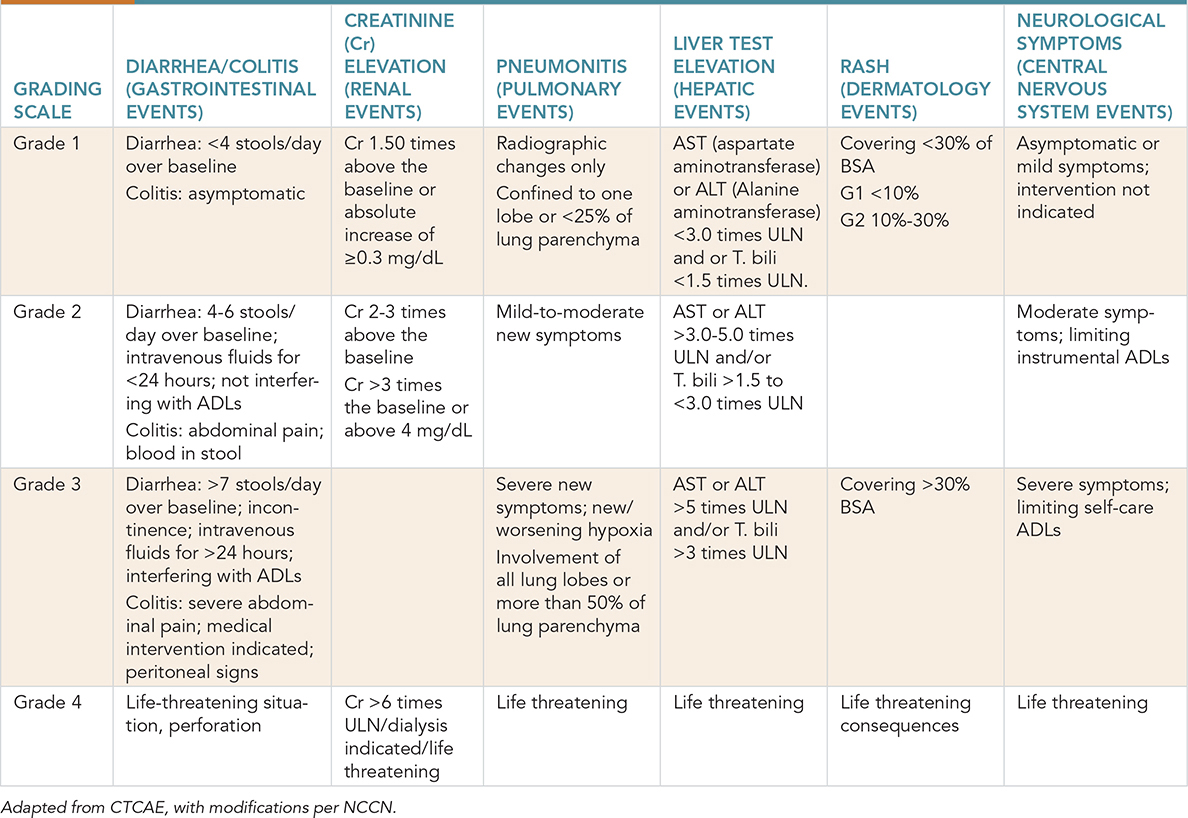
This case involves a 68-year-old man diagnosed with metastatic non–small cell lung cancer (NSCLC) and adenocarcinoma histology with a programmed death ligand 1 (PDL-1) score of 35%. He completed 4 cycles of carboplatin, pemetrexed, and pembrolizumab combination treatment and is currently on pemetrexed and pembrolizumab maintenance treatment. He presented to the hospital with abdominal cramps, 6-8 episodes of bloody diarrhea in a day, and fatigue. An infectious etiology has been ruled out, and immune-related colitis is speculated. What treatment should be offered to this patient? Learning Objectives: 1. What are common toxicities of immunotherapy? 2. How are the toxicities graded? 3. How are lower and higher grade toxicities of immunotherapy treated? In the past, limited success from traditional immunotherapy agents for most solid malignancies resulted in a perception that immunotherapy has only a limited role in oncology.1 However, with a better understanding of genetic patterns, predictive biomarkers such as PD-L1 and tumor mutational load have resulted in promising outcomes with immunotherapy.2–4 The development of this novel class of immune-based therapy presents new challenges in recognizing and managing a spectrum of treatment-related toxicities. The toxicity profiles of these agents, including those that block immune checkpoints, immunostimulatory agents, and adoptive T-cell therapy, are the result of hyperactivated T cells and a surge of cytokines directed against normal tissue.5,6 Immunotoxicity management is based on expert consensus, and a majority of the guidelines are obtained from the National Comprehensive Cancer Network (NCCN) with 2a category evidence (based on lower level evidence with a uniform consensus that intervention is appropriate). A detailed history and physical examination remain the key to approaching any new presenting complaints. Non-inflammatory (including infectious) etiology should be ruled out before considering drug toxicity. Hence, treatment-related toxicity should be a diagnosis of exclusion. An understanding of the timing, likelihood, and presentation of immune toxicity as well as how to manage the toxicity effectively will be a necessity for any health care provider dealing with cancer patients. Once the diagnosis of treatment-related toxicity is confirmed, management is based on a patient’s presenting grade. Common Terminology Criteria for Adverse Events v4.0 (CTCAE) provides a descriptive terminology that can be utilized for an adverse event (AE) and grading severity scale for each AE term (Table 21-1). TABLE 21-1 Grading Severity Scale for Adverse Events Hepatic toxicity is a common immunotoxicity present in about 2%-10% of cases treated with immunotherapy.7,8 This number increases to 25%-30% in combination immunotherapy, with presentation around 6-12 weeks.9 It is important to rule out other potential causes of liver dysfunction, including viral hepatitis, disease-related hepatic dysfunction, and drug-induced hepatic dysfunction. Any potential hepatotoxic medication should be discontinued. It is important to obtain an alcohol history. Other workup includes iron studies, liver ultrasound, and imaging for potential liver metastasis. Autoimmune hepatitis should be ruled out if suspicion is high; this can be done by checking serum antinuclear antibody (ANA), antineutrophil cytoplasmic antibody (ANCA), and anti–smooth muscle antibodies. For isolated elevation of transaminases, consider checking creatine kinase (CK) for other etiologies. In case of hepatotoxicity with total bilirubin (T. Bili) greater than 1.5 the upper limit of normal (ULN) and other causes of hepatic dysfunction ruled out, immunotherapy must be permanently discontinued. The patient should be admitted to the hospital, and prednisone/methylprednisone should be started at 2 mg/kg/day. Hepatology consultation should be obtained, and daily transaminases should be checked. If there is no improvement in 3 days, mycophenolate should be considered. Infliximab should not be used in hepatic AEs. In patients with hepatotoxicity with transaminitis only and normal bilirubin, management depends on the degree of severity of transaminitis. In Grade 1 (G1) transaminitis (<3 × ULN), immunotherapy should be continued, but liver function should be checked more frequently. For Grade 2 (G2) (3-5 × ULN) toxicity, immunotherapy should be held, and liver function should be assessed every 3-5 days. In case of worsening transaminitis, prednisone should be started at 0.5-1 mg/kg/day. Severe or Grade 3 (G3) toxicity (5-20 × ULN), inpatient care should be considered. Prednisone should be started at 1-2 mg/kg/day. For Grade 4 (G4) toxicity (>20 × ULN/life-threatening disease), prednisone should be started at 2 mg/kg/day. The patient should be treated as an inpatient, and mycophenolate should be considered. Infliximab should not be used due to potential concerns of hepatotoxicity, but there is no evidence to support this.10 One of the most common toxicities associated with immunotherapy is diarrhea from colitis. With cytotoxic T-lymphocyte–associated protein 4 (CTLA4) inhibitors, the incidence of diarrhea can be as high as 54%, and it is lower with programmed cell death protein 1 (PD1) inhibitors at around less than 19%.11 Colitis in this setting tends to mimic inflammatory bowel disease, and patients with colitis were more likely to have been prescribed nonsteroidal anti-inflammatory drugs (NSAIDs) as compared to patients without colitis.12 Appropriate management is necessary to prevent life-threatening complications from this AE. Mild or G1 colitis is consistent with fewer than 4 bowel movements more than the baseline. It is important to know the baseline rate of bowel movements per day since the grading depends on excess of that. For G1 colitis, immunotherapy can be continued; however, close monitoring is required, and in case of worsening symptoms, it is important to rule out any infectious etiology (stool studies, including testing for Clostridium difficile colitis). Moderate (G2) colitis is defined as 4-6 stools more than baseline not affecting the quality of life, and severe (G3-G4) is defined as more than 6 bowel movements above baseline or symptoms of colitis affecting activities of daily living (ADLs), causing hospitalizations, hemodynamic instability, and other severe complications (toxic megacolon, ischemic bowel disease, perforation). For moderate–severe colitis, it is important to rule out an infectious etiology with stool testing as previously discussed. Other investigations, such as computed tomographic (CT) imaging and esophagogastroduodenoscopy (EGD)/colonoscopy should be considered. If available, stool lactoferrin/calprotectin can be used to distinguish underlying inflammation from infection. Pancreatitis is a rare immunotoxicity but has been reported.13 If there are clinical signs/symptoms of acute pancreatitis and it is suspected, proper workup with CT imaging and laboratory tests should be done. Magnetic resonance cholangiopancreatography (MRCP) should be considered if otherwise radiographically negative but high suspicion is present. Gastrointestinal consultation should be considered. Management depends on the severity of pancreatitis. When there is elevation of amylase/lipase without signs/symptoms of pancreatitis, workup as mentioned is necessary to diagnose pancreatitis. In the absence of pancreatitis, resuming immunotherapy can be considered. This holds true for G1 or mild acute pancreatitis as well. Acute pancreatitis is considered mild when there are any of the three following present: signs/symptoms of pancreatitis, CT findings consistent with pancreatitis, or pancreatic enzymes (amylase/lipase) greater than three times the ULN. If two of these parameters are present, it is considered G2 or moderate pancreatitis, which warrants holding immunotherapy. In addition, methylprednisone/prednisone should be used at 0.5-1 mg/kg/day. Immunotherapy can be restarted with no radiographic evidence of pancreatitis and improvement in amylase/lipase. If all of the parameters are present or there is hemodynamic instability, this is considered G3 or severe toxicity, which warrants permanently stopping immunotherapy. Methylprednisone/prednisone at 1-2 mg/kg/day should be used, and mycophenolate mofetil can be considered if symptoms are not improving. Pneumonitis is an uncommon toxicity but is very challenging to manage, especially in patients with lung cancer. It is hard to diagnose pneumonitis radiographically in patients with lung cancer because of different confounding factors, such as radiation-related changes, lung cancer itself, lymphangitic spread, and more. The incidence of pneumonitis varies with different immunotherapy agents. Incidence ranges between 0% and 10% in anti-PD-1/PDL1 agents with an overall incidence of about 2.7% in anti-PD1 agents.14–17 The incidence is interestingly less with CTLA4 inhibitors.18 Pneumonitis is seen more with combination immunotherapy as compared to monotherapy. Some studies have shown higher odds of pneumonitis in NSCLC as compared to other cancers.16 Symptoms include cough, dyspnea, chest pain, leading to hypoxia and respiratory failure. Even though pneumonitis can have various manifestations radiographically, commonly seen are ground glass opacities and patchy nodular infiltrates.19 The timeline of symptoms also helps with diagnosing pneumonitis since it is rarely seen early on, and the median time of presentation is around 3 months.15 Workup includes proper history, physical examination, and imaging. If the clinical picture, including radiographic findings, is consistent with pneumonitis, treatment should be started. Biopsy is mostly unnecessary but can be done to rule out other etiologies in case of an unclear clinical picture. Generally, with any suspicion of pneumonitis, immunotherapy should be held. With G1 or mild pneumonitis (described in Table 21-1), the patient should be reassessed in 1-2 weeks, and chest imaging should be repeated in 3-4 weeks. G2 or moderate pneumonitis requires ruling out an infectious etiology with viral panels, cultures, and bronchoscopy and bronchoalveolar lavage (BAL) can be considered as well. Empiric antibiotics can be started if an infectious process is in the differential. Methylprednisone/prednisone at 1-2 mg/kg/day should be started, and close monitoring every 3-7 days is important. Steroids should be continued until the clinical picture is G1 or less and then tapered slowly over 4-6 weeks. Immunotherapy can be restarted once G1 or less is present with radiographic improvement/resolution. For G3-G4 pneumonitis, immunotherapy should be permanently withheld. Management should be in an inpatient setting with methylprednisone/prednisone at 1-2 mg/kg/day. Infectious workup as previously mentioned should be done to rule out infectious etiologies. If symptoms do not improve in 48 hours, agents such as infliximab, mycophenolate mofetil, or even intravenous immunoglobulin (IVIG) can be used. Pulmonary consultation should be obtained early in severe pneumonitis. A meta-analysis done recently showed that the incidence of endocrine AEs was around 10% with immune checkpoint inhibitor usage.20 Common manifestations include diabetes, thyroiditis, hypophysitis, and adrenal insufficiency. With hyperglycemia (fasting glucose level > 200 mg/dL), it is important to obtain a proper history and rule out underlying type 2 diabetes mellitus (DM). New-onset DM should be worked up with c-peptide levels. Anti-glutamic acid decarboxylase (anti-GAD), anti-islet cell antibodies should be considered, and diabetic ketoacidosis (DKA) workup should be done. In the case of DKA and new-onset DM, the patient should be managed as an inpatient with endocrinology consultation, and immunotherapy should be held. Immunotherapy can be restarted once the DKA is resolved and blood glucose stabilized. Thyroid abnormalities can manifest as either central hypothyroidism (normal or low thyroid-stimulating hormone [TSH], low free thyroxine [T4]) or primary hypothyroidism (elevated TSH). Subclinical hypothyroidism is asymptomatic, with elevated TSH levels and normal free T4 levels. Thyroid hormones should be evaluated every 4-6 weeks. Immunotherapy should be continued with subclinical hypothyroidism; however, levothyroxine can be started with TSH levels above 10. Even with primary hypothyroidism, immunotherapy can be continued. Endocrinology consultation should be obtained for the management of primary hypothyroidism. Adrenal insufficiency should be ruled out. Thyrotoxicosis can be rarely seen as well and management requires symptom control with beta-blockers until the thyrotoxicosis resolves. Thyroid function evaluations should be repeated in 4-6 weeks, and if thyrotoxicosis resolves, no further treatment is needed. In case TSH remains low with elevated free T4/total T3 (triiodothyronine), a radioiodine uptake scan should be done to differentiate between true hyperthyroidism versus Graves-like etiology. If hypothyroidism develops with TSH greater than 10, levothyroxine should be started. Primary adrenal insufficiency requires proper workup with morning cortisol levels, cortisol, 30- or 60-minute cortisol test, corticotropin (ACTH) levels, comprehensive metabolic panel (CMP), and renin levels. Immunotherapy should progress, and endocrinology consultation should be obtained. Corticosteroids should be started prior to hormone replacement to avoid adrenal crisis. Steroid replacement should be with either prednisone 7.5 mg/10 mg or hydrocortisone 20 mg in the morning and 10 mg in the evening with a slow taper. In addition, mineralocorticoid, fludrocortisone, should be started at 0.1 mg every other day and as needed titration. Patients with hemodynamic instability should be admitted and managed with intravenous fluids and stress dose steroids. Hypophysitis can present as headaches, fatigue, nausea/vomiting, and low sodium and potassium. Complete workup includes evaluation of ACTH, cortisol, FSH, luteinizing hormone (LH), testosterone/estrogen (male/premenopausal female), free T4. Brain magnetic resonance imaging (MRI) should be obtained in symptomatic patients. Immunotherapy should be held in these patients. Endocrinology consultation should be obtained, and hormone replacement given as appropriate. Methylprednisone/prednisone at 1-2 mg/kg/day should be started. Immunotherapy can be restarted once symptoms are controlled with a prednisone dose less than 10 mg daily or equivalent. Acute kidney injury (AKI) or nephritis is an uncommon AE with an incidence of about 1%-2% in monotherapy and about 4.5% in patients treated with combination immunotherapy.10 Grade 3 and above toxicity is less than 2%.21 Median time to onset for grade 3 or above nephritis is around 16.3 weeks.22 With any evidence of elevated creatinine, it is important to rule out any other etiologies, such as dehydration, potential nephrotoxic medications, or recent intravenous contrast use. It is also important to obtain a spot urine/creatinine ratio. For proteinuria greater than 3 g/24-hour, check ANA, rheumatoid factor (RF), anti-neutrophil cytoplasmic antibody (ANCA), anti-dsDNA (anti–double-stranded DNA), and serum complement 3 (C3), complement 4 (C4), and total complement (CH50) to rule out other etiologies. With other causes ruled out, management depends on the degree of severity. G1 or mild nephritis requires creatinine monitoring every 3-7 days. Immunotherapy can be held until improvement is seen. For G2 nephritis, immunotherapy should be held, and nephrology consultation should be obtained. Once other causes are ruled out, treatment should be started with prednisone 0.5-1 mg/kg/day. Creatinine and urine albumin creatinine ratio should be monitored every 3-7 days. If persistent over a week, the steroid dose should be increased to methylprednisone/prednisone 1-2 mg/kg/day. Treatment should be continued until symptoms improve to G1 or less and be tapered over 4-6 weeks. Immunotherapy can be restarted with improvement concomitantly with steroids. G3 or above nephropathy requires permanently discontinuing immunotherapy. The patient should be treated as an inpatient, and nephrology consultation should be obtained. Renal biopsy can be considered if the diagnosis is unclear. Treatment should be started with methylprednisone/prednisone at 1-2 mg/kg/day. If still greater than G1 after 1 week of steroids, other therapies (eg, azathioprine, cyclophosphamide [monthly], cyclosporine, infliximab, mycophenolate) should be considered. Dermatological toxicities are seen in almost 30%-50% of patients using immunotherapy,23 with 1%-3% grade 3 toxicities24 with the CTLA4 inhibitor ipilimumab. The PD-1 inhibitors (pembrolizumab and nivolumab) have a lower incidence of cutaneous toxicity.17 The presence of cutaneous AEs such as rash and vitiligo have interestingly shown a better prognosis in many studies.25–28 The different types of skin AEs include maculopapular rash, pruritus, blistering disorders such as bullous dermatitis, Steven-Johnson syndrome (SJS), and toxic epidermal necrolysis (TEN). It is important to obtain a complete history, including history of underlying dermatological diseases, as well as a full-body dermatological examination. Any atypical features warrant a skin biopsy and dermatological consultation. The maculopapular rash is the most common dermatological toxicity observed in patients undergoing treatment with immunotherapy. With grade 1 (<10% body surface area [BSA] involved) presenting dermatologic signs, it is recommended to continue with immunotherapy treatment and proceed with symptomatic management (topical steroids, emollients, or oral antihistamines) of skin symptoms. In the case of grade 2 (10%-30% BSA involvement), consider holding immunotherapy and treating with high-potency topical steroids and/or oral steroids (prednisone 0.5-1 mg/kg/day). High-potency topical steroids for shorter duration are preferred over longer treatment with low-potency steroids. Oral antihistamines and topical emollients can be used for symptomatic control. Once an improvement is noted, taper steroids over at least 1 month. Immunotherapy rechallenge can be done once symptoms become milder (≤G1). For grade 3-4 symptoms, it is recommended to hold the immunotherapy and prescribe prednisone at 0.5-1.0 mg/kg/day. Dosage can be increased if there is no improvement, and urgent dermatology evaluation is recommended. If symptoms improve to grade 1, taper steroids over 4-6 weeks. Another common AE is pruritis. This can be divided into mild or localized (G1); widespread, causing skin changes from scratching (G2); and G3, which includes intense pruritis limiting ADLs significantly and affecting sleep. For G1 pruritis, immunotherapy can be continued and symptoms controlled with high-potency topical steroids. Immunotherapy can be held for G2 until symptoms are G1 or less, and treatment is high-grade topical steroids and oral antihistamines. Dermatology consultation is recommended. G3 pruritis can have significant implications on quality of life; hence, it is recommended to hold immunotherapy. Serum immunoglobulin (Ig) E levels and histamine levels should be checked. Omalizumab can be used in case of elevated IgE levels, and antihistamines can be used in case of elevated histamine. Gabapentin and pregabalin can be used for symptom control, and aprepitant can be considered as well. Urgent dermatology referral should be made. Systemic steroids with prednisone/methylprednisone 0.5-1 mg/kg/day should be used. Bullous dermatitis can be seen as an AE in some cases, although rare. The grading of toxicity mimics maculopapular rash, with G1 less than 10% BSA, G2 10%-30% BSA, and G3 greater than 30% BSA.
21
IMMUNOTOXICITY
GRADING OF SEVERITY
HEPATIC ADVERSE EVENTS
GASTROINTESTINAL ADVERSE EVENTS
PANCREATIC ADVERSE EVENTS
PULMONARY ADVERSE EVENTS
ENDOCRINE ADVERSE EVENTS
Diabetes
Thyroid
Adrenal Insufficiency
Hypophysitis
RENAL ADVERSE EVENTS
CUTANEOUS ADVERSE EVENTS
Maculopapular Rash
Pruritis
Bullous Dermatitis
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree