 6.8 . The standard has been that if a positive SLN is found on one side, then a complete groin dissection should be done bilaterally; however, emerging evidence supports omission of full contralateral IFLND if the contralateral SLN is negative. Fig. 6.10 proposes a potential management algorithm for SLNB. Clearly, these management schemes require individualization and constant vigilance of emerging literature that may alter recommendations.
|
| Author, Study, Year | Patients (n) | SLN Detection Rate (%) | Sensitivity (%) | NPV (%) |
|---|---|---|---|---|
| 403 | NR a | 98 | 97 | |
| 452 | 93 | 92 | 96 b 98 c | |
| 1614 (aggregated) | 98 | 95 | 98 | |
| 1779 (aggregated) | 97.7 | 95 | 97.9 | |
| 981 (aggregated) | 94.4 | 92 | 98 | |
| 1087 (aggregated) | 87 | 94 | NR |
Technique of inguinofemoral lymphadenectomy
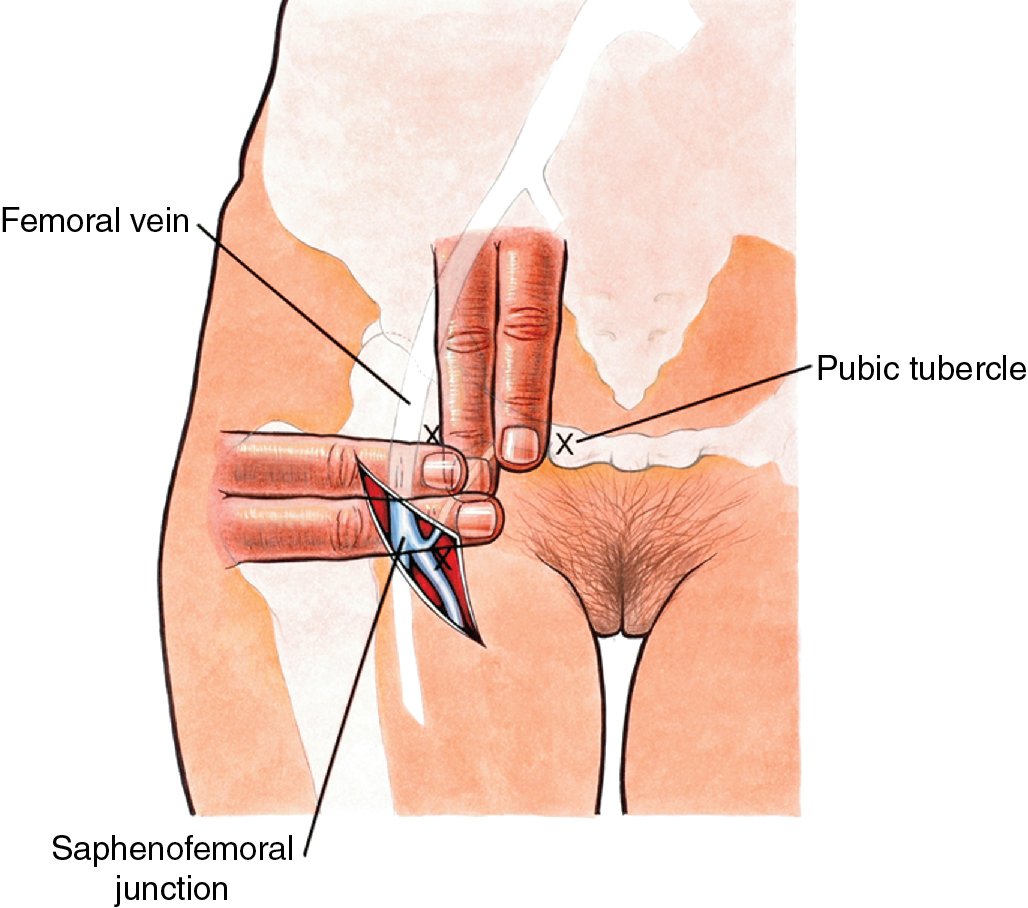
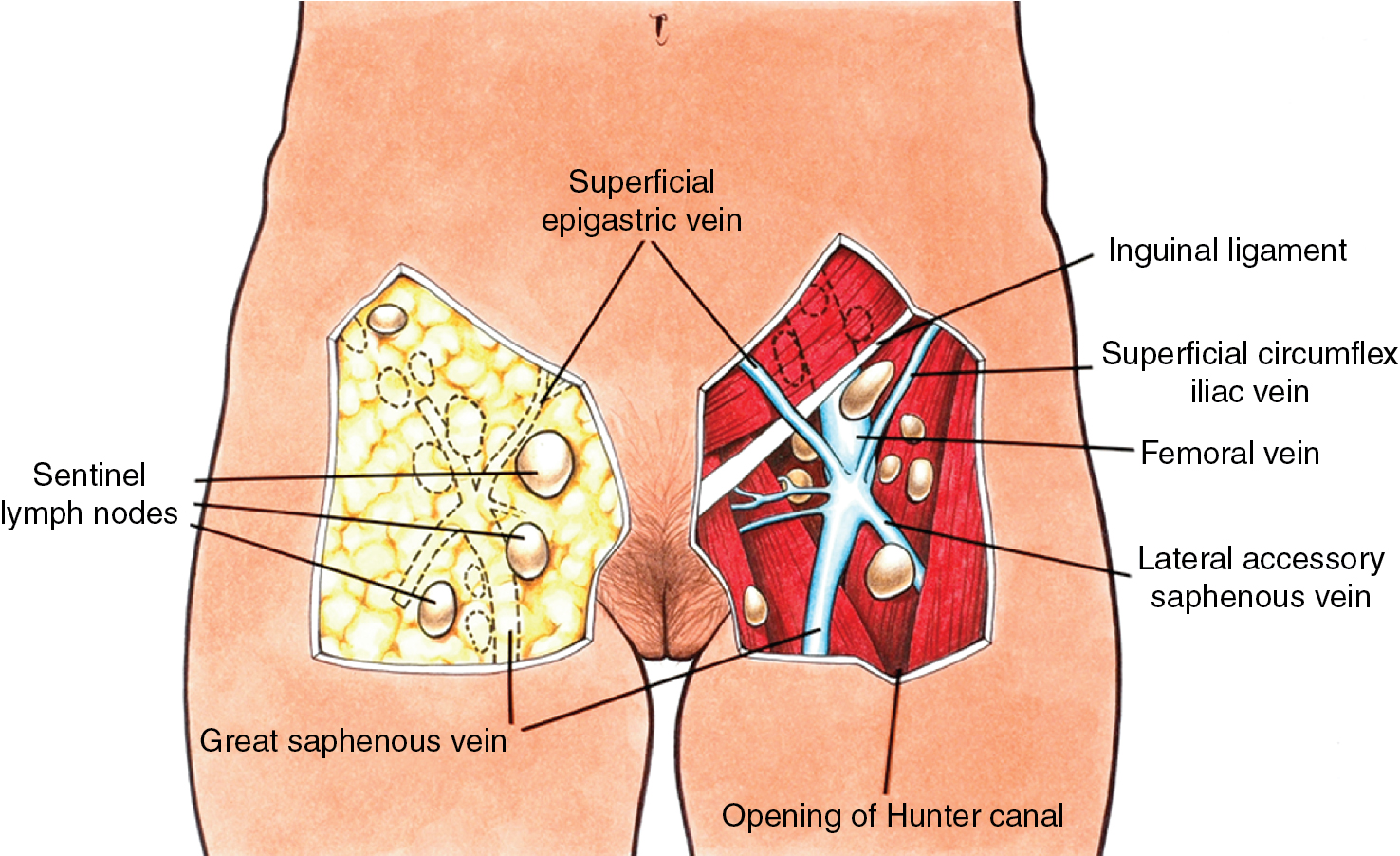
Although most women with clinical early-stage vulvar cancer will be candidates for SLN identification, complete inguinofemoral lymphadenectomy may be required for patients who fail to map and/or those with bulky adenopathy deemed resectable by the surgeon. An 8-cm incision is made parallel to the inguinal ligament two fingerbreadths (4 cm) beneath the inguinal ligament and two fingerbreadths (4 cm) lateral to the pubic tubercle ( Fig. 6.11 ). The incision is carried down through the Camper fascia, and at this point, skin flaps are bluntly and sharply dissected superiorly and inferiorly, allowing access to the fat pad containing the superficial nodes. The SLNs are in the fatty layer of tissue beneath the Camper fascia, in part anterior to the cribriform plate and protruding from beneath the fascia lata ( Fig. 6.12 ). The dissection should be carried superiorly to the inguinal ligament and inferiorly to a point approximately 2 cm proximal to the opening of the Hunter canal. The dissection should be carried laterally to the sartorius muscle and medially to the adductor longus muscle fascia ( Fig. 6.13 ). Blunt dissection facilitates identification of the cribriform fascia, which is most easily identified just below the inguinal ligament or in the area of the saphenous opening. The cribriform fascia unites with the fascia lata, and thus is contiguous with the fascia on the surface of the adductor longus and sartorius muscles; this may facilitate its identification. The portion of the fascia covering the femoral triangle is perforated by the saphenous vein, by lymph nodes of the vertical set, and by numerous blood and lymphatic vessels, hence the name cribriform fascia . If the dissection is carried out properly, the adventitia of the femoral vessels should not be clearly seen except through the vessel openings mentioned earlier.