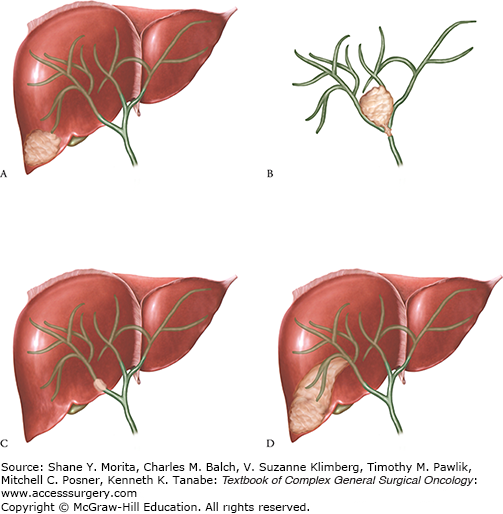
Intrahepatic cholangiocarcinoma (IHCC) is the second most frequent primary malignant tumor of the liver after hepatocellular carcinoma (HCC). IHCCs are considered as primary intrahepatic liver tumors. This differentiates them from malignancies of the biliary confluence (Klatskin tumors) that are classified as extrahepatic tumors, along with malignancies of the gallbladder and of the common bile duct. The definition of IHCC (primary tumor of the intrahepatic bile ducts) implies that it encompasses all malignancies arising from the intrahepatic bile ducts, starting from the second-order branches and up to the Hering ducts. This heterogeneity of origins readily explains that the clinical presentation and management of this tumor are quite heterogeneous, depending on whether the tumor originates from the end-order peripheral branches or from the juxtahilar branches. The former is likely to be more frequent than the latter, at least in part because as bile structures divide, their number increases. However, the proportion of each is still ill-defined because mass-forming-type IHCC may behave as hilar malignancies as a result of their location in the vicinity of the biliary confluence or centrifugal extension along the Glissonian pedicles. Three pathological subtypes have been described: mass-forming tumors, which is the most frequent form, described as tumors with clear borders between malignant and nonmalignant tissues; periductal tumors, described as more infiltrative and extended along peri-bile duct tissues without forming a discrete nodular mass; and intraductal tumors characterized by papillary growth within the lumen. These three subtypes seem to have different proliferative activity and different biological behavior: the mass-forming type develops intrahepatic metastases as a result of localized vascular invasion, whereas the periductal type has an infiltration spread via the Glisson capsule and pedicular lymph node metastases. In addition, frequency of lymph node metastases is higher in mass-forming and periductal types compared with intraductal type1 (Fig. 132-1).
Figure 132-1
Heterogeneity of the morphological presentation of IHCC. The two extreme situations depending on the origin of the tumor are the mass-forming type that originates from the end division of the intrahepatic bile ducts (A) and the periductal infiltrating type that originates from the proximal segmental or sectional bile ducts and resembles a Klatskin tumor (B). Some mass-forming type IHCCs may, however, behave as hilar tumor either when they develop from small bile ducts but in liver segments adjacent to the biliary confluence (C) or when they extend along the Glissonian pedicles (D).
It has been widely assumed in the past, in particular in surgical series, that IHCCs account for a very small proportion (approximately 10%) of all bile duct malignancies.2 In France, for example, the incidence of IHCC in 11 French registries ranged between 0.5 and 1.3 per 105 for men and 0.2 and 0.9 per 105 for women, whereas the respective incidences of all extrahepatic bile duct malignancies ranged between 0.6–1.3 and 0.7–2.3 per 105. Worldwide, the incidence of IHCCs varies greatly.3,4 Regions such as Thailand in Southeast Asia have the highest incidence of cholangiocarcinoma, as high as 113 per 100,000 in men and 50 per 100,000 in women, whereas in Western countries such as Australia, the incidence is low, at 0.2 per 100,000 in men and 0.1 per 100,000 in women.3,5 Differing exposure to risk factors is thought to account for the varying geographic incidences, with parasitic infections (Clonorchis sinensis and Opisthorchis viverrini) and hepatolithiasis being more prevalent in Asia.3,5 As far as primary liver tumors are concerned, it has recently been estimated that IHCC is 15 times less frequent than HCC. This figure is in between that observed in the United States (8 times) and that reported in Japan where the prevalence of HCC is particularly high (23 times). These variations are indeed more influenced by the numerator (i.e., the incidence of HCC which is highly variable in different areas of the world) than by the denominator (i.e., the incidence of IHCC which is fairly constant except in Southeast Asia where distomatosis is endemic).
The classically established risk factors for IHCCs include parasitic infections, Caroli’s disease, hepatolithiasis, and primary sclerosing cholangitis (PSC).6,7 These turn out, however, to account for a minor proportion of IHCCs in the West. New risk factors include hepatitis B virus (HBV) or hepatitis C virus (HCV) infection and obesity and diabetes. In most patients with IHCCs, however, no risk factor can currently be identified.
The hepatobiliary flukes, O. viverrini and C. sinensis, are associated with a very high risk of development of cholangiocarcinoma, particularly in Southeast Asia. In some areas of Thailand, the incidence may be as high as 100 per 100,000 inhabitants (as compared to 0.5 to 3 per 100,000 in most other areas of the world). A recent meta-analysis, performed by Shin et al,8 pooled 912 cases and 4909 controls and confirmed the strong association between C. sinensis and cholangiocarcinoma (OR = 4.7; 95% CI = 2.2–9.8). In endemic areas, the population-attributable risk, based on this study, was as high as 27.9% for men and 16.2% for women.
Bile duct cysts (i.e., choledochal) are rare congenital disorders characterized by cystic dilatation of the extra- and/or intrahepatic bile ducts. The risk of malignancy decreases after complete choledochal cyst excision; however, these patients are still at an increased risk of developing cholangiocarcinoma, compared with the general population.9–11 In a SEER-Medicare study, Welzel et al12 observed a strong association between choledochal cysts and increased risk of both IHCCs and extrahepatic cholangiocarcinomas (EHCCs), with ORs of 36.9 (95% CI = 22.7–59.7) and 47.1 (95% CI = 30.4–73.2), respectively.
Primary sclerosing cholangitis, an autoimmune disease that results in the stricturing of extra- and/or intrahepatic bile ducts, is an established risk factor for cholangiocarcinoma. Chronic inflammation, proliferation of biliary epithelium, production of endogenous bile mutagens, and bile stasis are postulated mechanisms of carcinogenesis.13 The lifetime incidence of cholangiocarcinoma among PSC patients ranges from 6% to 36%.14,15 Although PSC is known to be a strong risk factor for cholangiocarcinoma, no more than 10% of cholangiocarcinoma is attributed to PSC.15 It is unclear whether the duration of PSC correlates with the risk of developing cholangiocarcinoma; in fact, most cases present relatively soon after the diagnosis of PSC.6 Smoking and alcohol consumption have also been examined as potential risk factors for cholangiocarcinoma in patients with PSC. However, there are no definitive data to suggest that this is the case.6
Hepatolithiasis is the presence of calculi or concretions located proximal to the confluence of the right and left hepatic ducts. Hepatolithiasis is an established risk factor for IHCCs in Asian countries, with 2% to 10% of patients with hepatolithiasis developing IHCC.16 There are less data on the relationship between hepatolithiasis and IHCC in Western countries,17 but an Italian, hospital-based, case-control study also showed a significant association between hepatolithiasis and IHCC, with an OR of 6.7 (95% CI = 1.3–33.4).18
The currently banned carcinogenic agent, Thorotrast, a radiographic contrast agent used primarily from 1930 to 1960, has been strongly associated with an increased risk of developing cholangiocarcinoma.19
Newly established risk factors include HCV or HBV infection, cirrhosis, obesity, diabetes, and genetic polymorphisms. The data also suggest that in Western countries, HCV is consistently associated with IHCCs and not EHCCs. In Asian countries, it appears that HBV may be associated with IHCCs. Cirrhosis is the most consistently illustrated risk factor for IHCCs, but not EHCCs. A recent meta-analysis of published literature revealed the relative risks of HBV and HCV to be 2.6 (95% CI = 1.5–4.6) and 1.8 (95% CI = 1.4–2.4), respectively.20 Available data suggest that diabetes and heavy alcohol drinking may confer an increased risk for cholangiocarcinoma.21 Welzel et al22 recently showed that metabolic syndrome is a significant risk factor (OR = 1.56; 95% CI = 1.32–1.83, p < 0.0001) for development of IHCCs in the general U.S. population. The lack of an accurate, consistent cholangiocarcinoma classification system may have hindered the conduct and interpretation of risk factors in epidemiological studies.6
As a rule, IHCCs tend to be diagnosed at an advanced stage because the tumor remains clinically silent for a prolonged period. In contrast to HCC, where early screening of high-risk populations allows identification of earlier stage disease, no such strategy exists for IHCC. It is therefore important to know when the diagnosis should be raised.
The peak incidence occurs between the age of 55 and 75 years, and this tumor is extremely rare before the age of 45 (<10% of the patients). Although the sex ratio may fluctuate slightly around 1 in different areas of the world (0.9 in the Western world and 1.5 in Asia), this may simply reflect, at least in part, corresponding age-adjusted sex ratio of the population. In France, the male/female ratio is 1.2.23 Therefore, unlike HCCs, which is five to six times more frequent in males, there is no influence of gender for this tumor.
Patients with IHCCs tend to remain clinically well despite having large tumors. In surgical series where the mean tumor diameter typically ranges between 5 and 7 cm, one-third to one-half of the patients are asymptomatic. Circumstances of diagnosis therefore frequently include the evaluation of nonspecific abdominal symptoms or of abnormal liver function tests. Symptoms, when present, include abdominal pain, malaise, night sweats, asthenia, nausea, and weight loss. Interestingly, the presence of symptoms does not have the same detrimental impact on outcome that it has for other malignancies, such as HCCs or colorectal liver metastases. It should, however, be underlined that these data are derived from series of operated patients. If one considers all IHCC patients, most of whom are not amenable to surgery, the proportion of symptomatic patients is not unexpectedly much lower. Altogether, these data suggest that the course of the disease might be more indolent than previously thought and explains that this tumor is usually discovered at an advanced stage. When symptoms develop, the tumor is frequently unresectable.
Although IHCCs by definition exclude tumors located within the biliary confluence or first-order branches (left or right bile duct), jaundice is present in 10% to 15% of the patients. It may result from protrusion or migration of tumor material within the bile duct lumen or compression of the common bile duct by metastatic lymph nodes. However, the most frequent cause of bile duct obstruction is compression of the bile duct confluence by tumors located in the liver segments adjacent to the biliary confluence (segments 4 and 1) or carcinomatous infiltrating extension along the Glissonian sheath of segmental or sectional bile ducts.
Liver function tests are nonspecific, even though an increase in liver enzymes may be the only initial finding in some patients. Gamma glutamyl transferase (GGT) levels at least are always above the normal upper range, although this increase is often clinically slight. This feature is, however, common to any space-occupying lesion of the liver, with the exception of some benign tumors.
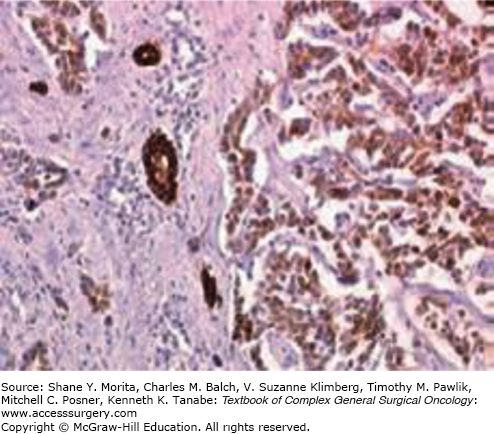
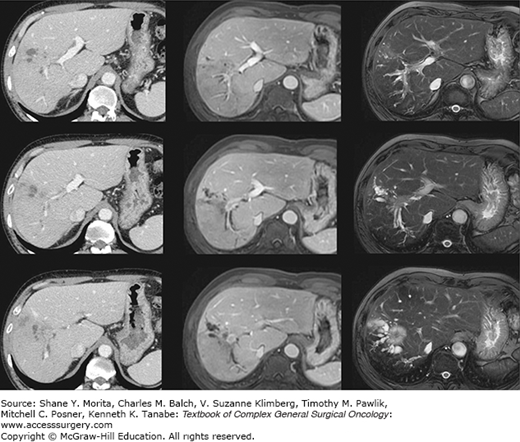
The diagnosis of IHCCs is usually fairly obvious on imaging studies (CT scan and/or MRI). It typically presents as a liver tumor that has three distinctive features: (1) it is fibrotic and therefore enhances at the delayed phase of the injection and is associated with a retraction of the capsule and an encasement of the vascular and biliary structures (Fig. 132-2); (2) it is frequently associated with satellite nodules that predominate at the periphery of the tumor; (3) enlarged lymph nodes in the hepatic pedicle (in particular at its right inferior part) or above the junction of the common and proper hepatic arteries are frequent. The main differential diagnosis is colorectal liver metastases (and to a lesser extent liver metastases of breast cancer). Depending on the context, there are two options to invasively confirm the diagnosis. The first is to perform a colonoscopy to rule out a colon cancer. The second is to perform a biopsy of the tumor (and of the adjacent liver) with immunostaining for cytokeratins (CK) 7 and 20. IHCCs are CK7+ CK20–, whereas colorectal metastases are CK7– CK20+ (Fig. 132-3). CK7 is indeed expressed relatively late during embryogenesis by cells that have differentiated into biliary cells (unlike CK19 which is expressed very early on and is a marker of undifferentiated cells).
Figure 132-2
Imaging findings. IHCC has three distinctive features: (1) it is fibrotic and therefore enhances at the delayed phase of the injection and is associated with a retraction of the capsula and an encasement of the vascular and biliary structures; (2) it is frequently associated with satellite nodules that predominate at the periphery of the tumor; (3) enlarged lymph nodes in the hepatic pedicle or above the junction of the common and proper hepatic arteries are frequent. Given the ill-defined borders that infiltrate the surrounding parenchyma and the lack of a clear tumor capsule, MRI allows better visualization of IHCC.
Tumor markers are of little help in the diagnosis of IHCC because of their lack of sensitivity as well as specificity. The most widely used are the carcinoembryogenic antigen (CEA) and CA19-9, but neither has in fact been very extensively studied. Overall, CEA levels are above 20 and above 100 in 15% and 5% of the patients, respectively.23 CA19-9 levels are between 100 and 1000 in 25% of the patients and above 1000 in a further 30%, but these values are often obtained in the presence of biliary obstruction, which is a known confounder. Alpha-fetoprotein (AFP) levels are below 200 ng/mL in 95% of the patients.23
In surgical series, IHCCs are often multiple; only 65% of IHCCs are unique, and this rate is similar in Japan and in Western countries. However, the multifocality of IHCC is unclear in different series because the terminology used is highly variable, such as “multifocal tumor,” “satellite nodules,” “intrahepatic metastases.” Indeed, it is difficult to differentiate multiple nodules corresponding to multifocal sites of carcinogenesis from multiple nodules corresponding to metastasis or direct extension from a primary tumor. This latter hypothesis seems to be the most common mode of spread. These satellite nodules are mainly observed in large,24 peripheral,25 and mass forming tumors.26
Unlike HCCs with vascular thrombus, the vascular extension of IHCCs is infiltrative in the vast majority of cases. However, macroscopic vascular thrombus has been reported.27,28 Frequency of vascular invasion in IHCCs (mainly portal invasion) was nearly 33% in Western series,29–31 58% in Japanese series,32,33 and 15% in the Korean series.34
Although peritoneal rupture is rare,35 peripheral IHCC is frequently associated (nearly 50%)23 with capsular retraction. Nevertheless, this capsular retraction is associated with adjacent organ invasion in nearly 25% of cases.36
Unlike HCC, biliary extension is observed in more than one-half of patients with IHCCs (versus 3% with HCCs),23 including common bile duct invasion in 8%.
Some Japanese surgeons claim that perineural extension has the same significance as lymphatic extension in IHCCs.37 Its frequency is inconstantly specified and ranges from 20% to 100%.32 Given the pedicular location of nerves, perineural extension is more frequent in proximal IHCC compared to peripheral IHCC (about 30% vs. 85%).38,39
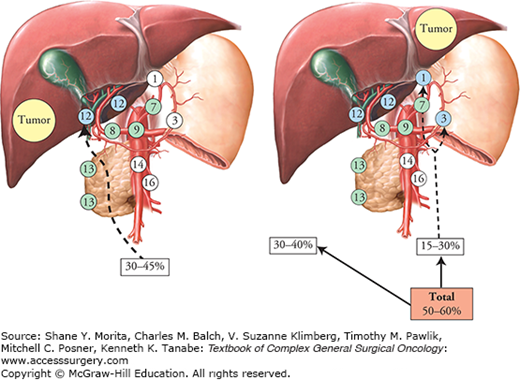
The proportion of positive lymph nodes is 40% in surgical series of IHCC when lymphadenectomy has been performed routinely. This high propensity for lymphatic extension has two consequences. The first is that the N status is likely to outweigh some of the “T” characteristics of IHCCs (a “small” tumor which is N+ will have a poorer prognosis than a “large” tumor which is N–). The second consequence, which is even more problematic, is that accurate surgical staging of IHCCs requires that all surgically treated patients undergo lymph node dissection.39 This is still not the case, whereas the accuracy of imaging studies to predict lymph node invasion is low. Hence, although some have considered patients who did not have lymph node dissection as being N–, these patients in fact have a prognosis intermediate between the true N+ and the true N–, suggesting that some of them were in fact N+. In a recent multicenter series including 11 institutions (from the United States, Switzerland, Italy, Belgium, and Portugal), only approximately one-half of patients undergoing surgery at any of the major hepatobiliary centers had their lymph nodes evaluated.40 To ensure accuracy of staging, lymph node sampling should be performed routinely at the time of surgery, as lymph node extension of IHCCs is much more prevalent than other hepatopancreatobiliary tumors.41 This lymphadenectomy should include harvesting of hepatoduodenal ligament for right-sided tumors and harvesting of cardia and lesser curvature nodes for left-sided tumors (Fig. 132-4). Although the removal of metastatic nodes may decrease locoregional recurrence, the implication of removing these nodes is mainly to allow accurate staging; the therapeutic benefit of lymphadenectomy for IHCCs is unproven.
Figure 132-4
Lymph node extension in IHCC. In patients with left-sided IHCC, nearly 20% had involvement of isolated lymph nodes of the cardia or of the lesser gastric curvature (16, 42, 45 rapport AFC). Lymph nodes: 1, right cardia; 3, lesser gastric curvature; 7, left gastric artery; 8, hepatic artery; 9, coeliac truck; 12, hepatoduodenal ligament; 13, retropancreatic; 14, mesenteric; 16, aortic.
In contrast to cholangiocarcinoma of the extrahepatic bile ducts, IHCCs are considered by the World Health Organization (WHO) as intrahepatic liver malignancies along with HCCs. Since HCCs are more frequent than IHCCs and the diagnosis of IHCCs was rather unreliable until the end of the 1980s, staging systems for intrahepatic malignancies have initially been developed based on the clinical, pathological, and therapeutic characteristics of HCCs. However, different staging systems for HCCs have proven to be both inappropriate and inaccurate for IHCCs because very few IHCCs are diagnosed when <2 cm in size. In 2010, the seventh edition42 of the TNM staging system of American Joint Committee on Cancer (AJCC) has implemented a unique staging for IHCCs. It was designed following a retrospective analysis of the Surveillance, Epidemiology, and End Results (SEER) database43 and was found superior to the two Western and two Japanese pTNM classifications that had previously been used, as detailed in Figure 132-5. This new edition of the AJCC ensured a more coherent distribution of TNM stages I, II, and III patients. Recently, this new system has been validated using a highly selected group of patients and shown to be more accurate in predicting survival after resection than the previous systems.44 According to this new edition, 28% of the patients had stage I tumors, and their 5-year survival was 62%. In contrast, 6% of the patients were stage IV, none of whom survived 2 years after surgery; therefore, the rationale for operating on the latter group is questionable. The remaining stages II and III patients, who comprised two-thirds of the population, had a median survival of 53 and 16 months, respectively, and their estimated 5-year survival was 27% and 14%, respectively. Although the seventh edition of the TNM staging system has introduced important changes resulting in a better contrast, the majority of IHCC patients are divided in T2 and T3 and standardized definition of the periductal infiltrating subtype is lacking.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree