40.1
Introduction
Fuller Albright speculated that removal of the stress and strain on the skeleton would result in bone loss . Of the total noninstitutionalized population in the United States in 1988, 3.8% (8.8 million persons) were estimated not to be able to perform any major activity . Immobilization osteoporosis represents a wide spectrum of conditions and disorders. A few representative examples illustrating the range of the rates of bone loss include bed rest at 0.1% per week, microgravity, 0.25% per week, and spinal cord injury (SCI), 1% per week for the initial months for the trabecular regions of the long bones of the lower extremities . For comparison, postmenopause, a nonimmobilizing condition associated with bone loss for which heightened awareness exists in the medical community, bone is lost at 3%–5% per year when an antiresorptive medication is not prescribed . Immobilizing conditions all have a reduction in mechanical load in common. Bone structure adapts to provide optimal strength for the variety of mechanical forces sustained in performing daily activities, which dramatically change during extended periods of immobilization or disuse. Changes in the total mass and spatial distribution of bone would be anticipated to change, with variable rates of cortical and trabecular bone loss. In addition to reduced load, neurally-mediated mechanisms, including influence of the autonomic nervous system and/or motor endplate activity, as well as vascular changes, may play a role in inducing and/or perpetuating bone loss. The etiology and severity of the immobilizing condition, anatomical region, age, gender, genetic factors, and duration of immobilization are but a few of the considerations that determine the location, magnitude, and characteristics of accelerated skeletal deterioration. If bone loss is quite rapid and marked, there is in an increased risk of fracture in the near term; if the bone loss is less severe, the risk of fracture may still be elevated when combined with age-related architectural changes and bone mineral loss. Although efficacious strategies have been developed for other forms of osteoporosis (e.g., sex-hormone deficient, glucocorticoid-induced, nutritional deficiency-related, etc.), the ability to maintain bone when load is markedly reduced acutely is, at present, far from clinically satisfactory.
The central role of the osteocyte in bone biology in sensing and transducing skeletal loads is now accepted dogma (see Chapter 7 : Osteocytes). Because of its location in the bone and its complex dendritic network, the osteocyte is the skeleton’s major mechanosensory cell. To sense load the osteocyte can use its cell body, dendritic processes, or bending of cilia. The mechanobiology of the osteocyte in responding to bone deformation, or its absence, is largely responsible for the conversion of a mechanical to a biochemical signal (see Chapter 14 : Cellular and molecular mechanotransduction in bone). Mechanical unloading directly influences osteocyte gene expression. A prime function of the osteocyte is to regulate mineral deposition and bone remodeling, and this function requires active osteoclasts. In many forms of severe acute immobilization, the increase in osteoclast numbers on the bone surface is marked . The release of receptor activator of nuclear factor kappa-B (NF-κB) ligand (RANKL) may be at least partially responsible for the differentiation, proliferation, and activation of osteoclasts in at least some forms of disuse-related bone loss . Another novel mechanotransduction pathway has been suggested: because the metabolic needs of the osteocyte are met by a combination of passive and enhanced diffusion that occur when the tissue is loaded during functional activity; depriving bone of mechanical loading has been hypothesized to curtail enhanced diffusion and quickly result in osteocyte hypoxia. Indeed, experimentally-induced immobilization has been shown to induce oxygen deprivation to the osteocyte, which rapidly promotes osteocyte apoptosis . The loss of osteocytes could be speculated to reduce the ability of bone to respond to load thereafter, even if subsequent applied loads are akin to forces experienced prior to the immobilizing condition. Several adult animal models and human conditions tend to confirm that once a chronic phase of immobilization has developed, restoration of bone mass and architecture is difficult to achieve, if possible at all .
A number of in vitro and animal models of immobilization have been investigated. Such models, it is appreciated, will never identically replicate the experimental or pathological conditions in man, whether spaceflight, voluntary bed rest, stroke, SCI, or other immobilizing conditions. However, animal models have afforded invaluable knowledge and understanding of the pathological processes that occur with skeletal unloading, and such investigative approaches have provided useful insight into analogous immobilizing conditions in man. Cellular, biochemical, and molecular studies in rodents have provided exciting new information that have supported the development of novel strategies to treat patients, astronauts, or individuals with temporary or more chronic conditions associated with unloading of bone. Our ability to define more clearly the cellular and biochemical derangements of immobilization from animal models, in combination with novel pharmaceutical approaches and improved imaging methodologies, should permit more accurate and reliable testing of scientifically appropriate, effective interventions to retard, prevent, or, possibly, reverse skeletal catabolism. It is envisioned that within the foreseeable future, highly efficacious approaches to prevent the bone loss of the most aggressive forms of immobilization-induced osteoporosis will finally be a reality.
40.2
Animal studies
40.2.1
Unloading and reloading on bone mass, metabolism, and architecture
Unloading of the skeleton results in rapid and marked loss of bone mass and strength. Animal studies have allowed systematic investigation of the characteristics of bone loss resulting from immobilization and the underlying cellular and molecular mechanisms. Many investigations have been conducted regarding the osseous effects of immobilization by placing a cast, tenotomy, or paralysis induced by nerve transection or SCI. These studies in experimental animal models have provided an understanding of their similarities and differences with regard to the effects of immobilization on osteoclast activation, osteoblast depression, uncoupling of remodeling, magnitude of loss in trabecular compared to cortical regions, rapidity of temporal loss, and the age-related ability of bone to regain substance and strength following remobilization. Increasing evidence supports the existence of humoral connections between muscle and bone that may influence bone responses to unloading. Insights from these studies have strongly influenced the development and application of therapeutic approaches to immobilization-related bone loss.
40.2.1.1
Limb casting
In rats, following immobilization of a hindlimb with a cast, bone mass assessed by dry weight of bone ash declined in tibia and femur . Bone loss continued for at least 24 weeks for the tibia but appeared to plateau for the femur . When the cast was removed, osteoporosis was still apparent after reloading for 4 but not after 16 weeks . In a more recent study, after 3 weeks of immobilization by casting, bone ash weights were reduced by 12% in tibia and 13% in femur. Torque strength of tibia and femur was reduced by approximately the same amounts . Significant improvements in bone mass were observed at 3 weeks after removing the cast, with near-normal bone mass observed by 10 weeks . In histomorphometric studies, female mice with a knee and ankle joint immobilized with a cast demonstrated marked loss of trabecular bone that was evident by 6 days, approaching 50% by 21 days and associated with smaller, though significant, reductions in cortical thickness and area with decreased bone strength. These changes were accompanied by histologic changes consistent with increased bone resorption and reduced bone formation. Osteoblast numbers were reduced by 3 days and declined by nearly 80% at 21 days, which was associated with decreases in mineral apposition and bone formation rates. Osteoclast numbers increased by threefold at 3 days and remained similarly elevated to 21 days .
40.2.1.2
Tenotomy
Studies of the effects of knee tendon transection on bone show early alterations in indices of bone resorption and formation similar to those reported after limb casting. In one such study using growing male rats, tenotomy reduced femoral bone mass by approximately 15% at 10 days with an approximate 50% reduction in trabecular bone volume . These alterations were preceded by increased osteoclast numbers at 30 and 72 hours and reduced mineral apposition rate in the secondary spongiosa. Effects of tenotomy on bone mass were similar to the effects of sciatic nerve transection assessed from parallel experiments. In a separate study in male rats, knee tenotomy increased osteoclast number and resorption surface in trabecular bone of the tibial metaphysis at 72 hours and reduced trabecular bone volume by approximately 50% at 240 hours; the bone formation rate, assessed by calcein labeling, was reduced at 240 hours by approximately 40% .
40.2.1.3
Spaceflight
In some studies, spaceflight reduced bone mass and bone formation rates for flights with durations of 10 days or more but not for flights of shorter durations . A decreased bone formation rate was associated with diminished osteoblast perimeter and osteoid perimeter . Of interest, there was no significant change in osteoclast number or activity during spaceflight. Efforts to understand why bone loss was observed after some flights but not others included assessments of the effects of caging animals individually or in groups. When rats were housed in groups, effects of spaceflight on bone mass and bone formation rates for weight-bearing bones were eliminated, suggesting that stress or other factors related to isolation of the animals, particularly the likelihood of diminished activity or stress-related social isolation when caged alone, are responsible for the observed reduced bone formation .
40.2.1.4
Hindlimb unweighting
Immobilization by tail suspension was developed as an animal model to simulate the effects of spaceflight on bone and results in decreased bone mass in unloaded hindlimbs . This hindlimb unloading model has been extensively studied with respect to effects on bone mass, architecture, metabolism, and quality. Both young rats that are rapidly growing and, consequently, have higher rates of bone remodeling and adult rats have been studied by this experimental approach. In studies of changes in bone after hindlimb unloading of 5-month-old male rats, micro-CT analysis was used to evaluate changes in bone mass and structure . Cortical bones of the tibia and femoral neck were similar for groups that were unloaded for 28 days, but significant decreases of 21% for proximal tibia and 20% for femoral neck were observed for cancellous bone. In a similar study of 6-month-old rats, 14 days of hindlimb suspension did not impair bone elongation rate but reduced tibial calcium content, ash weight and bone mineral content . In mice, hindlimb unloading by tail suspension for 10 days reduced cancellous bone volume of the proximal tibia, as assessed by micro-CT imaging .
Tail suspension has generally been found to reduce bone formation rates. In 6-month-old rats that were tail suspended , mean periosteal bone formation rates at the midshaft of the tibia were reduced by approximately 80% at 14 days. In the proximal tibia at 1 week the osteoblast numbers on the cancellous bone surface were reduced by 66% by unloading, and cancellous bone formation rate was reduced to a similar extent, with changes persisting at 3 and 5 weeks. In a different study that used an in vitro assay of the osteoblastic potential of bone marrow precursors, unloading reduced by nearly half the number of bone nodules observed .
The reductions in bone formation rate and osteogenic potential of marrow stromal cells reported after tail suspension have generally correlated with reduced expression of genes indicative of osteoblast differentiation and/or activation. During the first 7 days after unloading, osteocalcin mRNA levels decreased in metaphyseal bone and femoral midshaft , and these transcriptional changes were temporally related to reduced serum osteocalcin levels . Both gene expression and blood levels returned to normal by 14 days. A similar pattern of changes was reported for type 1 collagen in metaphyseal bone . Expression of IGF-I demonstrated a more complex pattern of alterations, with reduced mRNA levels early and then elevated levels compared to controls at later time points , which was interpreted as a compensatory response to diminished bone formation rates after hindlimb suspension. Of interest, exogenous IGF-I was reported to have a blunted capacity to stimulate bone formation in hindlimb suspended rats ; thus a probable defect in signaling responses may further reduce osteoblastic function in the presence of reduced local IGF-I levels. Hindlimb suspension has also been reported to reduce TGF-ß2 mRNA expression in osteoblasts and preosteoblasts of the cambial layers, and in osteocytes .
Tail suspension has been found to stimulate bone resorption and increase osteoclast numbers and osteoclast surface. In young rats, hindlimb suspension caused a transient increase in urinary excretion of deoxypyridinoline, a collagen breakdown product consistent with increased bone resorption. In both young and old rats, hindlimb suspension resulted in mild elevations in serum calcium, reduced parathyroid hormone (PTH) concentrations, and diminished circulating levels of 1,25 dihidroxyvitamin D 3 , a metabolic profile suggestive of resorptive hypercalcemia; reductions in 1,25-dihydroxyvitamin D 3 have been reported to be transient, consistent with the reports previously described of acute expansion of osteoclast numbers and/or surface after tail suspension with subsequent normalization of these indices . Studies in adult rats that were tail-suspended for up to 5 weeks failed to show evidence of increased bone resorption . Whether bone resorption was below detection thresholds in these analyses is not clear. A modest reduction in blood testosterone levels has also been reported in male rats after hindlimb suspension, and testosterone replacement reduced bone loss due to hindlimb suspension .
Reloading reverses many of the effects of hindlimb suspension, although the time required to reverse bone loss may be considerably longer than the period over which bones were unloaded. In a hindlimb unloading model, effects of reloading after 14 days of unloading on bone formation rates were examined. Unloading reduced bone formation rates at the tibiofibular junction, and this decrease was reversed by 6 days of reloading . In a similar study, 14 days of reloading normalized bone volume and partially normalized bone formation rate and osteoblast number . In adult rats, reloading for 28 days partly, though not completely, reversed reductions in bone weight, ash weight, and mineral content, resulting from 14 days of hindlimb suspension .
40.2.1.5
Spinal cord injury
Effects of SCI on bone have only recently been investigated in animal models. In one study, bone effects of sciatic nerve transection were compared to spinal cord transection in young, growing male rats . At 3 weeks after surgery, ash weights of femur and tibia were significantly reduced by nerve transection and by SCI, but SCI caused a greater decrease than neurectomy. Both SCI and nerve transection reduced areal bone mineral density (BMD) for proximal femur, distal femur, and proximal tibia, again with greater losses observed after SCI than after neurectomy. A similar pattern of changes was observed for bone geometric structure at the tibial metaphysis, where SCI produced marked decreases in bone volume and trabecular thickness and more modest decreases in cortical thickness. A provocative difference between SCI and nerve transection was that mineral apposition rate and bone formation rate/bone surface were normal to increased 3 weeks after SCI but decreased after nerve transection. Serum levels of N-telopeptide (NTx), an N-terminal type I collagen byproduct of bone resorption, were elevated at 3 weeks after SCI or neurectomy, with significantly higher levels observed after SCI. Bone loss after SCI was also found to be more severe than that induced by hindlimb immobilization with a cast . Thus in rats, as in man, bone loss after SCI is rapid, dramatic, and more severe than many, if not all other unloading-induced forms of bone loss.
A subsequent study evaluated skeletal changes and associated bone metabolism longitudinally for 6 months after SCI . Serum alkaline phosphatase and osteocalcin, two metabolic markers of osteoblast activity, were not significantly different between SCI and control rats at 3 or 6 weeks or at 6 months. Serum NTx levels were greater than twofold elevated at 3 weeks and remained elevated to similar levels at 6 weeks and 6 months, suggesting sustained and marked increase in bone resorption for at least the first 6 months after SCI in male rats. At 6 months, ash weights of femur and tibia were reduced by 46% and 35%, respectively. Areal BMD (aBMD) of the proximal tibia declined over time and by 6 months was reduced by 42%, associated with nearly proportionate reductions in Young’s modulus and strength. At the tibial metaphysis, bone formation rate and mineral apposition rate were normal or mildly elevated over the first 6 weeks, but significantly reduced at 6 months. The effect of SCI on properties of ex vivo cultures of bone marrow–derived osteoclasts and osteoblasts have been reported at 3 weeks after SCI by these investigators using the same rat model . Cultures of marrow stromal cells under conditions favoring their differentiation into osteoblasts revealed no differences between SCI and control animals. Conceptually, this finding agrees with reports from this same group that bone formation rates and mineral apposition rates are similar for SCI and controls at this time point. Ex vivo cultures of bone marrow cells from SCI animals revealed increased numbers of TRAP positive multinucleated cells and more than twofold increase in resorbed area in pit forming assays, consistent with increased osteoclastogenesis and osteoclast activity. Analysis of mRNA levels in ex vivo cultures of osteoblasts by Northern blotting revealed approximately a twofold increase in levels of RANKL mRNA and a small but significant reduction in osteoprotegerin (OPG) mRNA.
Similar results were obtained in a rat model of spinal cord contusion resulting in severe SCI . In this model a significant decline in BMD was observed at the distal femur of approximately 34% at 10 days after SCI. Micro-CT analysis of bone structure revealed a 48% reduction in trabecular bone and 30% decline in cortical thickness. Osteoclast numbers were increased in immunohistochemically stained sections of the growth plate by approximately fourfold. Mineral appositional rate was dramatically reduced by SCI. Why bone formation would be reduced at 4 weeks in the contusion model but not at 3 weeks in the transection model of SCI is unclear but is most likely due to differences in techniques used to measure bone formation rates.
The contusion model of SCI provides an opportunity to vary the severity of the injury by adjusting the force with which the spinal cord is contused. Contusion injuries to the spinal cord are achieved by dropping standardized conical weights from specific heights. A characteristic of this model is that some function of the hindlimbs will return over time in all but the most severe traumatic injuries to the spinal cord. This functional recovery can be quantified by behavioral testing, such as by the often used open field test of Basso et al. . The effects of graded contusion injuries on cancellous bone volume at 2–3 and 8 weeks after SCI have been studied in female rats. In these studies, animals injured with the lowest energy (6.25 g cm) retained stepping at all-time points, while more severe injuries compromised stepping for at least the first several days after injury, and the most severe injury prevented stepping at all-time points. Although there was significant variability in the data which precluded detection of small differences, only the severe injury led to significant declines in cancellous bone volume or indentation force, a measure of bone strength. Further study is needed to determine how milder forms of contusion SCI affect sublesional bone in rats.
40.2.1.6
Nerve transection
Cutting a nerve, or removing a segment of the nerve (neurectomy), produces paralysis and bone loss. Neurectomy has been found to have no effect on growth of long bones or vertebrae . Early work from Sevastik et al. demonstrated that the transection of the sciatic nerve in male rats reduced femur wet and ash weights . Weinreb et al. examined bones at times between 30 hours and 42 days after tenotomy or neurectomy . Bone loss in the two models was generally similar. Loss of trabecular bone at the metaphysis was large compared to overall bone loss, suggesting that trabecular bone was predominantly affected. Evaluating bone at 1–12 weeks after neurectomy in growing female rats, bone loss occurred over the first 4 weeks after the procedure, after which bone mass in the paralyzed limb stabilized, and some growth-related increases in bone mass appeared to occur .
In long bones of growing rats, neurectomy induces at least a brief period of increased bone resorption and more long-lasting period of reduced bone formation. While studies of blood levels of metabolic markers of bone resorption have not been performed, histomorphometric analysis of measures of osteoclast number and bone formation has been conducted after neurectomy. In growing male rats, sciatic nerve transection reduced the accretion of cortical bone area over the subsequent 56 days; at 7 days, sciatic nerve transection reduced periosteal bone formation assessed by labeling with tetracycline and diminished the periosteal mineral apposition rate . Similarly, in a study of growing female rats, bone formation assessed by the retention of 45 Ca was diminished at 21 days after bilateral neurectomy; bone resorption was increased over this period based on excretion of 45 Ca in the urine . Consistent with these findings, the osteoclast numbers increased after neurectomy but, perhaps surprisingly, only transiently at the sites measured. In growing female rats, neurectomy increased eroded surface progressively over the course of the 12-week study; periosteal and endosteal bone formation rates increased briefly at 1 week after neurectomy, then declined at 4 weeks before returning to baseline levels .
Results of analysis of mRNA levels have been consistent with reduced osteoblast activity and bone formation after neurectomy. Analysis of mRNA levels in isolated periosteal cells demonstrated that the expression of type 1 collagen, osteocalcin, and alkaline phosphatase was reduced at 1 and 2 weeks after nerve transection in cells isolated from either femur or tibia . In growing female rats, right-sided sciatic nerve transection reduced trabecular number in the metaphysis of the proximal tibia by nearly 50% at 4 weeks . This deficit persisted out to 12 weeks, although its magnitude decreased modestly, possibly reflecting the effects of continued growth of the animals.
40.2.1.7
Additive effects of multiple modes of immobilization
Whether combinations of multiple forms of immobilization result in greater bone loss is an interesting question that has been tested experimentally. In one study of rats, hind limb immobilization using a cast augmented bone loss observed following a severe spinal cord contusion . Similarly, interruption of neuromuscular transmission by injection of one hindlimb with botulinum toxin augmented bone loss observed with hindlimb unloading . It thus appears that, at least for cases where the skeleton is unloaded with preserved lower motor neurons and reflex circuits (e.g., contusion SCI or hindlimb unloading), that further unloading worsens bone loss.
40.2.1.8
Summary
Immobilization results in reduced mass of both vertebral and long bones, regardless of the etiology responsible for the condition. The best studied forms of immobilization in animals, hindlimb suspension and SCI, have shown similarities but also important differences with respect to alterations in bone metabolism. As has been described in man, bone loss after SCI in rodents is rapid and severe, may continue for many months, and is associated with a marked increase in osteoclast numbers and activity, with markedly increased bone resorption which eventually becomes uncoupled from bone formation. In agreement with findings in animal studies of other forms of bone loss due to unloading, following SCI there is an increased expression by ex vivo cultured osteoblasts of RANKL associated with reduced OPG expression. Bone loss of acute SCI appears to be greater than that occurring immediately after hindlimb immobilization or nerve transection; the reasons for this difference have not been adequately addressed but may be speculated to be due to local depression of neuromuscular factors. The effects of SCI on bone formation remain somewhat controversial very early after paralysis, with some studies showing no change, while others have reported a profound decrease. By contrast, following hindlimb suspension, there is a transient increase in bone resorption and a more long-lasting fall in bone formation. The underlying molecular basis for these differences is not well understood but potentially of great interest and directly relevant to improving the treatment of these disorders.
40.2.2
Cellular and molecular mechanisms of reduced bone formation after unloading
40.2.2.1
Osteocyte dysfunction and apoptosis
The osteocyte is the predominant mechanosensing cell and master regulator of bone formation and turnover and, therefore, would be expected to hold a critical role in immobilization-induced bone loss through release of mediators such as sclerostin and RANKL. Consistent with this prediction, several osteocyte abnormalities have been observed in animal models of immobilization. Scanning electron microscopy of etched bone surfaces has revealed marked derangements in osteocyte morphology in bone isolated from rats at 2 months after spinal cord transection . Osteocyte cell bodies were rounded, and both length and number of dendritic projections was reduced, suggesting diminished ability of osteocytes to communicate with each other or other cells in bone and bone marrow ( Fig. 40.1 ). Of interest, these abnormalities were blunted by administration of inhibitory antibodies against sclerostin beginning 1 week after SCI.
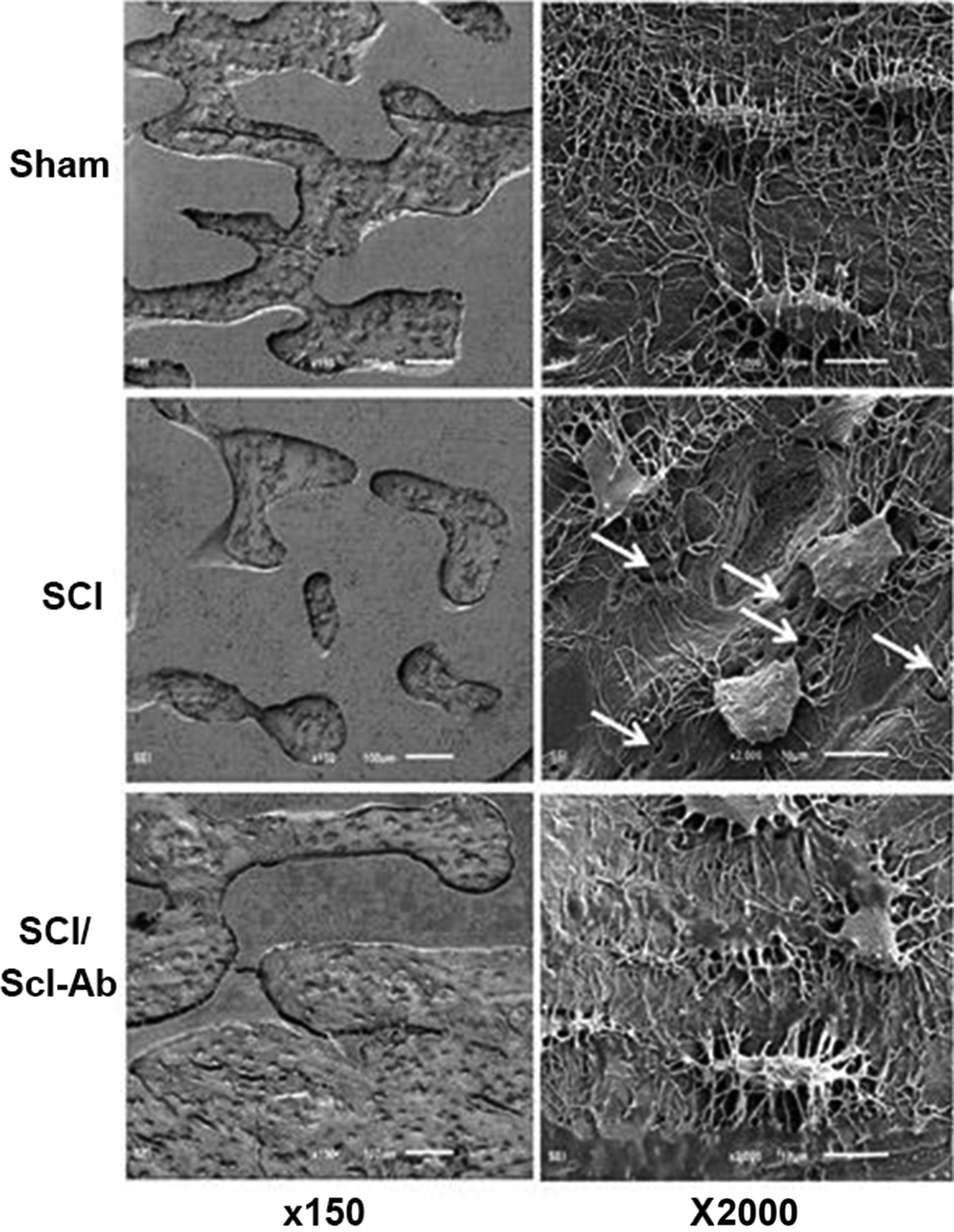
Abnormalities in osteocyte morphology in immobilized bone may be explained by autocrine, paracrine, and/or endocrine signals, as well as cellular stresses secondary to hypoxia or elevated cellular levels of reactive oxygen species (ROS). Hypoxia and ROS may impair mitochondrial and overall cellular function and impede maintenance of dendritic projections. As discussed in more detail below, osteocyte apoptosis has been observed in trabecular and cortical bone following hindlimb unloading by tail suspension .
A question that has attracted some attention is whether osteocyte apoptosis drives immobilization-related bone loss. One study evaluated the effect of quinolyl-valyl-O-methylaspartyl-[-2,6-difluorophenoxy]-methyl-ketone (QVD), an irreversible pan-caspase inhibitor that crosses the blood brain barrier, on bone loss after tail-suspension of mice . Administration of QVD reduced the percentage of caspase-3 positive osteocytes, percentage of cortical bone osteocytes positive for RANKL expression, and endosteal bone resorption at 14 days of hindlimb suspension. Mice treated with QVD also demonstrated at least partial preservation of BV/TV of trabecular bone at the distal femur. In a separate study using the tail-suspension model of hindlimb unloading, administration of either alendronate or IG9402 (a bisphosphonate analog that preserves osteoblast and osteocyte viability but does not induce osteoclast apoptosis in vitro ), both bisphosphonates, reduced osteocyte and osteoblast apoptosis and blunted the increase in RANKL expression in these cells . Alendronate (but not IG9402) protected against the loss of BMD of the spine and femur and loss of strength of vertebra; alendronate also greatly reduced osteoclast numbers in unloaded bone and lowered serum C-terminal cross-linked telopeptide (CTx) in control and unloaded mice while IG9402 did not. These findings suggest that protection against osteocyte apoptosis alone is insufficient to prevent unloading-related bone loss. Thus the role of osteocyte apoptosis in immobilization-related bone loss is as yet not fully understood.
40.2.2.2
Increased sympathetic tone
Several lines of investigation have been pursued to better understand the mechanism for the reduced bone-formation rate and decreased osteoblast number reported in animal models of immobilization. One interesting mechanism identified in hindlimb suspension models involves the upregulation of sympathetic nervous system activity in bone. Bone is richly innervated by sympathetic fibers. Pharmacologic and genetic evidence indicates that the activation of ß-2 adrenergic receptors on osteoblasts reduces bone formation rates . Administration of ß-2 adrenergic receptor antagonists, or hemizygotic disruption of the dopamine beta-hydroxylase gene, reduces bone loss and bone formation rate after hindlimb unloading . On the other hand, ß-1 adrenergic receptor agonists were reported to preserve cancellous bone microarchitecture and reduce osteocyte apoptosis in hindlimb unloaded male rats . While increased sympathetic tone may be important in bone loss due to stroke or spaceflight , it is less likely to be a contributing factor to diminished bone mass after nerve injury or SCI. Sympathetic nerve fibers to the lower extremities arise from sacral levels of the spinal cord. Thus depending somewhat on the precise nerve transected, sympathetic fibers to bone may be functionally interrupted and/or severed at the time of nerve injury. Thus as might be anticipated, propranolol did not significantly attenuate the bone loss that occurred at 30 days after sciatic neurectomy . After SCI, descending autonomic fibers are lost below the level of the lesion, resulting in marked decreases in sympathetic tone to tissues and, presumably, bone. Although pathological activation of sympathetic ganglia occurs after SCI (autonomic dysreflexia), sympathetic tone is generally depressed .
40.2.2.3
Diminished osteoblastic potential of marrow stromal cells
In studies of immobilization, diminished numbers of osteoblast progenitors or reduced capacity of such progenitors to differentiate have been observed. An elegant study by Barou et al. evaluated the effects of hindlimb suspension on the proliferation of osteoblastic cells by BrdU labeling and immunohistochemistry . Six days of hindlimb suspension significantly reduced bone volume, trabecular number and thickness, and bone formation rates. Hindlimb unloading reduced the numbers of BrdU labeled periosteal osteoblasts, peri-trabecular osteoblasts, and bone marrow cells. Similarly, Machwate et al. studied the effects of 14 days of hindlimb suspension on bone, bone metabolism, and proliferation of marrow osteoblast precursors and endosteal cells . Hindlimb suspension reduced plasma osteocalcin, mineral apposition rate, and bone formation rate. The proliferation rate of cultured endosteal cells was reduced, as was the number of alkaline phosphatase-labeled marrow stromal cells. Reductions in the osteogenic potential of bone marrow stromal cells have also been observed after sciatic neurectomy of young rats . At 11 days after neurectomy the number of adherent marrow stromal cells was reduced when these cells were cultured in vitro . Proliferation of marrow stromal cells was not affected by neurectomy. Alkaline phosphatase activity in cell lysates obtained from adherent cells cultured under conditions favoring osteoblastic differentiation was reduced by more than 25%, and the number of bone nodules formed was reduced by 70%.
Evidence of reduced osteoblastic differentiation of mesenchymal progenitors and of reduced maturation of osteoblasts has also been described. Once committed to an osteoblast fate, mesenchymal progenitors undergo initial proliferation followed by several phases of differentiation . C-fos expression is high during proliferation, elevations in alkaline phosphatase occur during early osteoblast differentiation and are associated with deposition of osteoid matrix prior to calcification, and calcification of matrix is associated with upregulation of osteocalcin. The ex vivo culture of marrow progenitor cells in the presence of dexamethasone and/or bone-morphogenetic protein 2 (BMP-2) has been found to stimulate osteoblastic differentiation of marrow precursors . Variations on this model system have been used in conjunction with immobilization models to understand some of the cellular defects that underlie immobilization-related declines in osteoblast numbers and bone formation rates. Zhang et al. examined the effect of 11 days of hindlimb suspension on osteoblastic differentiation of marrow stromal cells in rats . Hindlimb suspension reduced the number of marrow mesenchymal progenitor cells [colony forming unit-fibroblast (CFU-F)] by nearly 50%. Numbers of alkaline phosphatase positive cells present in cultures of marrow stromal cells were also reduced, and to a similar extent, reflecting decreased numbers of osteoblast precursors. Basso et al. reported that defects in in vitro differentiation of osteoblast precursors that resulted from hindlimb unloading could be reversed by reloading of hindlimbs for 14 days ; the reduction in alkaline phosphatase-positive bone marrow stromal cells observed in cultures was reduced by unloading and normalized by reloading.
In mice, Kondo et al. found that hindlimb unloading for 11 days reduced the numbers of bone marrow stromal cells that differentiated into bone-forming osteoblasts assessed as numbers of bone nodules present in the cell culture plates. Of interest, treatment with a ß-2 adrenergic receptor antagonist, propranolol, during hindlimb unloading normalized the number of osteoblast-like cells capable of forming bone nodules in ex vivo cultures, suggesting that this treatment also reversed the inhibitory effects of hindlimb unloading on numbers and differentiation of osteoblast progenitors . Similar effects were observed in mice that were hemizygous for the deletion of the dopamine ß-hydroxylase gene , which would serve to reduce sympathetic neurotransmission.
40.2.2.4
Sclerostin, receptor activator of nuclear factor kappa-B ligand, and osteoprotegerin
The discovery that mutations resulting in loss of sclerostin are responsible for bone sclerosing dysplasias and van Buchem disease focused attention on the role of this protein in controlling bone mass. Sclerostin is encoded by the SOST gene and is expressed primarily in osteocytes . Sclerostin inhibits Wnt signaling through binding with LRP5, LRP6, or LRP7 (see also Chapter 12 : Wnt signaling in skeletal homeostasis and diseases). Osteocyte expression of sclerostin is reduced by mechanical loading and increased by unloading . Downregulation of sclerostin is necessary for bone anabolic responses to mechanical loading . In studies of the effects of sclerostin on cultured cells, sclerostin has been reported to reduce in vitro mineralization by primary human osteoblasts, to block maturation of osteoblasts to a preosteocyte phenotype, and to regulate the expression of key genes involved in bone matrix mineralization (i.e., block cleavage by the endopeptidase that degrades peptides which bind to nascent bone mineral and inhibit mineral deposition) . Effects of an inactivating antibody on bone loss due to immobilization of the left hindlimb of 10-month-old adult rats have been reported . In this model, bone mass and bone formation rates were not appreciably reduced by unloading. Antibodies against sclerostin increased bone volume, trabecular surface, and bone formation rates and reduced eroded surface in normal and hindlimb-unloaded animals; bone volumes more than doubled after sclerostin antibody in normal animals and nearly doubled in hindlimb unloaded animals. A recent evaluation of the effect of anti-sclerostin antibodies in a mouse hindlimb unloading model in which adult mice were hindlimb-suspended for 7 days demonstrated that inhibition of sclerostin prevented the increase in a bone resorption marker, TRACP5b . When bone marrow stromal cells were cultured under osteoblastogenic conditions, unloading reduced the number of alkaline phosphatase positive colonies and the number of bone nodules; inhibition of sclerostin normalized these measures of progenitor function . In sclerostin knockout mice, bone loss after spinal cord transection was prevented . Similarly, the administration of inhibitory antibodies to rats reduced bone loss after spinal cord contusion or transection and restored BMD following spinal cord transection , although trabecular architecture of the new bone remained abnormal, demonstrating a more rod-like structure.
Cells of the osteoblast/osteocyte lineage release several mediators that stimulate osteoclastogenesis and osteoclast activation, one of which is RANKL. Together with M-CSF, RANKL induces osteoclast differentiation of hematopoietic precursors and activates mature osteoclasts . Osteocytes are believed to be a major source of RANKL in the remodeling of cancellous bone . Cells of the osteoblast lineage also release OPG, a soluble, decoy receptor for RANKL which reduces biological activity of RANKL . Administration of recombinant human OPG to rats reduced hindlimb suspension-related bone loss, excretion of collagen breakdown products, and the loss of bone strength . In tail-suspended mice, OPG also reduced unloading-related bone loss and decreases in bone strength . In rats at 7 days after sciatic neurectomy, OPG administration significantly attenuated declines in bone mass in the femur and tibia . In a similar study , dosing of OPG was first titrated in normal rats to find the minimal doses capable of increasing bone mass. Effects of OPG were then evaluated at 14 days after hindlimb suspension. OPG improved femoral elastic force, trabecular volume, and cortical thickness. A potentially significant observation was that at the dose used, OPG did not appear to reduce bone formation rates, in contrast to more usual findings with higher doses of antiresorptive therapies. Upregulation of RANKL expression appears likely to be involved in the persistent increase in bone resorption observed following SCI in rats . Expression of RANKL mRNA and protein have been reported to be increased in cultures of osteoblast-like cells derived by differentiation of marrow stromal cells of SCI rats, whereas expression of OPG mRNA was reduced in these cells .
Expression by cells of the osteoblast/osteocyte lineage of RANKL and OPG has been shown to be regulated by Wnt signaling . Genetic studies in mice have found that the osteocyte is responsible for the majority of RANKL release that occurs upon unloading of bone . Osteocytes are also the predominant source of sclerostin in bone . One function of sclerostin has been shown to be the upregulation of osteocyte RANKL expression , indicating an autocrine or paracrine mechanism, wherein unloading-induced upregulation of sclerostin expression in osteocytes drives increased osteocyte RANKL expression, thus promoting osteoclastogenesis and bone resorption.
40.2.2.5
Disruption of the hypothalamic pituitary axis
The endocrine system plays an important role in fine-tuning bone mass by the release of growth hormone and IGF-1 and the gonadal steroids, testosterone, and estrogen. It is, therefore, notable that immobilization has been found to lower the levels of testosterone. For example, hindlimb suspension raised corticosterone levels at 6 days while markedly reducing testosterone levels, and depressed testosterone levels were observed for at least 3 weeks . In a separate study, basal growth hormone levels were depressed at 1 and 8 weeks of hindlimb unloading without any change in levels of cortisol or testosterone . In a rat spinal cord transection model, serum follicular stimulating hormone (FSH), luteinizing hormone (LH) and testosterone levels were depressed for at least 2 weeks after injury. In the milder, moderate contusion injury model in rats, testosterone levels in blood were reduced by SCI for at least 2 months . Taken together, these data indicate a decrease in levels of hormones with anabolic effects on the skeleton acutely after immobilization, during the period of greatest and most rapid bone loss, that may exacerbate the direct effects of unloading on the skeleton.
40.2.2.6
Other signal transduction molecules
The development of technologies for creating cell-type restricted knockouts in bone has allowed investigations to address the roles of many potential signaling molecules involved in both paracrine/autocrine signaling and intracellular signaling in immobilization osteoporosis. Connexins (Cx) are membrane proteins involved in gap junction formation that also are able to form hemichannels that permit nonselective passage of cations and other small molecules between cytosol and extracellular space. Liberation of PGE2 via Cx43 (gene name Gja1) has been implicated in mechanotransduction by osteocytes . The effects of conditional knockout of Cx43 in bone cells under an osteocalcin-Cre on bone loss after hindlimb suspension was investigated. The knockout resulted in lower body weight, smaller muscles, and diminished cortical thickness and trabecular bone volume. Based on percentage bone lost over time after hindlimb unloading, the Cx43 knockout did not prevent bone loss. In a separate study the effects of a conditional knockout of Cx43 in osteoblasts and osteocytes under a Col-Cre on changes in bone parameters over time after botulinum toxin injection into muscle in mice were examined. While no significant effects of the knockout on loss of trabecular bone were observed, Cx43 knockouts lost less tibial cortical bone associated with higher tibial bone strength and a decrease in osteoclast surface , requiring further investigation to unravel the reasons for these contrasting findings in trabecular and cortical bone.
Several other signal transduction molecules have been interrogated for their roles in unloading-related bone loss. For example, expression of a constitutively active PTH receptor in osteoblasts blunted hindlimb suspension-induced bone resorption . Similarly, daily injections of recombinant PTH (1–34) significantly reduced the loss of trabecular and cortical bone in a mouse model of spinal cord transection . Injection of soluble BMP1A receptor on bone in mice after hindlimb suspension has shown that this recombinant molecule, which binds and inactivates BMP2 and BMP4, significantly lowered osteoclast surface and prevented unloading-related bone loss .
40.2.2.7
Muscle-bone interactions
Physical exertion directly and positively affects bone mass, and muscle mass is a potent determinant of bone mass. The reduction in normal mechanical loading of bone by muscle contraction and loss of gravitational and dynamic forces, such as heel strike, are also likely to contribute to declines in bone mass after immobilization. Following paralysis in the rat, muscle mass atrophies by about 50% after SCI and 90% after sciatic nerve transection . Maximal muscle contractile forces are reduced and, unless spasticity occurs, muscle contractile activity is greatly curtailed. The importance of gains in muscle mass to improvements in immobilization-related bone loss is demonstrated by studies investigating the effects of the ß-2 adrenergic agonist clenbuterol on muscle and bone in a rat model of sciatic nerve transection, in which clenbuterol protected against both the loss of muscle and bone. Tenotomy prevented the beneficial effect of clenbuterol on bone, indicating that reloading of bone by muscle was responsible for much or all of the osseous effects of clenbuterol .
The relationship between muscle and bone mass has also been explored in a model of temporary paralysis induced by injection of botulinum toxin into the calf. Manske et al. examined the temporal relationships between the restoration of muscle mass and the reversal of bone loss in mice treated in one hindlimb with botulinum toxin . Muscle and bone mass began to recover by 4 weeks after paralysis but remained significantly reduced at 16 weeks. Of interest, at 16 weeks soleus mass in botulinum toxin-injected animals was greater than controls while gastrocnemius mass remained reduced, suggesting that controlling for sporadic recovery of specific muscles may be an important consideration in the design of future studies of this type. Bone mass may become uncoupled from muscle mass and function. This uncoupling is illustrated by the changes in bone mass resulting from the loss of function mutations in the SOST gene encoding sclerostin, which increase bone mass, sometimes in a dramatic fashion .
The muscle-restricted growth inhibitory factor myostatin has been shown to be an important determinant of both muscle and bone mass. Myostatin is a member of the TGFß superfamily that signals through activin IIB receptors. Germline deletions of myostatin in mice resulted in an approximate doubling of muscle mass and increased bone mass of humerus, femur, and vertebra . Increases in both trabecular and cortical bone were observed in myostatin knockout mice. Increases in bone mass of humerus were localized to the regions of muscle insertions, and not the midshaft . Whether the effects of myostatin on bone mass reflect purely mechanical consequences of greater muscle mass or direct actions of myostatin on bone is not known. Myostatin deficiency increases muscle mass and bone strength, but this myokine has been found to influence the proliferation and fate of bone marrow progenitor cells in a manner that is complex because effects differ depending on cell type and the presence or absence of load. Bone marrow–derived mesenchymal stem cells (BMSCs) were exposed to hydrostatic compressive loading in vitro , which increased expression of osteogenic factors BMP-2, IGF-1, periostin, and RUNX-2, but expression of these factors was decreased with pretreatment with myostatin . BMSCs harvested from mice with myostatin deletion had increased osteogenic differentiation compared to wild-type mice, but animals that were unloaded for 7 days were found to have a 200% increase marrow adipocyte number with cell surfaces that were almost totally devoid of osteoblasts compared to normal animals . These findings suggest that the increased osteogenic differentiation of BMSCs from mice lacking myostatin is load-dependent: when loaded, myostatin deficiency results in increased bone strength, but when unloaded, a myostatin deficiency does not prevent, and may accelerate, bone catabolism . Myostatin was shown to support the proliferation of undifferentiated embryonic stem cells and to inhibit myogenic differentiation of rat bone marrow-derived stromal cells . The possibility that circulating myostatin derived from unloaded muscle may influence distant tissues is supported by findings that the known effects of myostatin knockouts in mice to reduce body fat mass is reproduced by muscle restricted disruption of myostatin, but not adipocyte restricted deletion . Whether myostatin contributes directly to immobilization-induced bone loss is not clear. Evidence against this possibility comes from a study of effects of a soluble activin receptor IIB on bone and muscle after spinal cord transection in mice in which the inhibitor did not alter bone parameters assessed by aBMD of distal femur . A potential important area for research will be the elucidation of the role of circulating myostatin in modulating bone mass and the proliferation and fate of bone progenitors.
Although myostatin release is increased by immobilization and depressed by activity, physical activity increases the release from muscle of the peptide mediator irisin, IL-6, and the small organic acid, ß-aminoisobutyric acid (BAIBA). An obvious question is whether one or more of these soluble mediators participates in coupling activation of muscle contraction to bone mass. Irisin is a peptide generated by cleavage of fibronectin type III domain containing protein 5 (Fndc5), a protein expressed on the surface of skeletal muscle and neurons that was first identified as a peptide involved in upregulating mitochondrial respiration in white adipose tissue (beiging) in response to physical exercise . Administration of irisin to mice at doses below those required to stimulate beiging of white adipose tissue stimulated an increase in cortical bone mass and strength of the femur attributable to increase in cortical periosteal surface but did not alter trabecular bone . Irisin reduced loss of trabecular and cortical bone at 4 weeks after hindlimb suspension of mice . When initiated after 4 weeks of hindlimb unloading, irisin also increased trabecular bone mass. Whether low irisin or interruption of signaling via irisin receptors in bone worsens immobilization bone loss has not been determined.
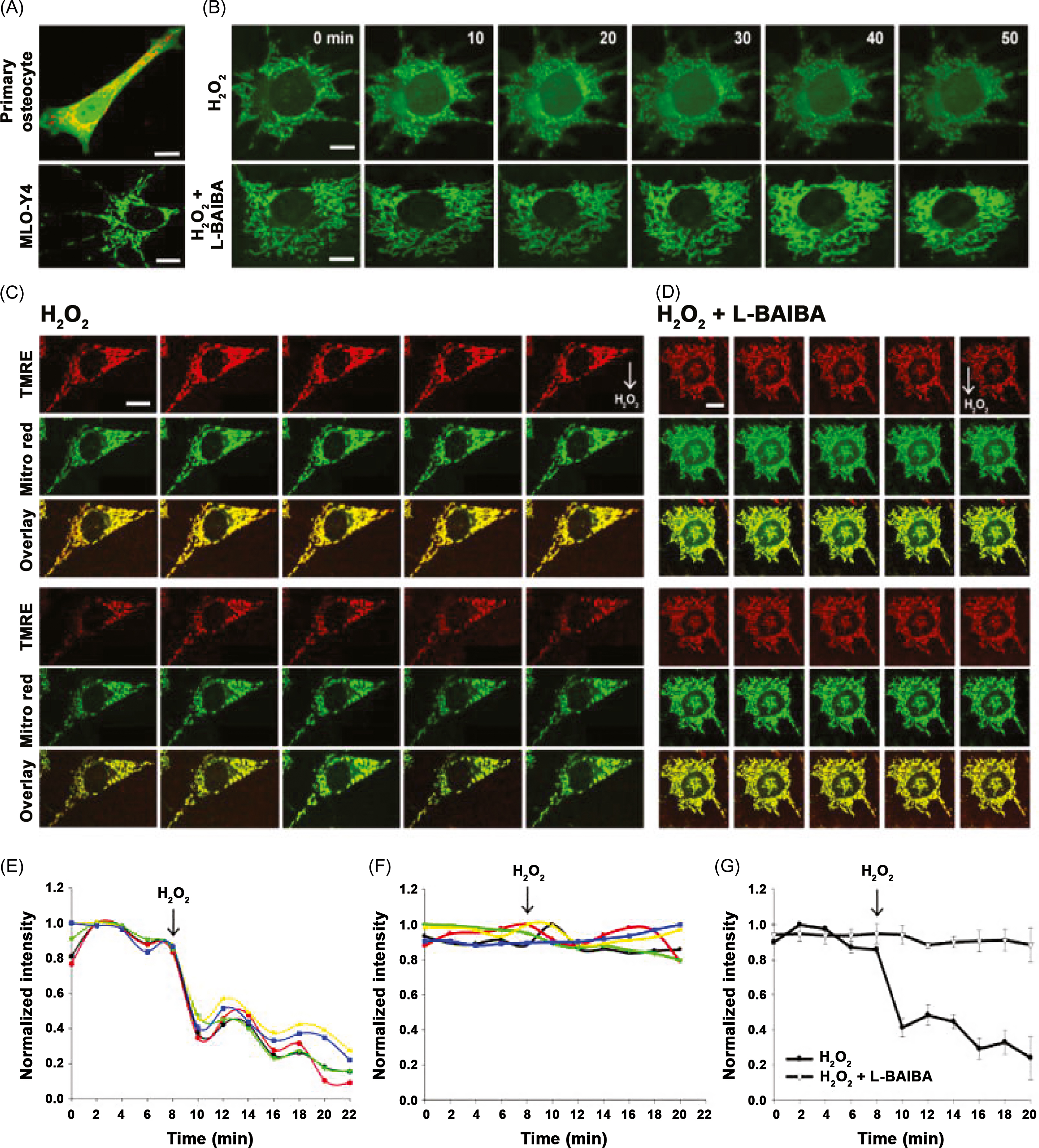
ß-aminoisobutyric acid (BAIBA) is another molecule produced by muscle during exercise and linked to browning of white fat and stimulating hepatic ß-oxidation ; loss of the insulin-sensitizing actions of BAIBA may be indirectly deleterious to bone by promoting the emergence of diabetes, a disorder linked to poor bone quality. After 2 weeks of hindlimb unloading in mice, BAIBA increased BV/TV and markers of osteocyte viability, an effect ascribed to protection against mitochondrial breakdown in response to oxidative stress ( Fig. 40.2 ) . These intriguing observations suggest that reduced BAIBA may contribute to osteocyte death during immobilization by the failure to protect against such oxidative stresses.
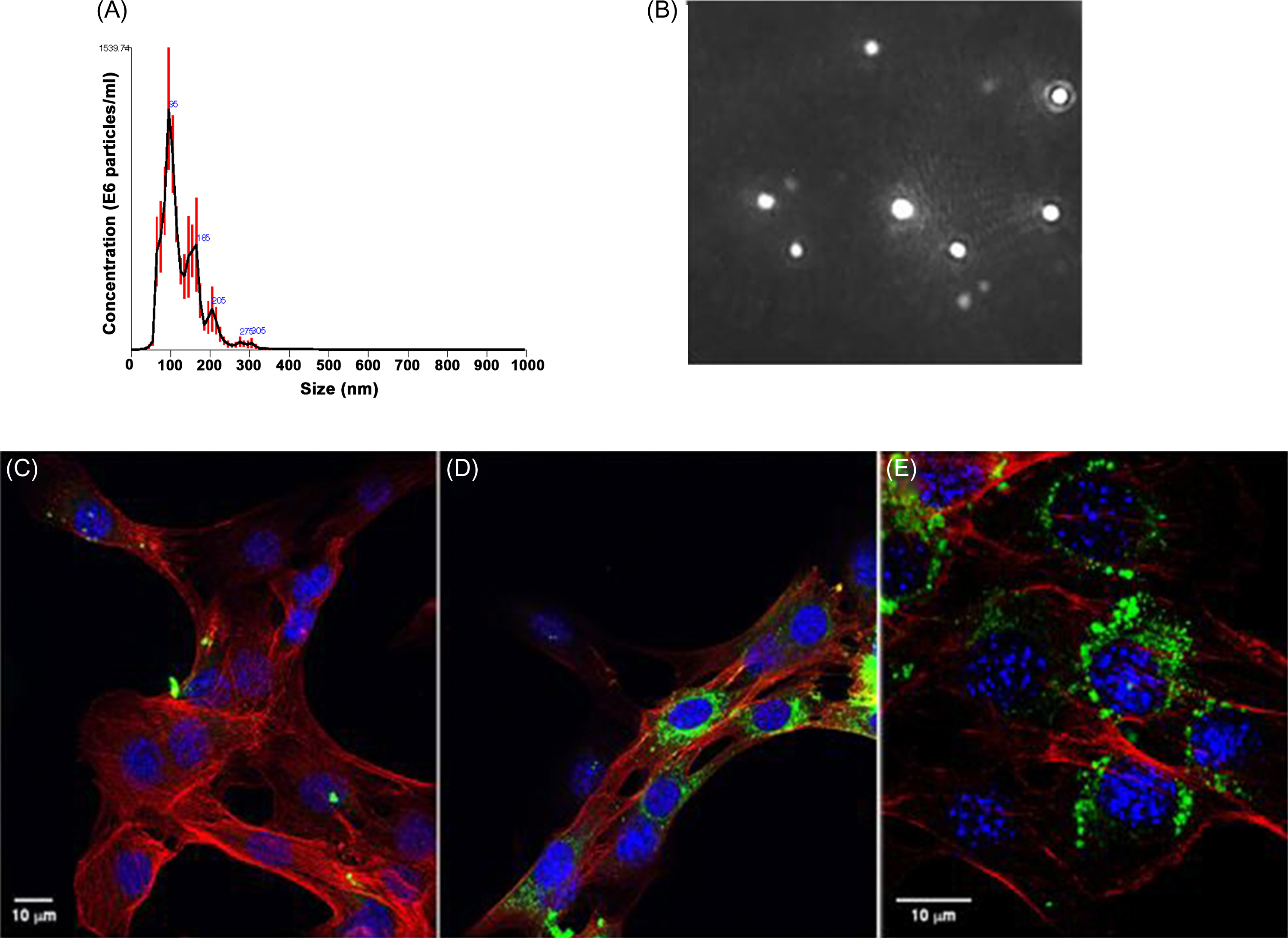
Exosomes are bilamellar vesicles formed and secreted by most eukaryotic cells which permit transport of diverse cargos between cells, including that of mRNA, micro-RNA, and proteins. It is now clear that exosomes produced by one tissue may have distant effects on others . Just how muscle-derived exosomes affect bone is unclear. Of note, recent studies have shown that skeletal muscle fibers release exosomes that are taken up by target cells and depress Runx2 protein levels ( Fig. 40.3 ). Denervation alters micro-RNA expression profiles in muscle-derived exosomes . Whether and how such exosomes modulate bone biology during immobilization will prove to be a fascinating area of inquiry.
40.2.2.8
Alterations of the cellular composition of the marrow cavity
Even brief periods of hindlimb suspension result in partial replacement of the bone marrow space by adipocytes that can be reversed by reloading of bone . Why infiltration of bone marrow by adipocytes occurs and what, if any, effects adipocytes have on the bone marrow osteogenic or osteoclastogenic precursors are provocative areas of future investigation. Reductions in mechanical deformation of progenitor cells may provide one explanation for the increased adipocyte numbers in unloaded bone, as cellular strain promoted osteoblastogenic differentiation of progenitors . Cyclical loading of progenitor cells elevated Runx2 expression and reduced that of the adipogenic transcription factor PPAR-gamma. In other experiments, cyclical loading prevented the adipogenic effect of PPAR-gamma agonists .
40.2.2.9
Summary
Elevations in osteocyte expression of sclerostin, as well as the autocrine and paracrine effects of sclerostin to increase osteocyte release of RANKL, are likely to be seminal events resulting in the early, dramatic increase in bone resorption after immobilization. The possibility that sclerostin also is responsible for the diminished osteoblastogenesis, osteoblast numbers, and bone formation observed after immobilization is an attractive possibility. In young rodents with more rapidly growing bones, reloading restores normal bone mass and bone cell properties. Restoration of normal muscle mass and function appears to be important determinants of the improvement of bone mass after periods of prolonged immobilization. Studies with recombinant OPG suggest that therapies directed at reducing the biological effects of RANKL, either by strategies to reduce release or disrupt end organ binding, may be effective in attenuating bone loss due to immobilization. Reducing the biological effect of RANKL may hold greater promise clinically for the efficacious treatment of severe immobilizing conditions than that of the current approach utilizing the relatively early administration of bisphosphonates. Preliminary investigations suggest that antisclerostin agents are also quite promising when immobilization-related bone loss has exceeded fracture thresholds and rebuilding of substantial amounts of bone is obligate to reduce risk of fracture. A growing body of evidence supports humoral coupling by which increases or decreases in muscle activity modulate serum levels of irisin, BAIPA, and myostatin, as well as perhaps several other unidentified factors may yet be shown to have important effects on bone mass. Impairments in osteocyte cellular and mitochondrial function develop after immobilization and may, in part, be rescued by BAIPA.
40.3
Human studies
40.3.1
Voluntary unloading
40.3.1.1
Bed rest
Voluntary bed rest in healthy individuals is an experimental model for muscle atrophy and bone loss. Bed rest has been employed to permit the study of the skeleton when the usual forces of gravity on the body have been altered. Head-down bed rest has been suggested to serve as a ground-based model of microgravity, with similarities to that of spaceflight. However, there are striking histopathological and bone compartment differences in skeletal response between these conditions that will be discussed in detail in this chapter.
In a study of eight healthy men placed at anti-orthostatic bed rest for 4 months, total and active iliac crest resorption surfaces and the number of osteoclasts per area of trabecular surfaces did not significantly change after bed rest. Trabecular bone volume and mean cortical thickness were not changed, even though the number of trabeculae was decreased; there was an increase in trabecular thickness that did not reach significance . Thus contrary to expectation, these investigators did not find a significant change in bone mass, or evidence of heightened osteoclastic function but suggested that bone architecture might be affected by the reduction in mechanical forces. The possibility certainly exists that, although relatively easy to biopsy, the iliac crest may not be representative of bone remodeling elsewhere in regions of greater interest, such as the lower extremities.
Resistive exercise during prolonged bed rest prevents muscle atrophy, but associated bone loss is far more difficult to prevent . Various physical interventions and drug therapies have been evaluated for their ability to prevent or reduce bone loss, with varying degrees of success.
Twenty healthy subjects participated in a 17-week horizontal bed rest study and were assigned to either exercise (five men and five women) or control (six men and four women) groups. Exercise consisted of a horizontal resistance exercise training machine that permitted the subject to exercise while supine; subjects exercised 6 days/week, consisting of alternating days of upper or lower body exercise. Mean net calcium balance was mildly positive in the exercise group (+21 mg/day) and negative in the control group (−199 mg/day), with ionized calcium and associated hormonal changes in the control group suggestive of resorptive hypercalciuria. In support of the notion that disuse uncouples bone remodeling, control subjects had an increase in biochemical markers of bone resorption but essentially no change in those for bone formation, whereas the exercise group had an increase of markers for both resorption and formation, suggesting bone remodeling in an appropriately coupled manner. Between-group differences for BMD by dual-energy X-ray absorptiometry (DXA) revealed no loss of bone in the exercise group, but in the control group a −3% loss at the total hip and pelvis and a −9% loss of the calcaneus was noted ( Table 40.1 ). Although there was some loss of muscle mass of the gastrocnemius and soleus in the exercise group, it was significantly less than in the control group. Twenty-five men were placed at strict bed rest with −6° head-down tilt for 90 days and randomized to receive either resistive exercise (exercise with a flywheel device every 2–3 days) or a bisphosphonate (pamidronate 60 mg intravenously 14 days prior to bed rest) or no intervention (control) . Calf muscle loss measured by cross-sectional area (CSA) determined by peripheral quantitative computed tomography (pQCT) was mitigated by exercise (−17%±2.7%) compared to the control and drug-treated groups (−25.6%±2.5% and −25.6%±3.7%, respectively). BMC losses of the tibia at the diaphysis and epiphysis were reduced by resistive exercise or pamidronate administration compared to controls, but only reached significance for the diaphyseal site. In another 90-day 6° head-down tilt bed rest study in young healthy men, significant bone resorption occurred in the control ( n =9) and resistive exercise ( n =9) groups with loss of proximal femoral BMD (albeit somewhat lessened by exercise), increased biochemical markers of resorption, urinary calcium excretion and renal stone formation, whereas the pamidronate-treated group ( n =7) maintained femoral BMD and had reduced bone resorption, urinary calcium losses, and the complete absence of renal stone formation .
| Male controls ( n =13), %change | Female controls ( n =5), %change | All controls ( n =18), %change | Male exercisers ( n =5), %change | Female exercisers ( n =4), %change | All exercisers ( n =9), %change | |
|---|---|---|---|---|---|---|
| Lumbar spine | −1.7±0.8 | −0.3±0.6 | −1.3±0.6 a | 4.4±1.8 | 2.2±0.8 | 3.4±1.1 a,b |
| Femoral neck | −1.9±0.9 | −0.6±1.0 | −1.5±0.7 | 0.3±1.1 | −0.1±0.7 | 0.1±0.7 |
| Trochanter | −3.6±0.7 | −3.6±1.0 | −3.6±0.6 a | −0.8±1.5 | −4.3±1.6 | −2.3±1.2 |
| Total hip | −3.9±0.7 a | −2.3±1.0 | −3.4±0.6 a | 0.3±1.2 | −2.4±1.4 | −0.9±1.0 b |
| Calcaneus | −10.3±2.3 | −6.1±3.1 | −9.2±1.9 a | 0.4±0.6 | 2.1±1.2 | 1.2±0.7 b |
| Distal radius | −0.5±0.6 c | 0.8±0.4 d | 0.0±0.4 | −0.7±0.8 | −1.3±0.6 | −1.0±0.5 |
| Proximal radius | −0.6±0.3 a | 0.6±0.6 d | −0.2±0.3 | 0.1±0.5 | 0.3±0.6 | 0.2±0.4 |
| Total body | −0.7±0.4 | −0.7±0.2 e | −0.7±0.3 a | 0.3±0.4 | −0.2±1.2 | 0.1±0.5 b |
| Pelvis | −3.6±0.8 | −2.6±1.0 e | −3.3±0.7 a | −0.4±1.7 | 1.7±0.7 | −0.5±1.0 b |
| Legs | −1.9±0.7 | −1.6±0.6 e | −1.8±0.6 a | −0.3±0.5 | −1.4±1.7 | −0.8±0.8 |
| Arms | −0.7±0.9 | −0.1±0.3 e | −0.6±0.7 | −0.8±0.5 | −0.1±1.1 | −0.5±0.5 |
a Baseline versus bed rest ( P <.05).
b Control versus exercise ( P <.05).
c Five concurrent controls only.
d Three concurrent controls only.
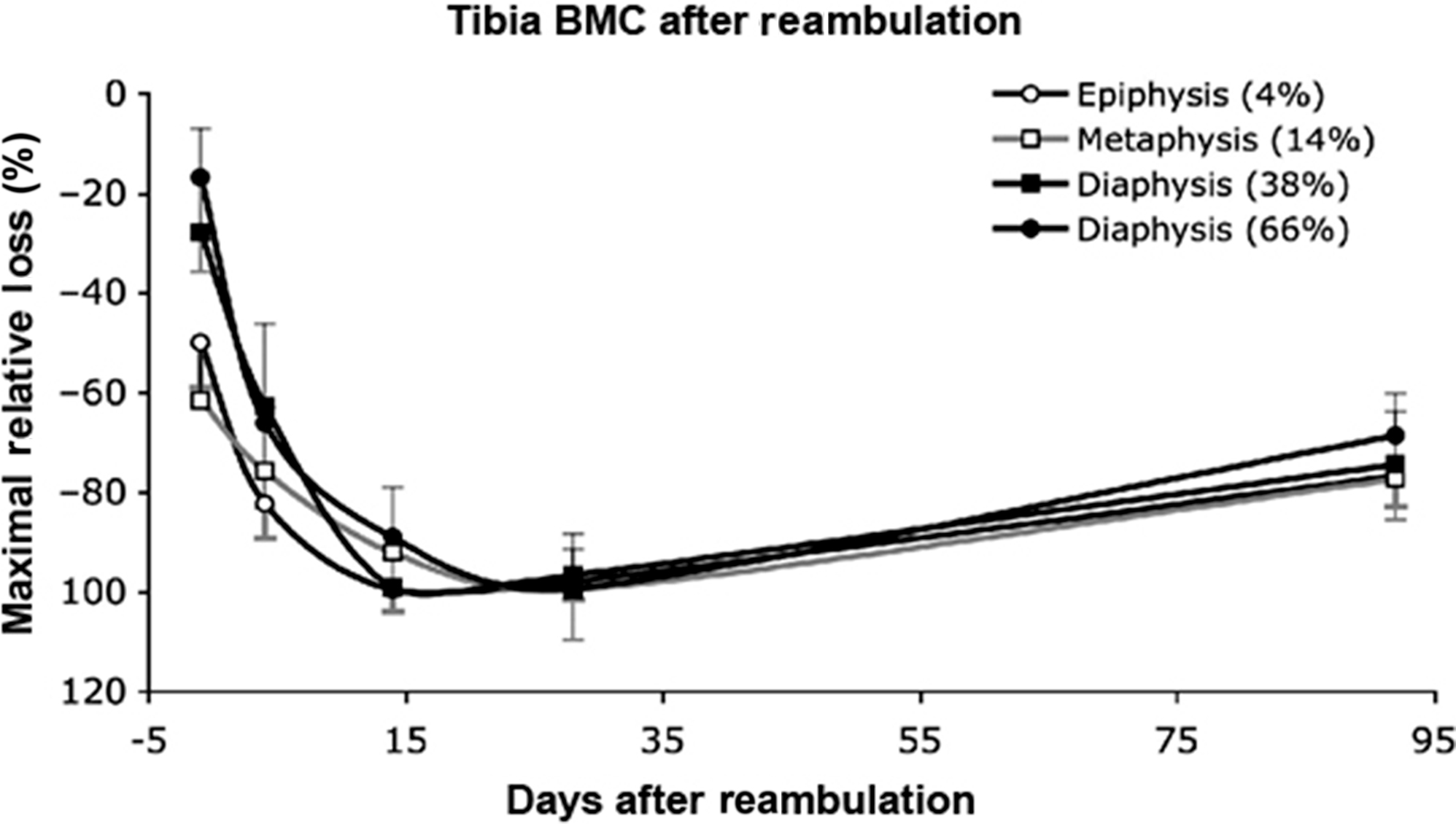
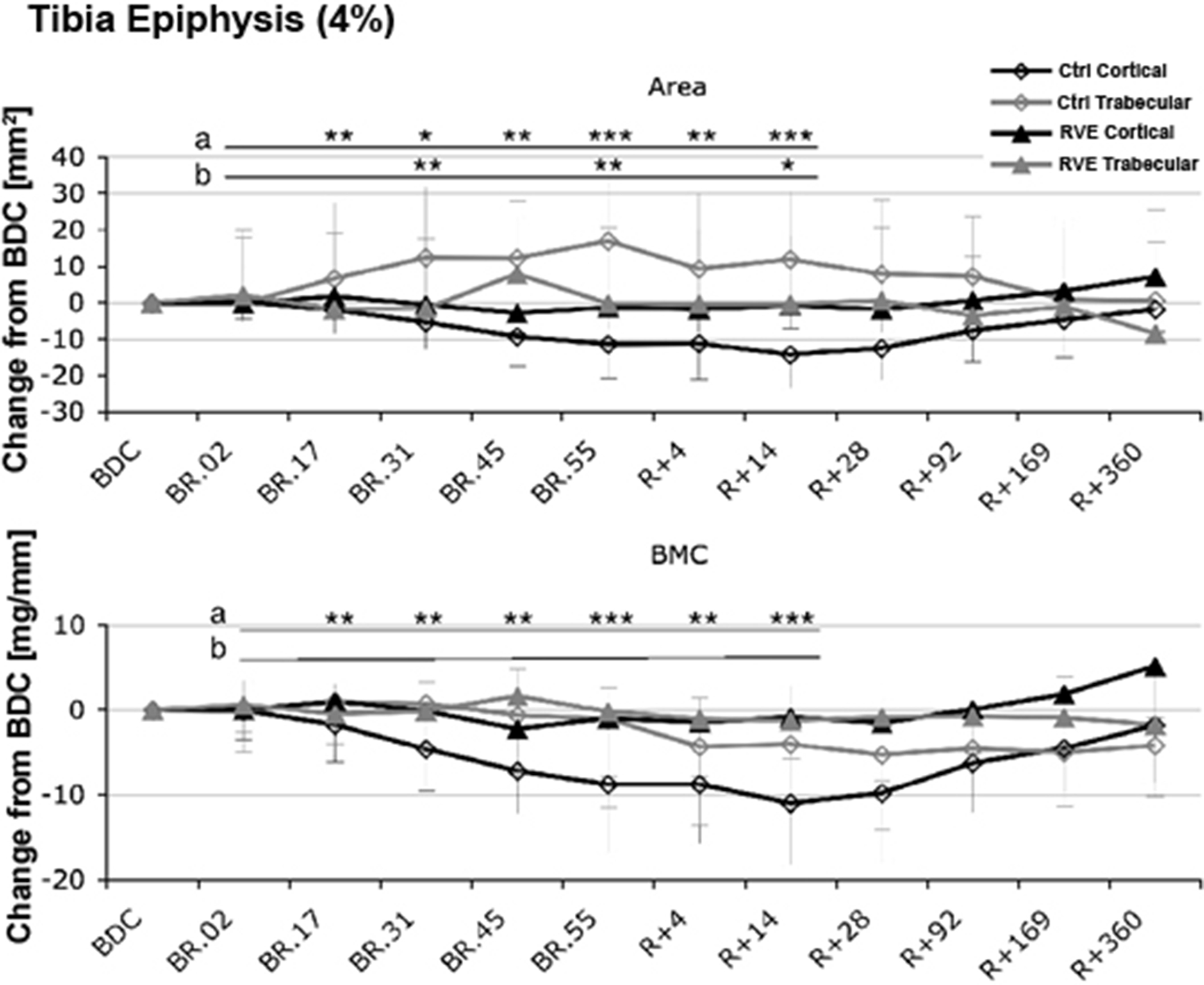
In addition to forms of resistive training, vibration has been studied in an attempt to reduce the bone loss of voluntary strict bed rest . Low-intensity, high frequency vibration delivered by an oscillating platform has been previously reported to reduce bone loss in children with disuse osteoporosis who were able to stand independently but had limited mobility associated with their disability . Twenty healthy men were placed at horizontal bed rest for 56 days and randomly assigned to vibration with resistive exercise 11-times per week or to no physical intervention. The vibration with resistive exercise was applied while in the supine position in a side-alternating manner (i.e., while one leg is shortening, the other leg is lengthening) to generate greater shock absorption; elastic springs generated a force that simulated gravity and an oscillating force was added by means of eccentric mass rotation under a teetering foot-board . Calf muscle mass CSA was significantly reduced in both groups but far less so by the exercise intervention. In the control group, bone losses at the tibial epiphyses were −2.3%±2.3% at day 55 and continued to decrease after reambulation at day 14 to −3.6%±1.9% ( Fig. 40.4 ), whereas changes at the tibial diaphysis were far less in magnitude (<1%) but still found to be significant. Of note, in the tibial epiphysis trabecular area increased and cortical area decreased ( Fig. 40.5 ). No significant change was observed in the exercise group for either tibial site. At 12 months after reambulation, the tibial epiphyses in the control group had significant losses of BMC, whereas the other sites, regardless of group, had full recovery.
A 90-day 6° head down tilt study was performed in seven healthy men . BMD at weight-bearing sites progressively declined at 28, 60, and 90 days after bed rest at a rate of 1%–2% per month that was associated with an increase in urinary calcium excretion and elevations of urinary N-telopeptide (NTx), deoxypridinoline and pyridinium crosslinks (e.g., biomarkers of bone resorption). A suppression in the levels of PTH was observed due to the bed rest–induced resorptive hypercalcemia. Serum sclerostin levels were elevated at all-time points after initiating bed rest, but neither RANKL and OPG levels were significantly changed nor were levels of osteocalcin or bone-specific alkaline phosphatase .
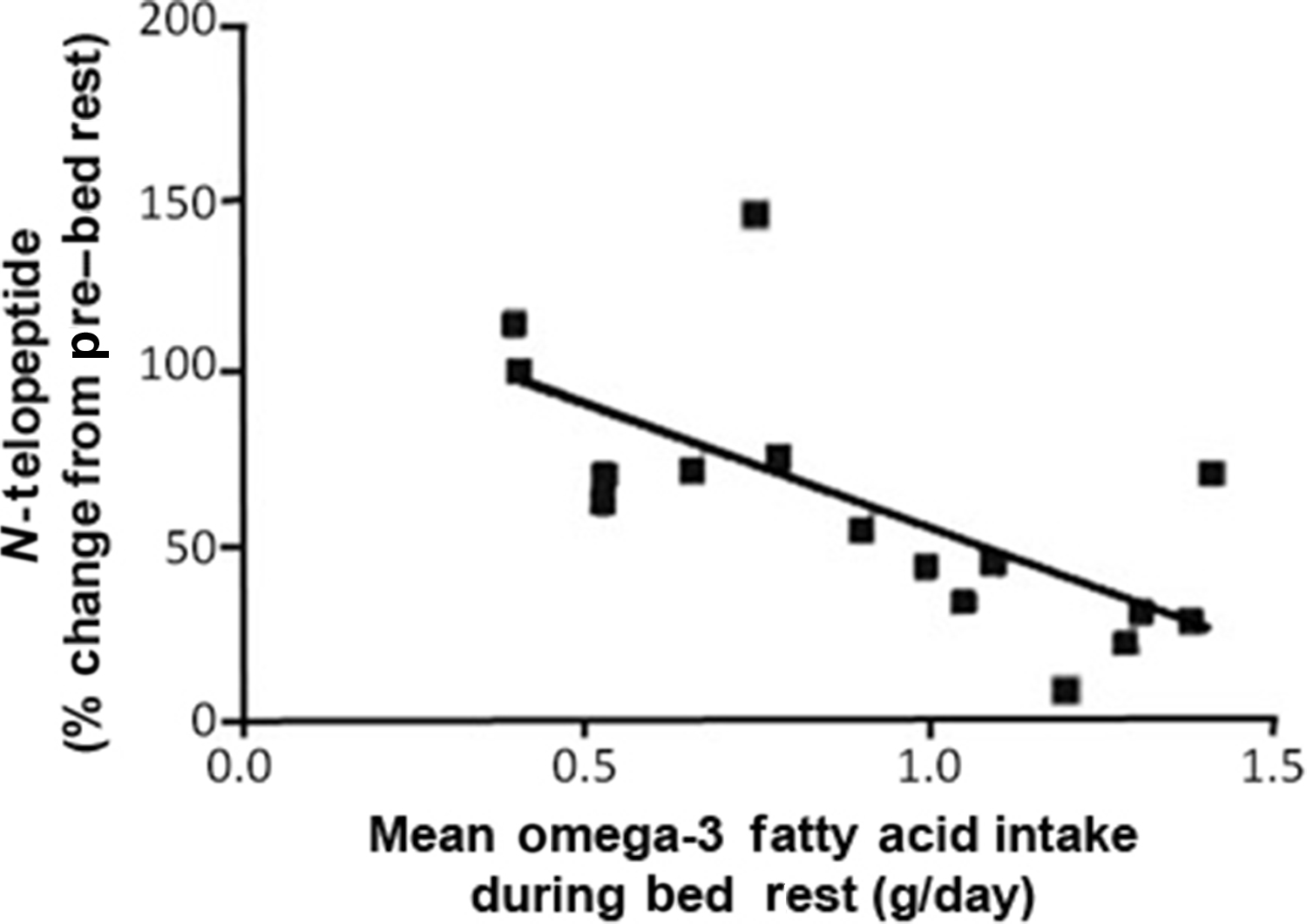
The activation of nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) has been reported to be associated with the induction of a myriad of gene expression changes that are vital to processes related to muscle catabolism and osteoclast production. In an effort to determine the suppression of NF-κB activation by simple dietary means, subjects participated in a 6° head-down tilt bed rest study of 60 or 90 days, wherein the amount of omega-3 fatty acid eicospentaenoic acid (EPA) was determined in the diet and correlated to a marker of bone resorption, NTx ( Fig. 40.6 ) . In a separate in vitro experiment, EPA was demonstrated to inhibit RANKL-induced differentiation of cells of the monocyte/macrophage lineage. Indeed, higher levels of EPA were inversely correlated with levels of urinary NTx excretion.
In a study of 24 healthy women who were placed at −6° head-down bed rest for 60 days, the mean fat marrow fraction was significantly expanded by 2.5%±1.1% . This increase in marrow fat persisted 1 year after resumption of normal upright activities. The observed 2.5% increase in the fraction of fat was 25-fold greater than that expected from historical ambulatory controls and was equal to approximately 4 years of bone marrow involution.
In summary, voluntary bed rest would be expected to result in uncoupling of osteoclast and osteoblast activity, although the single study of the histomorphometry of the iliac crest after prolonged bed rest did not confirm this assumption. Physical intervention of a resistive and/or vibratory-resistive modality, if sufficiently rigorous and frequent, can mitigate or prevent loss over several months of immobilization. Of interest the loss of bone appears to be cortical bone, with an actual gain in tibial epiphyseal trabecular area. Significant losses of bone appear to continue after reambulation, probably due to the effects of remodeling with resumption of normal upright activity. The accumulation of fat in the bone marrow after bed rest may be speculated to represent a diversion of mesenchymal stem cells from bone to fat, with possible broad and adverse implications for long-term bone health. The possibility of antagonizing RANKL to reduce the recruitment and activity of osteoclastic cells by merely increasing dietary EPA is provocative, but this observation suggests the utility of the more practical possibility of direct potent pharmacological inhibition of RANKL in clinical conditions that result in prolonged bed rest.
40.3.1.2
Spaceflight
Spaceflight induces a rapid, sustained, and profound loss of bone. Members of the International Space Station lost bone at several sites ranging from 0.8% to 1.5% per month. Several investigators have increased our appreciation of this phenomenon by describing the individual variability of bone loss and recovery, the specific sites and magnitude of bone loss, the altered geometry of bone after recovery, the calculated strength changes, and more accurate predictions of who is at risk for the greatest loss of bone. Spaceflight has been likened to accelerated aging of the skeleton, a realization that would place some of the astronauts at an appreciably heightened risk of sustaining a future fracture than if they had not been exposed to microgravity conditions.
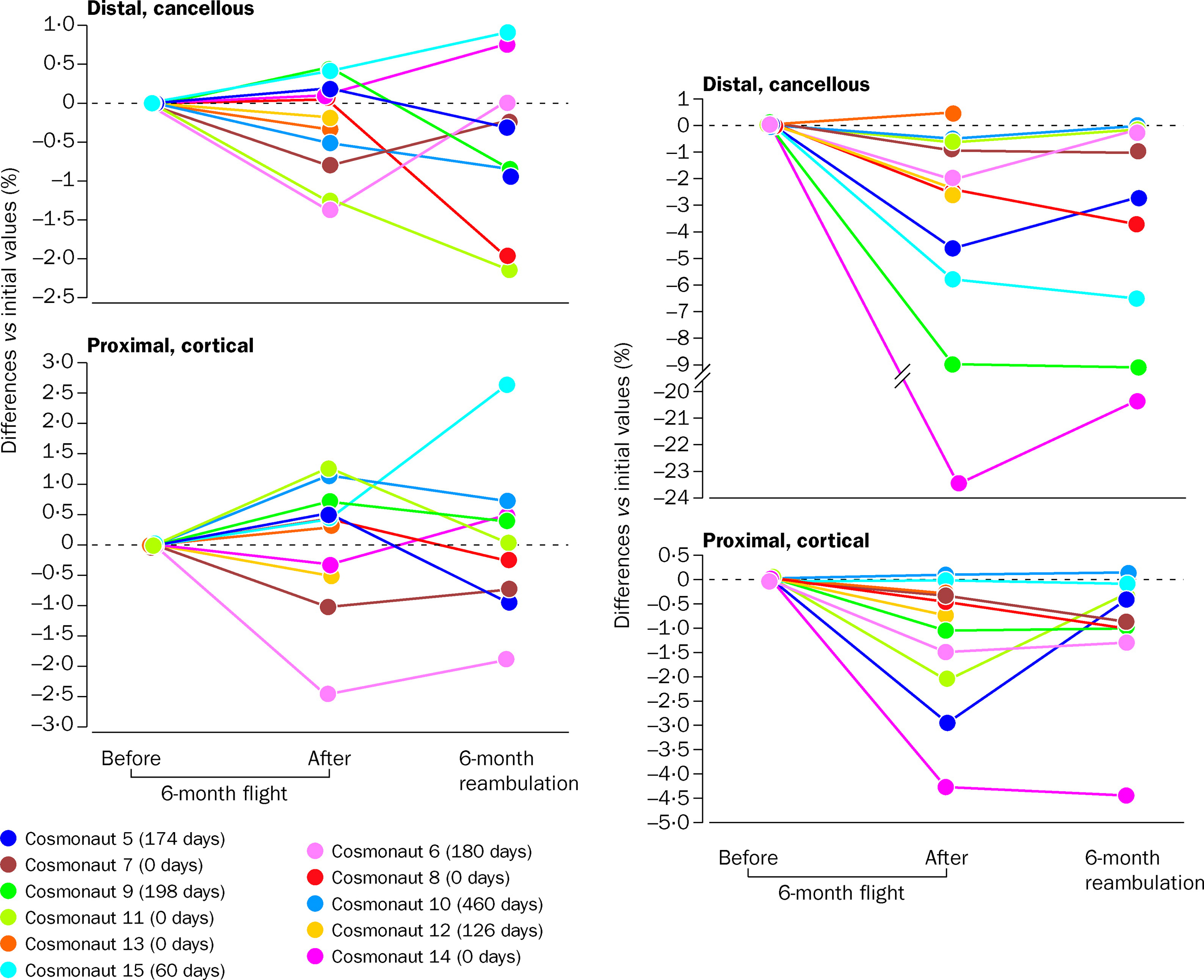
Fifteen cosmonauts who resided aboard the Russian Mir space station for as long as 6 months were studied for regional BMD measurements by pQCT . Loss of tibial cancellous BMD was evident after the first month of microgravity conditions and worsened with their continued mission. In those who were longest in space, tibial cancellous bone was, on average, markedly depleted but cortical bone was only slightly reduced. Mean cancellous and cortical bone of the radius was not significantly influenced by spaceflight, although individual variations in BMD at the distal cancellous and proximal cortical sites were observed. The individual variation of each of the cosmonauts was striking ( Fig. 40.7 ). Loss of BMD of the distal tibia ranged from 0% to −24% at the cancellous site from 0% to −4.5% at the proximal cortical site. With return to earth at 6 months, recovery of BMD at the tibia was also extremely variable, with the range being from no recovery to full recovery at the distal cancellous and proximal cortical sites of the tibia ( Fig. 40.7 ). In an effort to address this marked variability in bone loss with similar microgravity exposure, an analysis of polymorphism of genes related to bone metabolism was performed in relation to bone lost during spaceflight, with the findings suggesting that rapid bone loss may correlate with the TT genotype of the vitamin D receptor . During a 180-day stay in the Mir space station, biochemical markers on two male cosmonauts revealed uncoupling of bone remodeling with the depression of bone formation (i.e., decreased bone alkaline phosphatase by 27%, osteocalcin by 38%, and type 1 procollagen propeptide by 28%) and heightened bone resorption [i.e., increased C-telopeptide (CTx) by 78% and free deoxypyridinoline by 54%]; while in space, intact PTH was suppressed by 48%, suggesting increased bone resorption . Of interest the circadian variation of the biochemical markers of bone resorption was preserved during spaceflight, with an early morning peak and a low point late in the afternoon . The CTx concentration returned to preflight values by about 100 days on earth, whereas free and total deoxypyridinolines rapidly normalized only to rebound to supra-normal levels over the ensuing 100 days of follow-up . A subsequent investigation performed on 13 astronauts confirmed the aforementioned findings of uncoupling of bone remodeling by biochemical markers of formation and resorption, as well as extended the previous work on a presumptive resorptive state induced by spaceflight by the observations of suppressed PTH, increased urinary excretion of calcium, reduced 1,25-dihydroxyvitamin D levels, and also that of confirmatory calcium kinetic data .
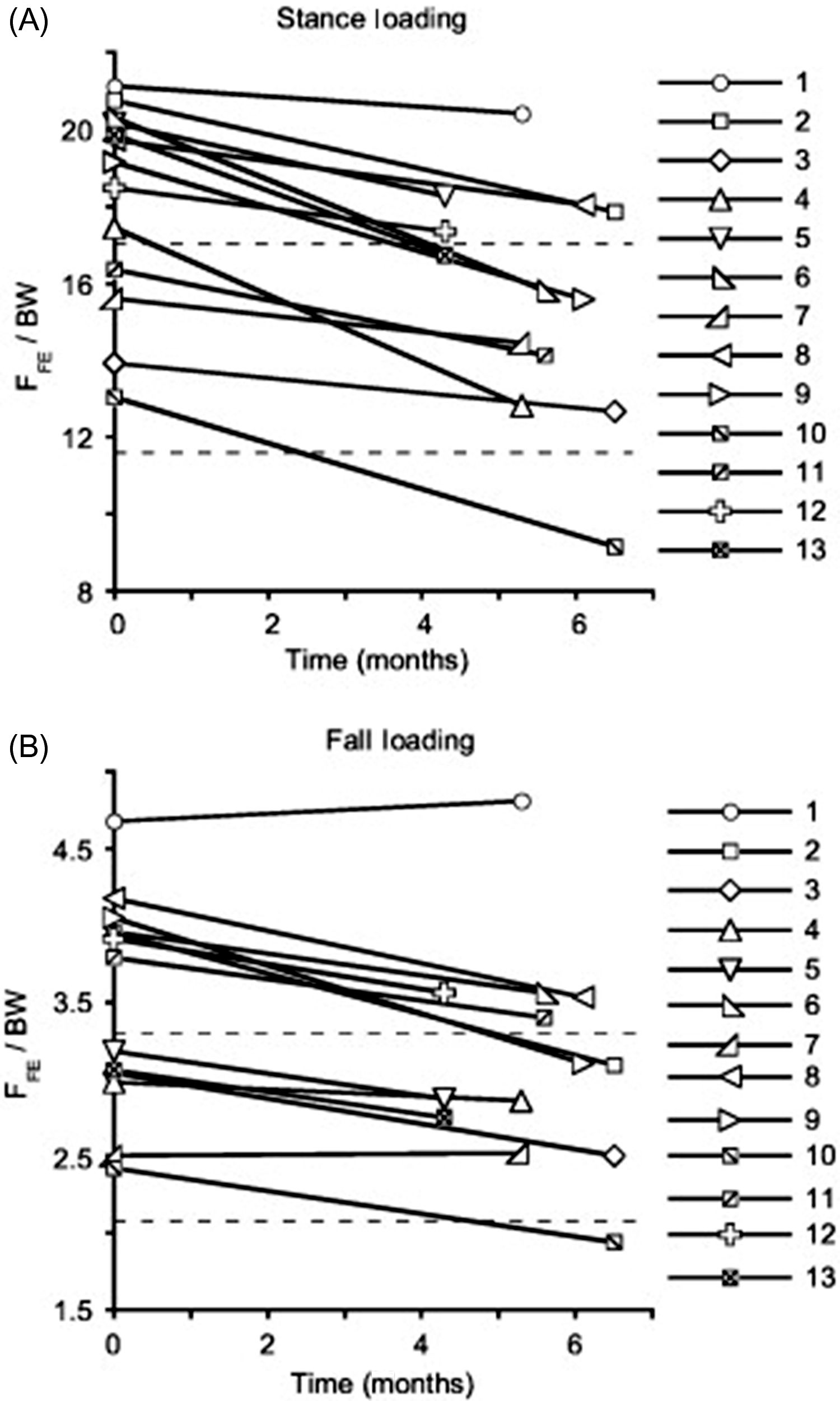
In a separate study, cortical and trabecular bone loss of 14 crewmembers on 4- to 6-month missions aboard the International Space Station were assessed by DXA for aBMD and by volumetric BMD (vBMD) of the hip and spine. The hip aBMD decreased at a rate of 1.5% per month and spine aBMD decreased at a rate of 0.9% per month (rates of bone loss of these regions are comparable to those reported for the Mir cosmonauts); hip integral, cortical, and trabecular BMD rates of loss were 1.2%–1.5%, 0.4%–0.5%, and 2.2%–2.7% per month, respectively; hip cortical bone loss resulted from cortical thinning . The same group of investigators performed additional studies on 14 astronauts shortly after their return from space and then at 1 year after return to earth . Previous magnitudes of bone loss were confirmed, with crewmembers losing about 11% of total bone mass of the proximal femora, with trabecular bone mass falling by 14.4%–16.5% and cortical mass, by 12%. Bone size remained constant during the spaceflight, indicating that observed cortical losses were the result of endocortical thinning. Bone mass of the femoral neck and overall proximal femoral regions returned to preflight values at 1 year; however, femoral neck CSA increased 2.4%, with associated increases in total bone tissue volume of 7.2% for the femoral neck and overall proximal femoral regions. Thus the vBMD of the femoral neck and overall proximal femoral regions were 91% and 93% of preflight values, with bending and compressive strength reduced 16% and 17%, respectively, below preflight values. Further analysis of the reduction in proximal femoral strength (F FE ) of astronauts on the International Space Station for 4–6 months by finite element modeling revealed that mean F FE under stance and fall loading decreased at rates of 2.6% and 2.0% per month, respectively ( Fig. 40.8 ) . Of note the magnitude of reduction in F FE was similar to estimated lifetime losses due to aging.