In 1893, William Bradley Coley reported the case of a German immigrant with a neck sarcoma that was deemed inoperable and yet disappeared after an erysipelas infection ). Coley hypothesized that the immune response provoked by the bacterial infection led to remission of the sarcoma. Therefore, he went on to develop Coley’s toxins, a mixture of initially live and later dead bacteria that included Streptococcus pyogenes and Serratia marcescens species, and used it for therapeutic purposes . The American surgeon and cancer researcher William Bradley Coley has been considered the “father of cancer immunotherapy.” Coley’s work is certainly noteworthy, but it should be noted that current opinion is that Coley’s toxins are not effective in treating cancer.
Immunotherapy for cancer is utilizing components of the immune system to induce anti-tumor responses. Cancer immunotherapy can be divided into active and passive immunotherapy. Passive immunotherapy enhances existing anti-tumor responses and includes the use of monoclonal antibodies, and cytokines. Active immunotherapy directs the immune system against specific cancer antigens and includes immune cell therapies and therapeutic cancer vaccines.
The cancer immunity cycle illustrates the stepwise process that activates tumor-specific immune responses ( Fig. 17.1 ). Solid tumors present and release tumor-associated antigens (TAAs) that are processed by professional antigen-presenting cells (APCs), e.g., dendritic cells. APCs present these antigens to T cells at local lymph nodes. The T cells are activated and primed to recognize the TAAs. T cells reach distant tumor sites via the systemic circulation, recognize specific TAAs on tumor cells, and infiltrate the tumor tissue. Cytotoxic T cells can elicit potent anti-tumor responses and cause regression of solid tumors.
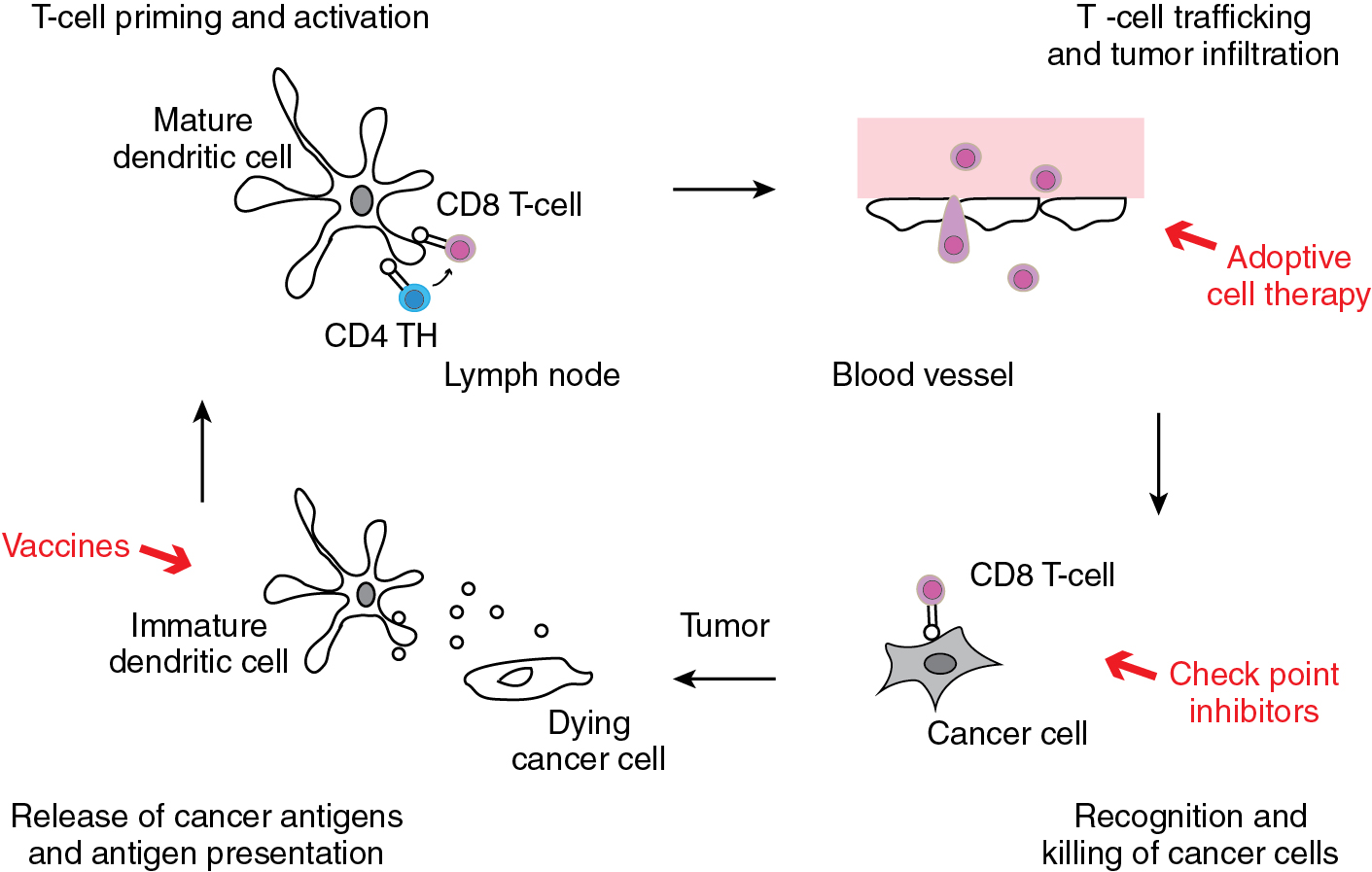
Every step in the process of antigen presentation, T cell activation, and tumor cell killing is regulated by complex mechanisms. Cytokines like interleukin (IL)-2, for example, can activate T cells. The interaction between B7 expressed on dendritic cells and CD28 on T cells generates activating signals, while the interaction between B7 and cytotoxic T lymphocyte-associated protein 4 (CTLA-4) inhibits the activation of T cells during antigen presentation. In the tumor microenvironment, various immune checkpoints modify the activity of T cells. The interaction between programmed cell death receptor 1 (PD-1) and the programmed cell death ligand 1 (PD-L1) inactivates T cells and suppresses anti-tumor immunity. Other checkpoint inhibitors including PD-L2, V-type immunoglobulin domain suppressor of T cell activation (VISTA), and lymphocyte-activation gene 3 (LAG-3) have similar T-cell suppressive function.
Several important immunologic concepts should be considered. Clinically, in ovarian cancer and other solid tumors, the neoantigen load can be considered a prognostic biomarker for survival. Strickland et al. reported a statistically significant difference in overall survival ( P = 0.0331) among patients with a high neoantigen load compared with those with a low neoantigen load ( ). The tumor microenvironment is also a dynamic region in which vascular endothelial growth factor has been noted to have immunosuppressive properties. Zhang et al. have observed improved clinical outcome with high levels of tumor-infiltrating lymphocytes (TILs) within tumor cell islets ( ). Indeed, the effectiveness of immunologic checkpoint inhibitors has been shown to track with infiltration by TILs.
When considering the different immune checkpoints described previously, it is important to invoke the hierarchy of co-inhibitory receptors. It appears that all checkpoint inhibitors have some effect on CD8+ T cell and natural kill (NK) cell effector function, PD-1 blockade is more robust than that of LAG-3, T cell immunoglobulin mucin domain-3 (TIM-3), or TIGIT blockade, with the latter three preferentially having an impact on tumor tissue Treg and IL-10-producing cells. The dendritic cell phenotype is also affected by TIM-3 and TIGIT inhibition. This suggests that different immune checkpoint inhibitors (ICIs) can be combined to achieve distinct immunologic responses.
The most commonly used immunotherapies utilize ICIs. The currently available ICIs are monoclonal antibodies that target PD-1, PD-L1, or CTLA-4. The blockade of these proteins by monoclonal antibodies will prevent the interaction with their respective binding partners and thereby prevent T-cell activation. Cell therapies utilize different subpopulations of white blood cells for treatment. T cells, for example, can be extracted from peripheral blood mononuclear cells or from tumor tissues. The latter approach generates so-called tumor infiltrating lymphocytes that can be expanded in cell culture and selected for the recognition of specific TAAs. Genetic engineering techniques generate T cells that express chimeric antigen receptors (CARs) or T-cell receptors (TCRs) for the recognition of specific TAAs. Vaccines stimulate anti-tumor immune responses by presenting TAAs to APCs. Both cell therapies and therapeutic vaccine are still under investigation in gynecologic malignancies and are currently not approved for use in patients.
Cytokines
Early immunotherapies in gynecologic cancers have used cytokines such as IL-2, IL-12, and interferon (IFN)-α and -γ as single-agents or in combination with other treatment modalities. These cytokines primarily activate cytotoxic T cells and enhance antigen presentation. A variety of clinical trials has demonstrated anti-tumor efficacy via intraperitoneal application. Despite these positive results, cytokine therapy is only used in clinical trials for gynecologic malignancies and has not been approved for standard treatment in patients. Cytokine therapy can induce significant side effects including fever and cardiovascular compromise often requiring intensive care unit management.
Interleukin-2
Intravenous infusion of recombinant IL-2 in high doses is associated with high morbidity. Weekly intraperitoneal infusions were reportedly better tolerated and yielded in a phase II trial of platinum-resistant ovarian cancer a 25% response rate. Four patients showed a complete and two a partial response with a long duration of response.
Interleukin-12
Intravenous and intraperitoneal application of recombinant IL-12 has, in the treatment of ovarian cancer, only modest success thus far. In a phase II trial of recombinant IL-12 , the maximum response was stable disease. Two patients showed stable disease and nine patients progression of disease.
Interferons
Intraperitoneal infusions of single-agent interferons and combination treatments of recombinant IFN-α and -γ plus chemotherapy for ovarian cancer have been mainly studied in the 1990s. Although these are mainly phase I trials, they demonstrated that immunotherapy in ovarian cancer can have efficacy.
Monoclonal antibodies
Monoclonal antibodies are tools of immunotherapy. Epidermal growth factor receptor (EGFR), HER-2/neu, and vascular endothelial growth factor (VEGF) antibodies predominantly interfere with overexpressed molecules that are critical for cancer cell proliferation and invasion; they do not stimulate the immune system. However, this classification becomes somewhat blurred because of partially overlapping mechanisms, e.g., trastuzumab triggers antibody-dependent cellular cytotoxicity (ADCC) and functions in part as an immune stimulator, while its main mechanism is thought to be the interference with an upregulating signaling cascade. Antibodies that stimulate the immune system are described in the following sections.
The WHO nomenclature provides an antibody classification. The stem “-mab” indicates monoclonal antibodies. The substem for the animal origin of the antibody was dropped in 2017 but is still part of older antibody names (-o- for mouse, -a- for rat, -e- for hamster, -i- for primates). For humanized antibodies, -zu- follows, or -xi- for chimeric antibodies (only fragment crystallizable [Fc] region replaced). The subset preceding the source of the antibody defines its target. Examples are -ciI- for circulatory system, -li(m) for immune system. The old system used different tumor designations, e.g., -go(v) for ovarian cancer, while the new system only employs the site agnostic -t(u). The leading first one to two syllables in an antibody name are without any meaning. With this classification in mind, antibody names can be dissected, as exemplified with the anti-folate receptor α (FRα) antibody farle-tu-zu-mab : according to the new classification a tumor site of the antibody’s anti-tumor effect is not designated (-tu-); however, it is a humanized (-zu-) monoclonal antibody (-mab).
Oregovomab
Oregovomab is a mouse monoclonal antibody directed against the membrane bound and soluble Ca-125. The antigen-antibody complexes trigger broad cellular and humoral immune responses. The Ca-125-oregovomab complexes can prime dendritic cells. Anti-idiotypic antibodies are formed against oregovomab and Ca-125 which are able to induce Fc-mediated tumor cell killing. Improved survival was noted in ovarian cancer patients who develop specific B- and T-cell responses after oregovomab injection. In a phase III trial, oregovomab maintenance therapy after standard adjuvant chemotherapy of primary ovarian cancer did not show any benefit. In another phase II trial in the same setting, however, simultaneous day infusion with oregovomab on alternate cycles with adjuvant platinum-based chemotherapy permitted an immune effect and significantly improved progression-free survival. Based on these data, a randomized phase III trial of carboplatin and paclitaxel with or without oregovomab has been initiated.
Human Milk Fat Globule 1
The Human Milk Fat Globule 1 (HMFG1) is a murine monoclonal antibody that recognizes an epitope on the extracellular domain of mucin 1 (MUC1). This antibody has been labeled with Yttrium 90 and used for radioimmunotherapy of ovarian cancer. After promising initial clinical studies on its intraperitoneal application, a phase III trial showed no difference between standard treatment of ovarian cancer and standard treatment plus a single intraperitoneal infusion of Y-90-labeled HFMG1 in progression-free and overall survival.
Catumaxomab
Catumaxomab is a trifunctional monoclonal antibody. It consists of one half (one heavy and one light chain) of an anti-EpCAM antibody and one half of an anti-CD3 antibody. It can simultaneously bind to EpCAM on the tumor cell and CD3 on the T cell. In addition, with its Fc region it can bind to accessory cells, including macrophages, NK and dendritic cells. In phase II and phase II/III trials, catumaxomab was effective in decreasing malignant ascites production in ovarian cancer, improved quality of life, and prolonged puncture-free interval with an acceptable safety profile. However, only a 5% response rate was seen in platinum-resistant ovarian cancer. Catumaxomab was voluntarily withdrawn in the United States in 2013 and for commercial reasons by the European Commission in 2017.
Farletuzumab (MORab003)
Farletuzumab is a humanized monoclonal IgG1 antibody directed against the FRα. Farletuzumab does not prevent folate binding to the receptor, nor inhibit receptor-mediated endocytosis. Instead it induces ADCC, complement-dependent cytotoxicity (CDC), and tumor cell autophagy. In a phase II trial, patients with recurrent platinum-sensitive ovarian cancer treated with farletuzumab and carboplatin and paclitaxel followed by farletuzumab maintenance therapy showed favorable responses compared to historic controls. However, in a subsequent phase III trial analyzing recurrent platinum-sensitive ovarian cancer treated with chemotherapy plus farletuzumab or plus placebo, the progression-free survival did not improve. Subsequent analyses showed that ovarian cancer patients with higher FRα levels may benefit more from farletuzumab use.
Antibody-drug conjugate
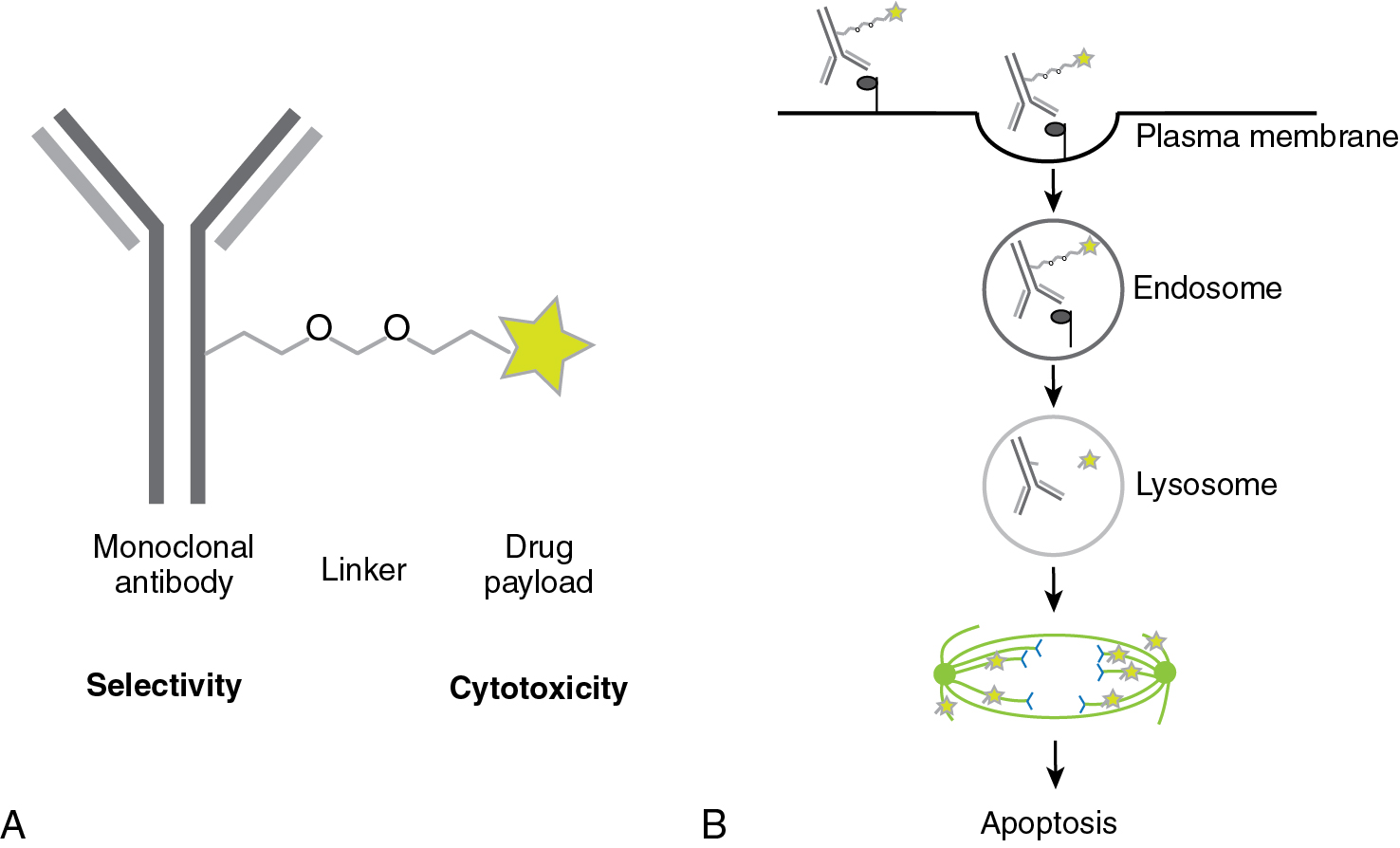
Antibody-drug conjugates (ADCs) are a new class of anticancer drugs which employ the specificity of an antibody in combination with the cytotoxicity of a small molecule anticancer drug. It does not enhance the immune response and thus does not meet the strict definition of immunotherapy; however, given the recent promising results of ADCs in gynecologic cancers, we would like to introduce their concept here. In ADCs, a monoclonal antibody is fused by a linker to a small cytotoxic molecule ( Fig. 17.2 ). The antibody provides selectivity. The cells take up the ADC by endocytosis. The cytotoxic drug, the payload, is released in intracellular compartments such as endosomes or lysosomes. The linker can be cleavable, e.g., by cathepsins that are located in lysosomes and activated by the low pH in lysosomes, or it can be non-cleavable. In the latter case, the antibody will be degraded intracellularly and only the cytotoxic agent remains and thereby becomes active. In the former case, the cytotoxic agent may evade the cell and kill tumor cells nearby as well, the so-called “bystander killing.”
Mirvetuximab soravtansine (IMGN853)
This is an ADC combining a monoclonal antibody against the FRα with the maytansinoid DM4 (N(2´)-deacetyl-N(2´)-(4-mercapto-4-methyl-1-oxopentyl)-maytansine) via a sulfo-SPDB linker (N-succinimidyl 4-(2-pyridyldithio)-2-sulfobutanoate). Maytansine inhibits microtubule assembly and induces mitotic arrest, and is thereby cytotoxic.
Clinical use of antibody drug conjugates
Ovarian cancer
Following a promising dose-escalation study on single-agent mirvetuximab, a recently published phase Ib trial of mirvetuximab soravtansine (6 mg/kg adjusted ideal body weight every 3 weeks) combined with bevacizumab in platinum-resistant ovarian cancer showed that this combination was well tolerated and effective. The objective response rate was 39% (including 5 complete and 21 partial responses) and the median progression-free survival 6.9 months.
Cervical cancer
Tisotumab vedotin targets tissue factor, a protein highly expressed in cervical cancer. This ADC has demonstrable activity in pretreated recurrent and metastatic cervical cancer and is discussed in detail in the chapter 3 ( ).
Immune checkpoint inhibitors
Negative regulators of the immune system, so-called immune checkpoints, prevent an overshooting immune response with possible self-recognition and subsequent autoimmune phenomena. Since cancer antigens may be at times similar to self, immune checkpoints also limit anti-tumor responses of the immune system. The inhibition of these negative regulators unleashes the immune anti-tumor response ( Fig. 17.3 ).
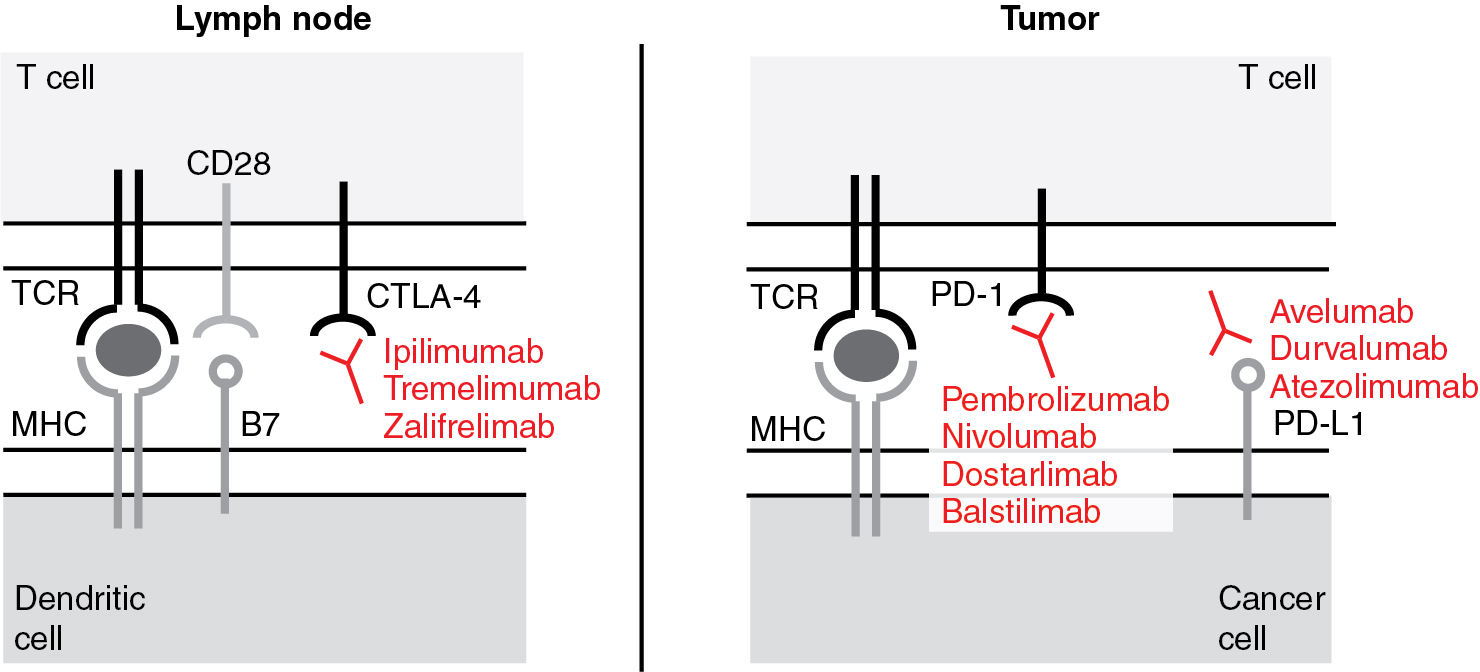
Anti-cytotoxic T lymphocyte-associated protein 4 antibodies
In 1994, it was demonstrated for the first time that CTLA-4 plays an inhibitory role in regulating the T-cell response. CTLA-4 is primarily an intracellular protein. Its cell surface expression is tightly regulated by restricted trafficking and rapid internalization. It is inducibly expressed by native T cells and constitutively expressed by FoxP3 + regulatory T cells (Tregs). Upon T-cell activation, i.e., T-cell receptor engagement and co-stimulation with CD28, CTLA-4 translocates to the plasma membrane. Here, CTLA-4 outcompetes CD28, prevents co-stimulatory signals from binding, and thereby inhibits the T cell. CTLA-4 activation arrests T-cell proliferation and activation. Mice lacking CTLA-4 die of a fulminant lymphocytic infiltration of almost all organs. In 1996, James Allison and colleagues blocked CTLA-4 with antibodies to inhibit its immune suppressive effects and showed an increased anti-tumor response. Early clinical trials yielded durable anti-tumor responses in solid tumors but also mechanism-related toxicities including autoimmune enterocolitis, hepatitis, and dermatitis. Algorithmic use of steroids alleviated the autoimmune side effects of anti – CTLA-4 antibody treatment without abrogating its antitumor response.
Ipilimumab
Ipilimumab is a fully human monoclonal anti-CTLA-4 IgG1 antibody. It has been hypothesized that the anti-CTLA-4 antibodies disinhibit the immune response by two possible mechanisms: (i) interference of the CTLA-4/ B7 binding and (ii) depletion of immunosuppressive Tregs via Fc-mediated antibody dependent cell-mediated cytotoxicity (ADCC) and CDC.
Tremelimumab
Like ipilimumab, tremelimumab is a fully human monoclonal anti-CTLA-4 antibody. However, it is the non-complement fixing isotype IgG2 and may have fewer effects on the density of Tregs (see earlier). The experience with tremelimumab in gynecologic malignancies is thus far limited.
Zalifrelimab
Zalifrelimab (AGEN1884) is another fully human monoclonal anti-CTLA-4 IgG1 antibody. As an IgG1 antibody it displays the same mechanism of action as proposed for ipilimumab .
Clinical use of anti-CTLA-4 antibodies
Ovarian cancer, cervical cancer
Single-agent ipilimumab has been used in recurrent ovarian cancer with only modest success. Similarly, ipilimumab did not show significant single-agent activity in recurrent cervical cancer, even though it was well tolerated and able to induce an immune response. The combined use of e.g., CTLA-4 antibodies with PD-L1 antibodies showed more clinical benefit (see later).
Anti-programmed cell death receptor-1 antibodies
In 1992, PD-1 (or CD279) was first described by Tasuku Honjo and colleagues. The name programmed cell death receptor was chosen, since the receptor was believed to be involved in T-cell death. Later, PD-1 was found to be an immune checkpoint. The tyrosine phosphatases SHP-1 and SHP-2 ( S rc h omology region 2 domain-containing p hosphatase) mediate PD-1’s inhibitory function and dephosphorylate signaling molecules downstream of the TCR and thereby inhibit cytokine production, e.g., IL-2 and IFN-γ, and T-cell proliferation.
PD-1 is a cell surface receptor and, in contrast to CTLA-4, is expressed on a wide variety of cells, including CD4 and CD8 T cells, B cells, monocytes, NK cells, and dendritic cells. PD-1 is not expressed on resting T cells but can be induced upon activation. It can also be induced on APCs, PD-1 has two ligands, PD-L1 (or CD274 or B7-H1) and PD-L2 (or CD273 or B7-DC). PD-L1 is expressed by many cell types including epithelial, endothelial, and stromal cells. Its expression is induced by pro-inflammatory cytokines such as interferons, tumor necrosis factor (TNF), and VEGF. PD-L2 is expressed by APCs.
Upon TCR activation, T cells produce IFN-γ, the strongest stimulator of reactive PD-L1 expression. Repeated exposure to cognate antigens thereby results in high PD-L1 expression and continuous PD-1 signaling which continuously counteracts the stimulating effect of the antigen and eventually induces T-cell exhaustion. T-cell exhaustion is a state of acquired T-cell dysfunction and a hallmark of chronic infection and cancer. It is defined by progressive poor effector function and sustained expression of inhibitory receptors. Immune checkpoint inhibition using PD-1 and PD-L1 inhibitors aims to reverse T-cell exhaustion.
Currently available monoclonal antibodies recognize the receptor PD-1 and the ligand PD-L1.
Pembrolizumab (MK-3475)
Pembrolizumab is a humanized monoclonal anti-PD-1 IgG4 antibody. It does not activate complement or bind Fc receptors, and thereby avoids cytotoxic effects on T cells. It binds to the PD-1 receptor and blocks the interaction with PD-L1.
Nivolumab
Nivolumab is a fully human monoclonal anti-PD-1 IgG4 antibody. Comparison of the amino acid sequence of pembrolizumab and nivolumab shows that they are essentially identical except for the variable regions that bind the antigen. Crystal structures of PD-L1 bound to PD-1 as well as crystal structures of the PD-1 ectodomain in complex with the Fab fragments of pembrolizumab and nivolumab are available. These crystal structures suggest a similar mechanism for both antibodies; they competitively block PD-L1 binding by steric hindrance. However, pembrolizumab shows a greater overlap with the PD-L1 binding site than nivolumab . In fact, there is apparently no overlap between the binding sites of pembrolizumab and nivolumab on the PD-1 molecule, so that based on the crystal structure, simultaneous binding of the two PD-1 antibodies is possible.
Dostarlimab
Dostarlimab (TSR-042) is a humanized monoclonal anti-PD-1 IgG4 antibody that blocks the binding of PD-L1 and PD-L2. Contrasting other anti-PD-1 antibodies, dostarlimab is sufficiently dosed every 4 weeks for four treatment cycles and then every 6 weeks for maintenance treatment.
Balstilimab
Balstilimab (AGEN2034) is a fully human monoclonal anti-PD-1 IgG4 antibody.
Cemiplimab
The hinge-stabilized anti-PD-1 cemiplimab was recently reported to have significantly increased overall survival compared to physician’s choice chemotherapy in women with recurrent and metastatic cervical cancer who had progressed following treatment with platinum-based chemotherapy. These survival results are derived from the EMPOWER-Cervical 1/GOG-3016/ENGOT-cx9 trial and appear to not be biomarker-driven by PD-L1 expression and the survival benefit conferred includes both squamous cell carcinoma and adenocarcinoma ( ).
Anti-PD-L1 (programmed cell death receptor ligand-1) antibodies
Durvalumab
Durvalumab is a human monoclonal IgG1 antibody. The crystal structure of the PD-L1/ durvalumab complex is available.
Avelumab
Avelumab is a human monoclonal IgG1 antibody. The crystal structure of the PD-L1 / avelumab complex is available. The binding site of avelumab on PD-L1 partially overlaps with PD-L1’s binding site to the PD-1 receptor, thereby ligand-receptor binding is sterically hindered.
Atezolizumab
Atezolizumab is a fully humanized monoclonal IgG1 antibody. The crystal structure of the PD-L1/ atezolizumab complex is available. Atezolizumab competes with PD-1 for the same PD-L1 surface.
Clinical use of anti-PD-(L)1 antibodies
Endometrial cancer
The multi-cohort trial KEYNOTE-016 tested pembrolizumab in mismatch repair protein–deficient (MMRd) solid tumors. Cohort C of this trial, i.e., MMRd non-colorectal cancers, included two endometrial cancer patients; one showed partial and one complete response. In a similar trial design, KEYNOTE-158 studied pembrolizumab in microsatellite instability-high (MSI-H) solid tumors which included four endometrial cancers (4/21). The objective response rate for MSI-H non-colorectal cancers was 42.9% and the disease control rate 66.7%. In KEYNOTE-028, 13% heavily pretreated PD-L1 positive endometrial cancer patients showed partial response to pembrolizumab with the duration of response not reached and stable disease in another 13% of patients. The ongoing GARNET trial analyzed dostarlimab in PD-L1 positive advanced endometrial cancers. In preliminary result communications, 9/17 patients (53%) showed partial responses and an additional case (6%) stable disease.
Ovarian cancer
Objective response rates for single-agent pembrolizumab , nivolumab , and avelumab in advanced ovarian cancer were 11.5%, 15%, and 9.5%, respectively. The efficacy of single immune checkpoint inhibition in ovarian cancer is therefore less than observed in other solid malignancies like melanoma or renal cell carcinoma. The KEYNOTE-100 study is one of the largest studies that examined the clinical activity of single-agent pembrolizumab (anti-PD-1) in patients with recurrent epithelial ovarian cancer. Patients were divided into two different cohorts defined as one to three prior lines of treatment (cohort A, 285 patients), and patients with four to six prior lines of treatment (cohort B, 91 patients). The results showed an objective response rate of 7.4% for cohort A and 9.9% for cohort B, similar to other larger studies using single agent immune checkpoint inhibition. PD-L1 expression was assessed in tumor tissue by immunohistochemistry calculating the number of all PD-L1 positive cells including tumor cells, lymphocytes, and macrophages divided by the total number of viable cells which provides the Combined Positivity Score (CPS). Overall, patients with a CPS less than 1 ( n = 141) had a response rate of 5.0%, while the subgroup of patients with tumor CPS of greater than 10 ( n = 82) showed a higher response rate of 17.1%.
Cervical cancer
Nivolumab in PD-L1 unselected cervical cancer is being studied in the ongoing multicohort phase I/II CheckMate-358 trial which included 19 recurrent or metastatic cervical cancer patients. The objective response rate in cervical cancer was 26.3%, regardless of PD-L1 or HPV status and number of prior lines. The disease control rate was 70.8%, progression-free survival 5.5 months. The ongoing basket KEYNOTE-158 trial on pembrolizumab includes 11 PD-L1 positive cancer types. In this trial, PD-L1 positivity is defined as CPS ≥ 1. The updated analysis of the cervical cancer cohort with 98 patients showed an objective response rate overall of 13%, and 16% in PD-L1 positive cancers, disease control rate was 30.6%. At a median follow up of 10 months, the duration of response was not reached.
At the European Society for Medical Oncology (ESMO) 2020 conference, preliminary results on the phase II trial using single-agent balstilimab for recurrent metastatic cervical cancer were presented by O’Malley et al. Objective response rate was 14%, with 3/160 complete and 20/160 partial responses, and a duration of response of 15.4 months. Balstilimab showed more effect in PDL-1 positive cancers.
Gestational trophoblastic neoplasia
Ghorani et al. reported the use of pembrolizumab in four patients with unresectable drug-resistant gestational trophoblastic neoplasia (GTN). PD-L1 and human leukocyte antigen G (HLA-G) status as well as amount of TIL may be important identifiers of tumor response according to the case report. The authors cautioned potentially lasting fertility impairment given the persistent anti-trophoblastic immunity.
You et al. recently reported on the activity of the anti-PD-L1 checkpoint inhibitor avelumab. In the TROPHIMMUN phase 2 trial, 50% of patients with single-agent chemorefractory gestational trophoblastic disease were cured with avelumab. The safety profile was also favorable ( ).
Clinical use of combined immune checkpoint inhibition
Endometrial cancer
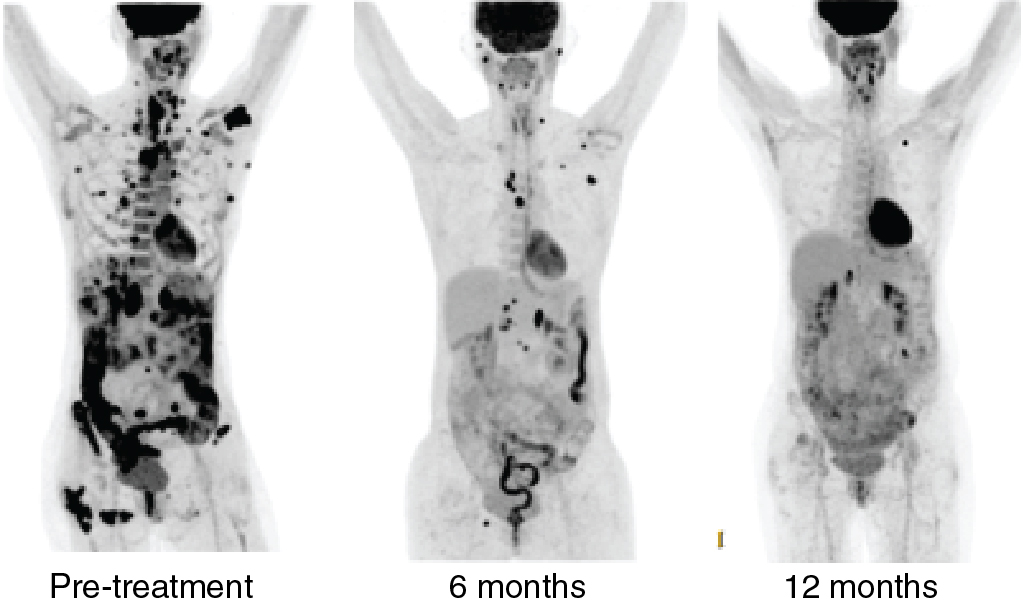
In a phase II trial, the combination of the multikinase inhibitor lenvatinib and pembrolizumab was tested in a PD-L1 and MSI-H unselected patient population with metastatic endometrial cancer. Objective response rate at 24 weeks was 39.6%. Thirty percent of patients had serious side effects, and one treatment-related death (intracranial hemorrhage) occurred. In the phase 3 randomized KEYNOTE-775 study, this combination was reported to have resulted in a statistically significant improvement in overall survival, progression-free survival, and objective response rate. Fig. 17.4 illustrates the treatment response seen in a patient with the combination of pembrolizumab and lenvatinib.
Ovarian cancer
There are emerging data on the combined use of the two different ICIs, i.e., ipilimumab, a CTLA-4 antibody, and nivolumab , a PD-1 antibody. It has been hypothesized that the different mechanisms of the checkpoint immune inhibitors potentiate their effect. While anti- CTLA-4 antibodies improve early activation and priming of T cells in lymph nodes, antibodies targeting the PD-(L)1 system act later in the process of T-cell tumor attack and locally in the tumor microenvironment. A recent phase II trial of this combination treatment in advanced rare malignancies included a cohort of 17 patients with gynecologic malignancies. The response rate was reported 41%, and stable disease in 29%. Another randomized phase II trial reported a 31.4% response rate for this combination treatment in recurrent ovarian cancer; however, the progression-free survival was only noted to be 3.9 versus 2.0 months. Serious adverse events were seen in 49% of patients. Interestingly, the response rate in patients with clear cell carcinomas was higher compared to high-grade serous ovarian cancer, an observation that has been reported in other studies. The reason for the possibly higher response rate in ovarian clear cell cancer is uncertain, but might be related to more efficient antigen presentation and greater T-cell infiltration.
Cervical cancer
Preliminary results of the ongoing phase I/II CheckMate-358 trial on the combination of ipilimumab and nivolumab in recurrent/metastatic cervical cancer regardless of PD-L1 status were presented at the ESMO 2019 meeting ( ). Two different application regimens were tested. Both showed objective response rates ranging from 30% to 46% dependent upon if the patient received prior systemic treatment. Twelve-month progression-free survival was found to be as high as 52.6% in patients without prior systemic treatment.
During ESMO 2020, preliminary results were not only presented for single-agent balstilimab but also for the phase II trial on the combination treatment using zalifrelimab/balstilimab . Objective response was 22% with 8/143 complete and 23/143 partial responses. The duration of response was not reached. Again, better responses were seen in PD-L1 positive cancers.
Resistance mechanisms to immune checkpoint inhibition
Various resistance mechanisms to ICIs have been described and an understanding of their complexity is emerging:
- 1.
Immunoediting, i.e., interactions between immune system and cancer cells, results in the selection of cell clones that lack neoantigens and exhibit poor immunogenicity.
- 2.
Downregulation of the major histocompatibility complex-I (MHC-I) and thereby loss of antigen presentation or loss of β2-microglobulin function which disrupts proper MHC-I folding and MHC-I transport to the cell surface.
- 3.
Establishing an immunosuppressive and tumor promoting microenvironment characterized by increased infiltration with Tregs and M2 macrophages mediated by immune modulating cytokines including TGF-β and VEGF-A.
- 4.
Expression of the indoleamine 2,3-dioxygenase 1 (IDO1) that converts tryptophan to kynurenine. Kynurenine accumulation has been associated with suppression of T-cell function.
- 5.
Enhanced co-expression of multiple immune checkpoints including TIM-3, LAG-3, and B and T lymphocyte attenuator (BTLA), which are associated with severe T-cell exhaustion.
- 6.
Antibiotic use can shift the relative abundances of bacterial species in the intestinal flora, reduce its diversity and thereby induce immune checkpoint inhibitor resistance, which is likely based on decreased cross-reactivity between the microbiome and tumor antigens, antigen presentation, and cytokine production.
Therapeutic cancer vaccines
Most cancer vaccines are designed to induce anti-tumor immune responses against specific TAAs. TAAs may be (i) antigens that are overexpressed in cancers, such as HER-2/neu, or mesothelin, (ii) cancer/germline antigens that are only expressed in germline cells, but can be re-expressed in cancer cells, e.g., melanoma associated antigen (MAGE-A1), New York esophageal squamous cell carcinoma 1 (NY-ESO-1), and (iii) cell lineage differentiation antigens, such as tyrosinase, and gp100. Therapeutic cancer vaccines can be classified by the vaccinating agent, i.e., how the TAA is delivered, (i) peptide/protein-based, (ii) cell-based, (iii) DNA/RNA-based, and (iv) glycan-based. Vaccines can be given alone or alongside cytokines or other stimulating factors. The development of therapeutic cancer vaccines has faced the following challenges: (i) low immunogenicity, (ii) established disease burden, and (iii) immunosuppressive tumor microenvironment. Examples of therapeutic cancer vaccines that have been used for gynecologic malignancies are presented ( Fig. 17.5 ).
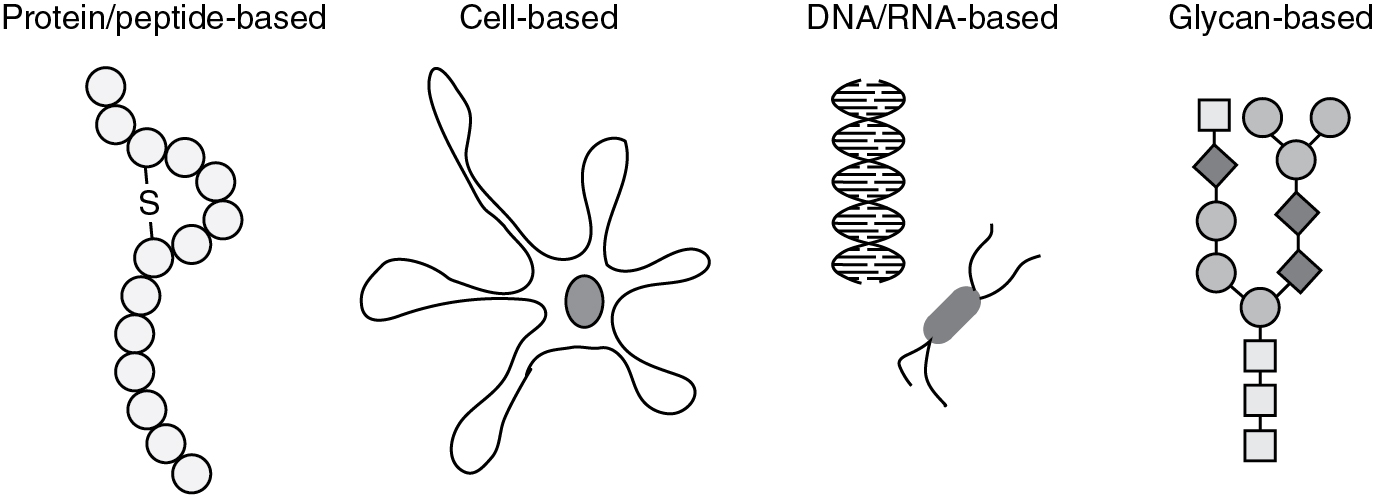
Peptide/protein-based vaccines
Coley’s first “vaccination,” the Coley’s toxin, falls into this category. Mixtures of ovarian cancer peptides or peptide fragments of the HER-2/neu receptor have been tested, mainly in the preclinical setting.
Ovarian cancer
One of the first proteins examined for an ovarian cancer vaccine therapeutic was HER-2/neu. Overexpression of the oncogene HER-2/neu is found in 15% to 30% of ovarian adenocarcinomas. Studies in humans have demonstrated that HER-2/neu MHC class I epitopes can induce IFN-γ-producing CD8+ T cells. HER-2/neu protein immunization promotes native HER-2/neu immunity as well as antibody epitope spreading. To date, there are various ongoing clinical trials involving HER-2/neu vaccines in ovarian cancer.
The tumor-suppressor protein p53 is overexpressed in almost all high-grade serous ovarian cancers. Antibodies against mutated p53 have been identified in approximately 25% of ovarian cancer patients. Though induction of p53-specific immunity has been achieved with well-tolerated vaccines, the clinical efficacy has been only modest thus far. The overall lack of clinical benefit with a p53-specific vaccine prompted strategies for combination therapies with immunomodulatory agents. Chemotherapy, specifically cyclophosphamide, has been shown to suppress Treg function. The presence of Tregs in ovarian cancer is a negative prognostic factor associated with decreased survival. Vermeij et al. combined their p53-SLP vaccine with cyclophosphamide and demonstrated a 20% stable disease rate ( ).
The Wnt/β-catenin pathway has been implicated in the alteration of the ovarian cancer tumor microenvironment through immune cell modulation by improving dendritic cell, T cell, and macrophage function. In ovarian cancer, WT1 expression is related to tumor type, grade, and stage, with WT1 expression highly associated with poor overall survival. Ohno and colleagues administered a modified WT1 peptide vaccine to 12 gynecological cancer patients with 3 patients demonstrating stable disease. In a phase II trial by Miyatake et al. 40 patients with gynecologic malignancies were given a WT1 peptide vaccine with 40% showing stable disease.
CA-125’s peptide epitope MUC16 promotes malignant cell growth and inhibits anti-tumor immune responses. In a large study of 119 advanced or recurrent ovarian carcinoma patients, Reinartz et al. utilized an anti-idiotypic antibody vaccine (ACA125) which mimics the CA-125 antigen. Overall, 68.1% were found to have an immunological response to the vaccine, with median overall survival (OS) of 19.4 months (range, 0.5 to 56.1 months). The subset of patients with antibodies to ACA125 had significantly longer survival times compared with negative responders (median 23.4 vs. 4.9 months, respectively).
The receptor tyrosine kinase Flt3 has also been studied as a potential vaccine antigen. In murine models, the Flt3 ligand enhances APC function and stimulates growth of NK cell precursors. In a pilot study by Freedman and colleagues, the Flt3 ligand was administered to patients with ovarian cancer and mesothelioma via intraperitoneal and subcutaneous routes; however, no objective responses were found.
Kalli and colleagues vaccinated ovarian and breast cancer patients with peptides based on FRα, a tumor antigen expressed in a variety of cancers such as ovarian, breast, and lung cancers. Following vaccination, IFN-γ-producing T cells were enhanced; however, no antibody responses were noted. All patients were alive at last follow-up of at least 2 years with a median relapse-free survival of 528 days in ovarian cancer patients in first remission and median survival was not reached for those in second remission.
The presentation of multiple peptides in a vaccine might theoretically increase the likelihood of generating T cell responses against a heterogenous tumor cell population compared to mono-valent vaccines. A polyvalent vaccine conjugated with keyhole limpet hemocyanin (KLH) and administered with OPT-821, an immunological adjuvant derived from the soapbark tree, was used in patients with ovarian, tubal, or primary peritoneal carcinoma of any stage. Positive IgM responses were found in less than 50% of patients with median OS of 47 months.
Efforts have been made to customize cancer vaccines based on preexisting tumor-specific antigens. Tsuda and colleagues reported on two regimens involving peptide vaccination in recurrent gynecologic cancers. In their first study, patients were administered predesignated peptide vaccines, while the second study vaccinated patients with peptides to which preexisting peptide-specific cytotoxic T lymphocyte precursors in peripheral blood were confirmed. No clinical responses were found with the first regimen; however, in the second approach 7 of 10 patients had enhanced peptide-specific cytotoxic T lymphocytes to additional peptides. Kawano and colleagues used a personalized peptide vaccine where antigens were selected based on preexisting host immunity. IgG responses were augmented in 96.7% of patients following the 12th vaccination; however, 31 of 37 cases showed disease progression.
New york esophageal squamous cell carcinoma 1
NY-ESO-1 is a so-called cancer-testis antigen (CTA). Its expression is restricted to testicular germ cells and placenta trophoblasts with no or only low expression in normal adult somatic cells. However, it is re-expressed in numerous cancer types and thereby is a good target for cancer immunotherapy.
Ovarian cancer.
For ovarian cancer vaccinations, epitopes of NY-ESO-1 as well as the full protein have been used. Diefenbach et al. vaccinated “high-risk” ovarian cancer patients (suboptimal tumor debulking, failure of CA-125 to normalize after three cycles of chemotherapy, or positive second-look surgery) with NY-ESO-1b peptide and Montanide ISA-51, a vaccine adjuvant. Median progression-free survival was found to be 13 months. Sabbatini and colleagues investigated the use of overlapping long peptides from NY-ESO-1 in combination with two different vaccine adjuvants in ovarian cancer patients in second or third remission. Of the 28 patients enrolled, 6 had no evidence of disease with a progression-free survival range of 17 to 46 months.
NY-ESO-1 is regulated by DNA methylation, and preclinical studies have demonstrated enhanced NY-ESO-1 expression and NY-ESO-1-specific cytotoxic T lymphocyte-mediated responses in ovarian cancer cell lines when treated with decitabine, a DNA methyltransferase inhibitor. This observation provided the rationale for a clinical trial by Odunsi et al. in ovarian cancer patients. NY-ESO-1 vaccine, decitabine, and GM-CSF were administered to determine if epigenetic modulatory drugs improved antitumor response. Of the 10 patients evaluable for clinical response, 1 had a partial response/disease remission and 5 had stable disease. The limited success is possibly related to the low immunogenicity of the vaccine.
DPX-survivac
DPX-survivac is a mix of human leukocyte antigen (HLA) class I peptides designed to trigger T-cell response against survivin.
Ovarian cancer.
This vaccine was delivered for recurrent ovarian cancer in combination with low-dose cyclophosphamide and epacadostat. The vaccine was well tolerated and showed antitumor response with disease control in 9 of 13 patients. A correlation between tumor size and response was noted with lesions <5 cm displaying increased tumor regression. This phase Ib/II trial is ongoing, with a phase II part that randomizes DPX-survivac and cyclophosphamide with and without epacadostat , an inhibitor of indoleamine 2, 3-dioxygenase-1 (IDO-1) that may enhance effector T-cell proliferation. Preliminary results revealed that DPX-survivac with cyclophosphamide induces strong T-cell response even without epacadostat.
Cell-based vaccines
The majority of cell-based vaccines have used dendritic cells. These APCs can be derived from a patient’s own monocytes. After leukapheresis, the monocytes are differentiated ex vivo into immature dendritic cells which are then stimulated and loaded with antigens and thereby matured. The mature dendritic cells present tumor antigens on their MHCs; finally, these mature dendritic cells are administered to the patient. Once re-infused, the mature dendritic cells will migrate to lymphoid organs and activate effector cells of the immune system, primarily T and B cells. Thereby, cancer immunosuppression might be overcome and immunosurveillance reinstated. Dendritic cells have been loaded with (i) tumor lysates or (ii) synthetic peptides, e.g., fusion proteins of HER-2/neu and granulocyte-macrophage colony-stimulating factor (GM-CSF) or peptides derived from WT-1, MUC-1, and Ca-125.
Ovarian cancer
Brossart and colleagues administered HER-2/neu or MUC1-derived peptide-pulsed dendritic cells in heavily pretreated metastatic breast and ovarian cancer patients. One patient with progressive ovarian cancer had stable disease over 8 months while on therapy. This study paved the way for additional peptide-pulsed dendritic cells vaccination therapies. Loveland et al. used dendritic cells pulsed with mannan-MUC1 fusion protein in 11 patients with adenocarcinomas. One ovarian cancer patient showed stable disease over 3 years of treatment. Peethambaram et al. administered dendritic cells loaded with recombinant HER-2/neu peptide and a GM-CSF domain. Two out of four ovarian cancer patients demonstrated stable disease over 15.7 to 18.3 months. WT1 peptide vaccines have had modest efficacy as demonstrated in various studies. The addition of low-dose cyclophosphamide prior to vaccination can potentially enhance vaccine potency. One study by Chu et al. used a HER-2/neu, hTERT, and PADRE peptide pulsed vaccine for maintenance therapy of recurrent ovarian cancer. Six of 11 patients had no evidence of disease at 36 months, and the 3-year progression-free survival was 80% with cyclophosphamide compared with 40% without. More recently, Gray et al. applied a dendritic cell vaccine as maintenance therapy in ovarian cancer patients previously treated with 1 or 2 lines of conventional chemotherapy in complete remission. CAN-003 was a phase 2b trial using a MUC-1 protein-targeted dendritic cell vaccine. The treatment did not result in an overall increase in progression-free or OS; however, a subgroup analysis revealed that patients in complete remission after second-line therapy had improved OS with vaccination compared to control (median OS 25.5 months with standard therapy vs. OS not yet reached with vaccination).
Morisaki and colleagues administered neoantigen peptide-pulsed dendritic cells in a case study of a woman with advanced-stage ovarian cancer. Following four rounds of vaccination, the patient had a significant decline in CA-125 levels with evidence of neoantigen-specific cytotoxic T lymphocytes induced by vaccination.
Dendritic cell vaccines electroporated with mRNA that is subsequently translated into protein have also been studied. Hernando and colleagues transfected dendritic cells with mRNA-encoded FR-α. Another study by Coosemans et al. loaded dendritic cells with WT1 mRNA and found a 2-month progression-free and 64-months OS in patients with serous ovarian cancer.
Whole tumor lysate-loaded dendritic cells harness a variety of antigens associated with a specific tumor. In theory, using neoepitopes from tumor mutations will increase efficacy over single-antigen vaccines. Bapsy et al. administered a whole tumor lysate-pulsed dendritic cell vaccine to 51 patients with advanced solid malignancies. Of the seven ovarian cancer patients, one had a partial response and two had stable disease while on therapy. Hernando et al. vaccinated patients with advanced gynecologic malignancies with dendritic cells pulsed with KLH and autologous tumor cell lysate. Mean progression-free interval while under vaccination was 25.5 months for patients with progressive or recurrent ovarian cancer.
Tanyi et al. tested a personalized vaccine generated by autologous dendritic cells pulsed with oxidized autologous whole-tumor cell lysate. The vaccine was injected into accessible lymph nodes in recurrent ovarian cancer patients and either administered alone, in combination with bevacizumab, or with bevacizumab plus low-dose intravenous cyclophosphamide. The treatment induced T-cell responses to autologous tumor antigens and amplified T-cell responses against mutated neoepitopes previously unrecognized. Overall survival of patients who showed vaccine treatment responses was 100% at 2 years compared with 25% in non-responders.
For the DCVAC/ OvCa randomized phase II trial, dendritic cells were loaded with antigens obtained from ovarian cancer cell line lysates ( ). The interim analysis of this trial showed an increase of the progression-free survival by 6 months and a trend toward increased OS in the group that received chemotherapy plus dendritic cell vaccine maintenance treatment after primary debulking surgery ( ).
Ongoing studies are underway to compare autologous oxidized tumor-lysate–loaded dendritic cells with a 10-peptide neoantigen based dendritic cell vaccine.
DNA/RNA-based vaccines
Recombinant vaccinia and fowlpox vectors
Recombinant vaccinia and fowlpox vectors for prime and booster vaccinations have been used to induce in vivo expression of the TAAs in somatic cells such as keratinocytes or myocytes. This approach has been tested in ovarian cancer using the genetic information of NY-ESO-1 and the combination of carcinoembryonic antigen (CEA) and MUC-1 combined with co-stimulatory proteins.
Ovarian cancer.
Jager et al. utilized recombinant vaccinia-NY-ESO-1 (rV-NY-ESO-1) and recombinant fowlpox-NY-ESO-1 (rF-NY-ESO-1) vaccines in patients with NY-ESO-1-expressing tumors. In this study, patients were treated with rV-NY-ESO-1, rF-NY-ESO-1, or rV-NY-ESO-1 followed by rF-NY-ESO-1. One advanced ovarian cancer patient included in the cohort treated with rV-NY-ESO-1 had a clinical response and remained disease-free for 8 months following treatment. Odunsi and colleagues used rV-NY-ESO-1 and rF-NY-ESO-1 in advanced epithelial ovarian cancer and melanoma. Of the 22 ovarian cancer patients, 42% had antibody seroconversions with spontaneous CD4+ T-cell responses detected in 68% of patients. Fourteen percent had preexisting CD8+ T-cell responses, which increased to 45% post vaccination.
Gulley and colleagues conducted a pilot study with PANVAC, a recombinant poxviral vaccine containing CEA and MUC-1 transgenes in combination with 3 co-stimulatory molecules (B7.1, intracellular adhesion molecule-1, and lymphocyte function-associated antigen 3—collectively known as TRICOM). The antigens were expressed by a vaccinia virus (PANVAC-V) for primary vaccination and fowlpox (PANVAC-F) for multiple booster vaccinations. Of the ovarian cancer patients treated with PANVAC, median progression-free survival was 18 months and median OS 19 (range, 6–21). A follow-up study using PANVAC in a heavily pretreated cohort of progressive metastatic breast and ovarian cancer was reported by Mohebtash and colleagues. In 14 ovarian cancer patients, median progression-free survival was 2 months and median OS 15.0 months.
More recently, Hardwick and colleagues evaluated a Modified Vaccinia Ankara vaccine, an attenuated poxvirus vaccine, delivering wild-type p53 (p53MVA) in platinum-resistant ovarian cancer. Patients received a combination of p53MVA and gemcitabine. There was one partial response and three patients with stable disease, with a median progression-free survival of 3 months. Five of the 11 patients demonstrated increased p53-reactive CD4+ and CD8+ T cells. In a subset analysis, there was a significant difference in median progression-free survival between responders and non-responders (7.0 vs. 2.3 months, respectively).
Engineered listeria monocytogenes
Attenuated live Listeria monocytogenes have been used as bacterial vectors. Listeria are facultative intracellular bacteria. In the cytoplasm, Listeria can induce MHC class I and II pathways and thereby T-cell responses and decrease in Tregs.
Cervical cancer, ovarian cancer.
In early cervical cancer studies, Listeria was used to deliver an HPV E7 plasmid; however, the risk of plasmid loss or antibiotic resistance was noted. More recently Listeria has been engineered to express a listeriolysin-E7 fusion protein (ADX11-001) which has been successfully applied in preclinical models. A phase II trial in recurrent and persistent cervical cancer showed an overall survival of 8.3 versus 8.8 months of ADX11-001 without and with cisplatin and a promising 34.9% combined 12-month overall survival rate. In ovarian cancer, Listeria has been used to express mesothelin.
Glycan-based vaccines
Most cancer cells display an altered glycosylation pattern. In fact, cancer cells are likely using unique glycosylation patterns for immune evasion. Pure glycan-based vaccines, however, showed only modest success because of poor immunogenicity and need to be conjugated to helper T-cell epitopes.
Lewis(y)
In a phase I trial, the synthetic penta-saccharide Lewis was coupled to the KLH carrier protein and used to vaccinate patients with recurrent and persistent ovarian cancer. At 18 months follow-up, 5 of 24 patients showed a complete response.
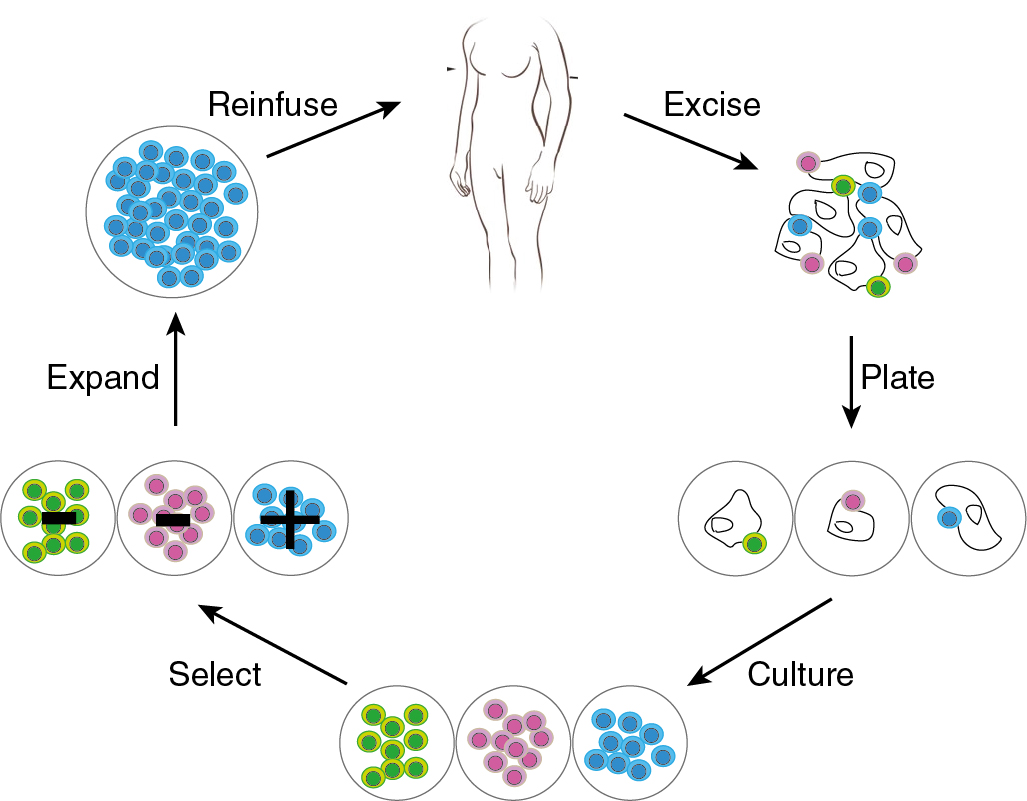
Adoptive cell therapy
Adoptive cell therapy is also known as cellular immunotherapy. Adoptive cell therapy uses patients’ own immune cells by (i) extracting T infiltrating lymphocytes from tumor tissue (TILs) or by (ii) engineering autologous T cells with tumor antigen specific surface receptor molecules. TCR therapy, CAR T cell therapy, and more recently, engineered NK cells, are being studied in various clinical trials. Among the most common targets for TCR- or CAR-directed cell therapy are NY-ESO1, mesothelin, and the folate receptor.
Tumor-infiltrating lymphocytes therapy
In the 1980s, Steven Rosenberg and colleagues performed the first preclinical studies in mice with TILs. The TILs were isolated, ex vivo stimulated, and re-infused. Similar to then described experiments, the current TIL therapy includes ex vivo expansion of TILs from resected primary tumor material, and re-infusion of expanded TILs. Cell infusion is preceded by a lymphocyte-depleting conditioning regimen and followed by high-dose IL-2 support ( Fig. 17.6 ).