Fig. 9.1
Immunohistochemical staining for ALK is typically diffuse with granular cytoplasmic staining (ALK D5F3, 200×). Image courtesy of Dr. Natasha Rekhtman
c-ros Oncogene 1 (ROS1)
The prevalence of patients with ROS1 rearrangements is 2% or less. FIG-ROS1 fusion was the first to be described although numerous other fusion partners such as SLC34A2, CD74, and TPM3 are actually more commonly identified in lung cancer and the number of translocation partners will likely continue to grow. Like ALK-rearranged tumors, carcinomas with ROS1 rearrangements are largely limited to adenocarcinomas, with similar caveats regarding testing flexibility. Patients harboring ROS1 rearrangements have been shown to have an objective response rate of 72% to crizotinib, and as such, the drug was approved by the FDA for use in these patients in 2016 [9, 16, 19, 20].
ROS1 rearrangements are generally detected by FISH testing using a break apart probe, although RT-PCR methods, particularly those using capture-based sequencing strategies, may also be employed. Given the low prevalence of ROS1 rearrangement in lung cancer, a cost-effective screening method is desirable. Studies evaluating ROS1 immunohistochemistry have used a single commercially available antibody clone, D4D6 (Cell Signaling Technology). Most studies demonstrate a sensitivity of 100% relative to FISH or RT-PCR but the specificity ranges from 92 to 100%. Several cutoffs and scoring systems have been used, but a meta-analysis determined a sensitivity of 95.87% and a specificity of 93.52% when a staining intensity of at least 2+ was used. Indeed, most tumors with confirmed ROS1 rearrangements by FISH or PCR have moderately intense staining expression; however, there is no universally accepted scoring system [9, 16, 19–24]. Further, unlike ALK IHC, ROS1 IHC can be much more difficult to interpret. While most often cytoplasmic and diffuse (Fig. 9.2), ROS1 staining can be patchy and weak and may show different staining patterns depending on the fusion present (i.e., granular/globular in CD74-ROS1 fusions and weak membranous staining in EZR-ROS1 fusions). Additionally, weak patchy staining can be seen in up to a third of tumors that do not have an underlying rearrangement. An additional complication is that the low frequency of ROS1-rearranged tumors limits the availability of material needed for validation [19, 20, 23–25]. Based on these limitations, ROS1 IHC may be used as a screening tool, but forthcoming guidelines recommend that ROS1 IHC-positive results undergo confirmation with FISH or a molecular method prior to considering a patient a candidate for targeted therapy. However, it is felt that there is strong enough evidence on the high sensitivity of the ROS1 IHC that tumors that lack ROS1 staining can be interpreted as negative for ROS1 fusion [12].
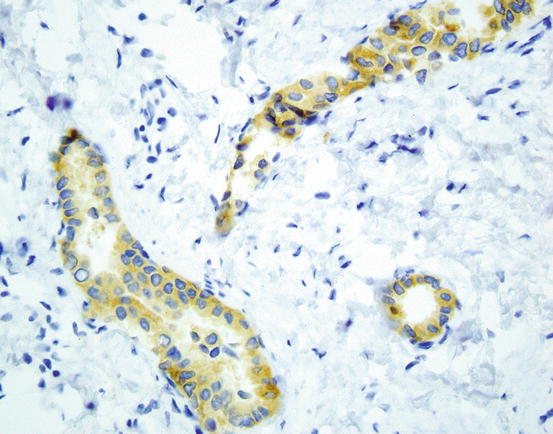

Fig. 9.2
Immunostaining for ROS-1 shows diffuse cytoplasmic staining in most positive cases (ROS-1 D4D6 400×). Image courtesy of Dr. Natasha Rekhtman
Epidermal Growth Factor Receptor (EGFR)
While the 2013 CAP/IASLC/AMP guideline allowed for the use of EGFR IHC with antibodies directed against specific mutant epitopes, the overall performance of such antibodies is generally suboptimal for consistently reliable detection of EGFR mutations with the exception of L858R which is highly specific for ELREA746_750 deletion [26–30]. While there may be a role for IHC in extremely limited samples or limited resource settings, advances in molecular technology enable analysis of very limited samples and circulating tumor DNA so routine use of EGFR antibodies is not advisable for routine use in selecting lung cancer patients for EGFR directed therapies.
BRAF
BRAF mutations in NSCLC include the c.1799 T > A9p.V600E point mutation seen in many other cancer such as melanoma; however, may other BRAF mutations occur in lung adenocarcinoma including both mutations in nearby amino acids as well as other substitutions at V600. Therefore, the use of immunohistochemical stains typically used in evaluation of melanoma which detect on V600E mutations will miss non-V600E mutations encountered in lung cancer and a method which evaluates the entire BRAF coding region should be utilized instead [31–35].
Other Genes
Potentially targetable abnormalities that occur in relatively small percentages of lung cancers are ever increasing. In general, it is recommended that these mutations should be tested as part of a multiplex testing panel and not performed routinely as a stand-alone test [12]. Immunostains applicable to most of these abnormalities (RET, ERBB2, MET) have generally not provided consistent results in comparison to molecular testing methodologies [25, 36]. However, similar to other low-level mutations, a low-cost screening method is attractive, particularly in low-resource settings and will likely continue to evolve. In regard to RET, mutations are rare, and while initial trials showed promise, there is currently limited evidence of therapeutic benefit although it remains under investigation [25]. RET IHC (ab134100 abcam, Cambridge, UK, with detections systems EnVision + DAKO, Denmark) produces diffusely granular cytoplasmic staining of moderate to strong intensity, occasionally with membranous or perinuclear accentuation. This antibody reported in sensitivity of 100% and a specificity of 88% in comparison to other methods but further investigation is needed [31, 36]. Conversely, due to the variety of mutations present, IHC testing for ERBB2 (Her2) as is commonly performed in breast cancer cases is not recommended for lung cancer cases [37].
Programmed Cell Death Ligand 1 (PD-L1)
Immunotherapy for treatment of lung cancer has rapidly evolved to become part of the standard of care for many patients with advanced NSCLC. The principle of immunomodulatory therapy is based on its ability to disrupt inhibitory signals between tumor and immune cells, usually T-cells. While other signaling processes exist, current lung cancer therapies focus primarily on the interaction between PD-L1 , expressed on tumor cells and programmed cell death protein 1 (PD-1) on T-cells, the interaction of which enables the tumor cell to evade the T-cell response to the tumor. By blocking either PD-1 or PD-L1, T-cells are then able to recognize and respond to foreign antigens on the cancer cells.
In recent years, the landscape of immunotherapy for lung carcinoma as well as other malignancies has evolved rapidly, but has resulted in complexities for pathologists in regard to testing tumors to determine potential eligibility for therapy. Currently, there are five different inhibitors of either PD1 or PD-L1, each of which currently has a paired proprietary anti-PD-L1 immunohistochemical antibody. Currently, in the United States, nivolumab is paired with Dako 28.8 PharmDx kit, pembrolizumab with Dako 22C3 PharmDx kit, atezolizumab with Spring Bioscience SP142 clone, durvalumab with Ventana SP263 clone, and avelumab with Dako 73-10 clone, the first three of which are currently FDA-approved for use in non-small cell lung cancer. In addition to the fact that each drug is paired with a proprietary antibody, each has different scoring systems for positivity, and the antibodies should be run on specified staining platforms (Table 9.1). Positive tumor staining for each antibody is based on a tumor proportion score defined as partial or complete membranous staining of any intensity (Figs. 9.3 and 9.4), while interpretation of the SP142 antibody additionally requires recording the percentage of positive tumor immune cells. Additionally, other non-FDA-approved antibodies also exist as laboratory developed tests (LDT) , such as E1L3N rabbit monoclonal and 9A11 mouse IgG1 clones from Cell Signaling Technologies; ab58810 rabbit polyconal from Abcam; MIH1 mouse monoclonal from Thermo Fisher Scientific; 015 rabbit IgG from Sino Biological; 7G11 mouse IgG1 from Freeman Laboratory, Dana Farber Cancer Institute; and 5H1 mouse monoclonal from Lieping Chen Laboratory Yale School of Medicine [38]. Further, drug approval and availability may differ in different parts of the world. In the United States, Dako 22C3 is FDA approved as a companion diagnostic (i.e., required) for prescribing pembrolizumab, while Dako 28-8 and Ventana SP142 are FDA approved as complimentary (recommended) diagnostic tests for nivolumab and atezolizumab, respectively. The Ventana SP263 clone is currently FDA approved as a complementary diagnostic for durvalumab in the United States for bladder cancer with approval for lung cancer anticipated at the time of this writing; however, this antibody is CE marked in Europe for determining eligibility for nivolumab and, more recently, pembrolizumab in non-small cell lung cancer. While the current text is up to date at the time of writing, given the rapidly evolving approval processes and recommendations for patient testing, it is recommended that the reader additionally refer to locally applicable resources for the latest testing guidelines and recommendations.
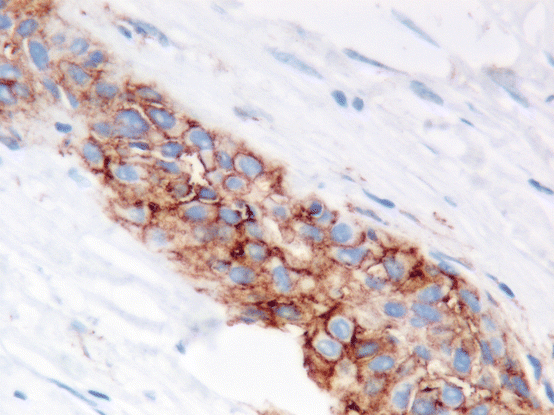
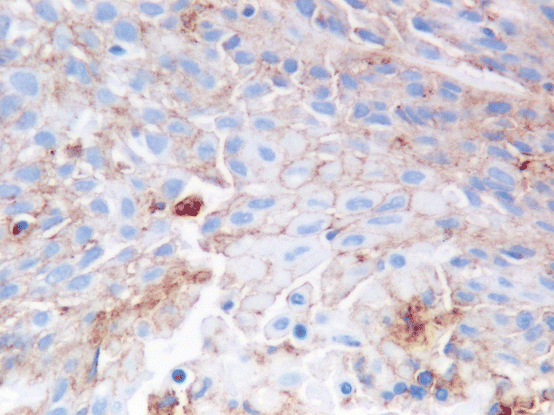
Table 9.1
Summary of immune therapy drugs , corresponding anti-PD-L1 antibody, staining platform, and scoring parameters
Drug | Antibody clone | Developer | Platform/detection system | Tumor cells scored | Immune cells scored | Current scoring for positive test |
|---|---|---|---|---|---|---|
Nivolumab | 28-8 | Dako | Dako Link 48 Envision Flex | Yes | No | ≥1% |
Pembrolizumab | 22C3 | Dako | Dako Link 48 Envision Flex | Yes | No | ≥50%-first line ≥1%-second line |
Atezolizumab | SP142 | Spring Bioscience | Ventana BenchMark | Yes | Yes | TC3/IC3 PD-L1 ≥50% TC2/IC2 PD-L1 5–49% TC1/IC1 PD-L1 1–4% TC0/IC0 PD-L1 <1% |
Durvalumab | SP263 | Ventana | Ventana BenchMark | Yes | No | ≥25% |
Avelumab | 73-10 | Dako | Dako Link 48 Envision Flex | Yes | No | ≥1% |

Fig. 9.3
Positive staining for PD-L1 is defined as partial or complete membranous staining of any intensity. This example shows strong membranous staining. Cytoplasmic staining should be discounted (Dako 22C3 Pharm Dx kit 400×)

Fig. 9.4
In this example, PD-L1 staining is weaker and only partially stains the cells membrane, but would still be considered as positive staining (Dako 22C3 Pharm Dx kit 400×)
Challenges to the pathologist with the currently approved testing approach are myriad given the various antibodies, scoring systems, and testing platforms. As such, a practical solution, preferably with a single PD-L1 antibody which may be run and validated on a range of staining platforms, would be desirable. Several efforts to compare antibodies have been undertaken or are currently ongoing. To date, studies evaluating Dako 22C3 and 28-8 antibody kits have shown fairly good concordance of staining between these two antibodies. For example, in the BLUEPRINT phase 1 study, 14% of cases stained with 28-8 and 22C3 were discordant, and there was 72% concordance in the German harmonization study. Both studies demonstrate a lower percentage of staining of tumor cells with the SP142 antibody [39, 40]. SP263 has also been shown to demonstrate good concordance with 22C3 and 28-8 antibodies [39]. Similar results regarding concordance among 22C3, 28-8, and SP263 but not with SP142 have also been reported by Ratcliffe et al. and Rimm et al. [41, 42]. The Rimm study also found good concordance with the LDT E1L3N [42]. This particular LDT has been shown to have good sensitivity compared to other LDTs but further study is needed for this group of tests [38]. Another major issue is the requirements for particular staining platforms/detection system for a given antibody, a prospect that is generally not realistic for most laboratories. Initial studies evaluating cross platform concordance for 28-8, 22C3, and SP263 antibodies have shown good concordance [43].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree