Chapter Outline
PULMONARY AND INTRATHORACIC TUMORS
IMAGING TREATMENT COMPLICATIONS
INTERVENTIONAL RADIOLOGY IN PEDIATRIC ONCOLOGY
The incidence of cancer in childhood is relatively rare when compared to the incidence in adults; approximately 6500 new cases are diagnosed in the United States each year. Nevertheless, cancer remains the second most common cause of death during childhood. Beginning in the mid-1960s treatment of childhood cancer has witnessed remarkable advances in chemotherapy, radiotherapy, and surgical intervention, with improvements in survival for the majority of pediatric malignancies. The imaging of children with malignancy has also evolved, with significant technical advances in both diagnostic and interventional radiology techniques. Faster magnetic resonance (MR) and computed tomography (CT) scanners, three-dimensional (3D) acquisition and postprocessing techniques, and an increasing number of hybrid imaging technologies allow practitioners to image children more quickly, accurately, and safely, in addition to providing options for image-guided diagnostic and therapeutic procedures that historically required surgical intervention. This chapter provides an introductory overview of the various diagnostic imaging techniques available and a review of the imaging findings commonly seen in a broad spectrum of pediatric tumors. The chapter concludes with a discussion of diagnostic and therapeutic interventional radiologic techniques, surveillance imaging considerations, and finally a brief introduction to the evolving field of molecular imaging and novel imaging technologies.
Technical Considerations
Conventional Radiography
The decision to perform an imaging study on a child and the type of imaging that is selected very much depend on the patient’s clinical history and physical examination findings. In young children with cancer, initial presenting symptoms and complaints are often nonspecific. An easily performed, relatively inexpensive imaging assessment like an x-ray is therefore appropriate as a first step in the evaluation. Conventional radiography is still commonly used as the first imaging examination undertaken both in children with suspected malignancy and in patients with nonspecific complaints that ultimately turn out to be related to neoplastic disease. These initial imaging studies provide valuable initial information that can help suggest a differential diagnosis and inform decision makers about subsequent imaging evaluations.
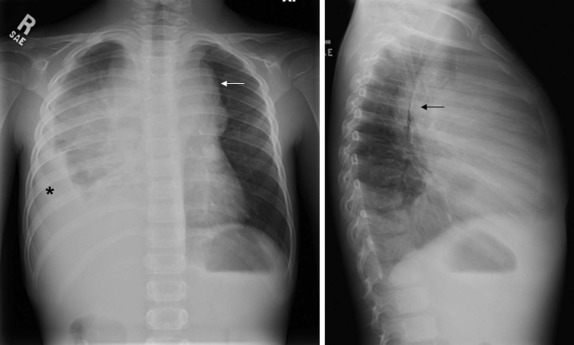
Although it is tempting to forego obtaining conventional radiographs in favor of cross-sectional imaging modalities such as ultrasound and CT, it is still appropriate in most instances to obtain conventional radiographs as a first step. Normal x-rays in certain settings may provide needed reassurance. Abnormal radiographs, on the other hand, provide a sensitive and accurate initial means of identifying pathology. For example, abdominal films allow assessment for bowel obstruction or perforation and may show solid mass lesions, abdominal calcifications, or areas of bone destruction ( Fig. 66-1 ). Chest radiographs often reveal primary intrathoracic or mediastinal tumors and pulmonary metastases. Chest radiographs are also critical in suggesting impending airway compromise, pneumothorax, or pneumomediastinum and are rapidly obtained, easy to perform, and fairly quickly interpreted. Oncologic emergencies are fortunately uncommon; however, cardiorespiratory emergencies in a child, such as pneumothorax or pneumomediastinum, airway compression secondary to a large mediastinal mass ( Fig. 66-2 ), and malignant pericardial or pleural effusions can be effectively evaluated in the emergent setting by properly executed chest and abdominal radiographs. Indeed, a CT scan may be contraindicated if radiographs indicate the presence of significant airway compromise in the setting of a large mediastinal mass, because supine positioning with or without sedation may lead to further airway compression, impaired venous return, and acute cardiorespiratory collapse.
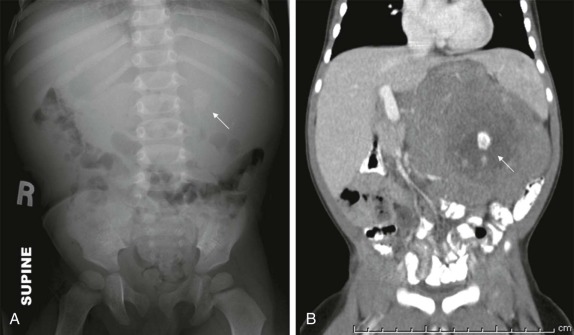
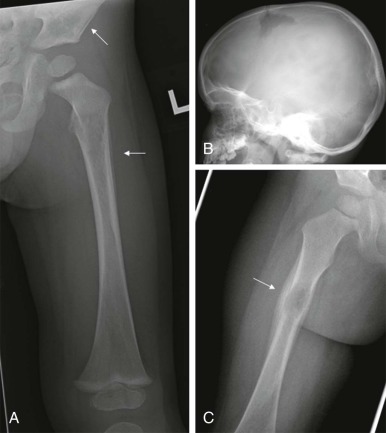
Radiographs are essential for initial imaging of nonspecific orthopedic complaints such as pain and limp and may lead to the diagnosis of marrow infiltrative disorders such as leukemia and neuroblastoma ( Fig. 66-3, A ). Radiographs are also necessary for evaluation of primary bone tumors such as Ewing sarcoma and osteogenic sarcoma, where the presence of calcifications, periosteal reaction, bone destruction, and soft-tissue involvement are important in formulating an initial impression and plan. Conventional radiography also plays a role in specific conditions such as Langerhans cell histiocytosis, where skeletal surveys are still used to detect the lytic lesions characteristic of this disease (see Fig. 66-3, B ). Whether whole body MRI or 18-fluorodeoxyglucose positron emission tomography (FDG-PET) scanning can be used to improve the sensitively and specificity of detecting these lesions has been the subject of several investigations.
The role of fluoroscopy in the primary evaluation of pediatric oncology patients is limited and has been largely replaced by CT. CT provides the advantage of visualizing both endoluminal and surrounding mesenteric/intraperitoneal/retroperitoneal processes, for which traditional fluoroscopic evaluations are limited.
Ultrasound
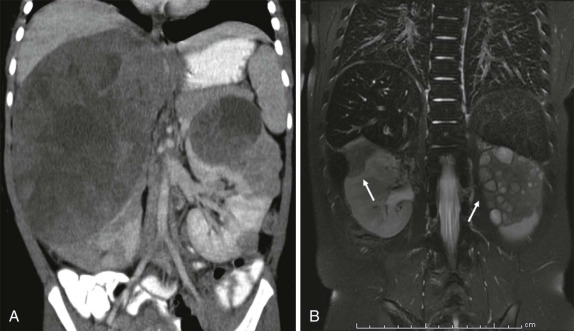
The use of ultrasound in pediatric radiology is ubiquitous. Ultrasonography is relatively inexpensive, can be done portably, involves no radiation, and in skilled hands can be used to accurately assess an anxious, moving child for whom sedation may pose additional risk. Technically ultrasound relies on the use of variable penetration and attenuation of sound waves by different tissue types. The routine ultrasound examination typically involves the combination of B-mode (brightness mode) scanning and either color or pulsed wave/duplex Doppler sonography to assess flow within vascular structures. As an initial screening modality, ultrasound is very sensitive at evaluating intraabdominal solid organ viscera and is the initial imaging modality of choice in the evaluation of Wilms tumor, neuroblastoma, and hepatoblastoma. Pelvic neoplasms, intraperitoneal free fluid, and musculoskeletal soft-tissue tumors can all be effectively evaluated by ultrasound.
Newer ultrasound imaging techniques rely on a large array of transducers, which afford high-resolution images independent of the size of the patient and depth of the lesion. Extended field-of-view image-processing algorithms offer the capability of visualizing large masses in their entirety along with their relationships to normal structures. Although not in routine clinical use, 3D ultrasound allows images to be displayed in multiple planes and may be useful in demonstrating relationships between solid-organ malignancies and adjacent normal structures. Ultrasound contrast agents are in common use experimentally and have the potential to add to the diagnostic information obtained by conventional ultrasound, but they are not yet being used routinely in clinical practice.
Ultrasound is also used intraoperatively to help identify lesions that are not visible, not palpable, or undetectable using other imaging techniques. Intraoperative ultrasound can also play an essential role in helping the surgeon identify a plane between tumor and adjacent normal tissue, which can be vital when tissue-sparing surgical techniques are desirable, such as nephron-sparing partial nephrectomy (see “ Renal Masses ”) and partial hepatectomy.
Computed Tomography
After ultrasound, CT scanning remains the primary imaging modality for evaluating most pediatric solid tumors. It is readily available in the majority of institutions, can be performed rapidly (often without sedation), and with newer generation scanners provides high spatial resolution and multiplanar viewing capabilities. CT scanning involves the use of a fan-shaped or cone-shaped x-ray beam generated by the x-ray tube rotating 360 degrees around the body part being examined. At the same time the x-ray tube rotation is occurring, the patient is moving longitudinally through the scanner, allowing the sequential and nearly simultaneous examination of multiple contiguous body parts. Once the x-rays pass through the patient, they strike an array of photodetectors that convert the x-ray energy into electrical signals, which are then used to reconstruct images from the region of the body through which the x-ray beam penetrated. The current generation of scanners are called multidetector-row helical CT scanners and rely on multiple detector rows (up to 256) to simultaneously acquire imaging information from larger segments of tissue. This allows for more rapid scanning and also allows the reconstruction of imaging data in multiple planes with minimal artifact and image distortion. These multiplanar reconstructions also allow volumetric data to be evaluated, which may be important in assessing response to therapy. In addition, the ability to view images in multiple planes with two-dimensional (2D) and 3D reconstructions and the ability to export images to robotic-assisted surgical devices is increasingly used by surgeons for both preoperative planning and intraoperative guidance in complicated cases.
For infants and anxious young children, it may be necessary for patients to be sedated in order to acquire high-quality imaging. In the majority of patients, sedation can be performed safely and effectively with skilled nursing staff and monitoring equipment. In particular, sedation is usually necessary before infusion of intravenous contrast agents, because the contrast infusion may be startling to an otherwise calm child, and motion artifact severely compromises the image quality.
The use of intravenous contrast agents, particularly for the majority of pediatric solid tumors, is necessary to properly show relationships between primary neoplasms and adjacent structures. Although centers less experienced in the care of pediatric patients may consider performing scans without the use of intravenous contrast based on perceived increased risks of contrast reactions in children, a retrospective review of CT scanning results from over 12,000 contrast-enhanced studies performed over 5 years showed less than 0.5% incidence of contrast reactions (57 reported adverse events), with the vast majority being minor reactions. No severe contrast reactions occurred in infants or very young children. Based on this and the results from other pediatric centers there is little data to support the routine omission of intravenous contrast in the evaluation of children suspected of having malignancy. In particular, the use of nonionic low osmolar agents can be performed very safely in the majority of patients and are the contrast agents of choice in most large pediatric radiology departments. Enteric contrast is also recommended to opacify the bowel lumen, allowing better discrimination between normal viscera and potential sites of mesenteric or retroperitoneal lymph-node enlargement. Historically barium had been used, but this has been largely replaced by low-osmolar water-soluble contrast agents that can be mixed with water or juice, produce fewer streak artifacts, and do not affect other imaging studies such as PET and single-photon emission CT (SPECT) imaging.
Optimal imaging of the thorax for the purpose of evaluating the mediastinum and hilar structures requires the use intravenous contrast, whereas CT scanning of the chest to assess for pulmonary metastatic disease can generally be performed without the use of intravenous contrast. The use of multiplanar reconstructions and the ability to review slices in very thin sections improves detection of pulmonary nodules, although the ability to discriminate benign from malignant pulmonary nodules based on CT is still limited. In infants and small children who are either asleep or in whom quiet breathing can be encouraged, the speed with which current generation scanners can acquire images may allow noncontrast examinations of the chest to be performed without sedation. In older children for whom breath-holding is possible, image quality and sensitivity/specificity of pulmonary nodule detection is improved when images are obtained at the end of inspiration. If breath-holding is required for a child requiring sedation, anesthesia consultation should be obtained to determine the need for general anesthesia and endotracheal intubation versus using positive pressure laryngeal mask ventilation.
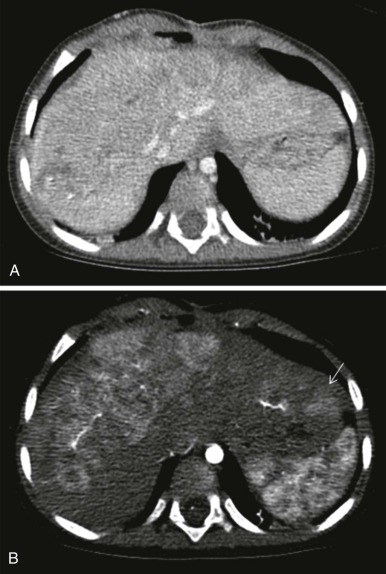
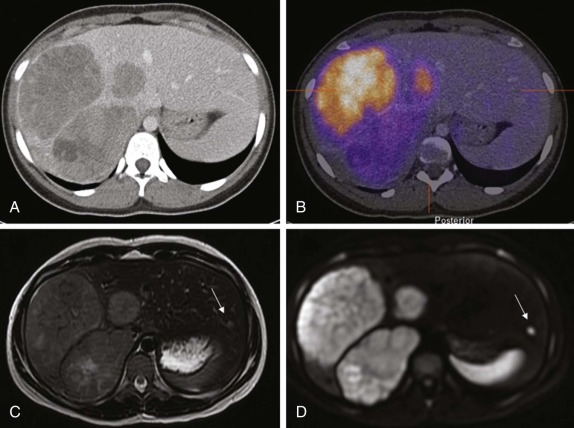
The use of intravenous contrast agents also requires skill on the part of the radiologist in interpreting the examination, with awareness of potential causes of artifact. In particular, the spleen has a variable pattern of enhancement that is well appreciated but can be confused for splenic disease, particularly in the setting of lymphoma. Delayed scanning can usually help in discriminating artifact from disease. In addition, acquisition of images at multiple phases after contrast infusion is often helpful in detecting subtle foci of disease that may only be seen at early arterial phases of enhancement, particularly in the liver ( Fig. 66-4 ). Other lesions, in contrast, may only become evident after delayed enhancement, and knowledge of the typical patterns of enhancement for the particular disease that is being evaluated or suspected is important in determining the scanning technique. It is still common to perform evaluations for hepatoblastoma in both hepatic arterial and portal venous phases in order to maximize sensitivity of lesion detection, delineate vascular anatomy, and provide optimal images to aid in surgical planning, although in many centers, improvements in abdominal MR image quality together with concerns about radiation related to repeated CT scanning have resulted in increasing use of MR imaging (MRI).
Discussions of radiation dose in the setting of diagnostic CT scanning have become widespread in both the imaging and wider medical literature. The data indicate measurable increases in incidence of malignancy attributable to radiation doses during diagnostic CT scanning, with cumulative increases in risk with increasing numbers of scans. Although these concerns are not to be underemphasized, particularly for otherwise well patients undergoing screening examinations, for patients with known malignancy or in whom malignancy is suspected, the relative benefit derived from the high-quality information achieved after diagnostic CT scanning almost always outweighs the modest incremental increase in risk associated with these techniques. Nonetheless every effort should be made to limit the examination to involved regions of the body and to minimize the frequency of scanning in these heavily evaluated patients, using the “as low as reasonably achievable” (ALARA) principle as guidance.
Magnetic Resonance Imaging
The physical principles of MR image formation are beyond the scope of this chapter and can be reviewed in articles devoted to this subject. MRI involves the use of an external magnetic field to orient protons (primarily contained in water molecules) within the body, after which radiofrequency pulses are applied with specific frequencies and orientations. These radiofrequency pulses force the protons aligned in the magnetic field to come out of alignment. After the radiofrequency energy is withdrawn, the protons gradually reassume their original alignment, or resonate, within the magnetic field. The process of relaxation is multidimensional, and the rate with which longitudinal and transverse relaxation (T1 and T2 relaxation times, respectively) occurs is dependent on the properties of the specific tissue. The energy released during this realignment process can be measured much in same way that a standard radio receiver acquires the signal (radiofrequency energy) from a radio station transmitter. This radiofrequency energy can be characterized both in terms of its spatial location and frequency, and this information in turn can be used to reconstruct images. The result is high resolution MR images obtained in multiple orientations and tissue planes with signal properties that reflect the tissue microenvironments within which the protons reside.
High field-strength and low field-strength magnets are in common clinical use. In general terms, with higher field strength there is a higher signal-to-noise ratio. Despite this, the high field-strength magnets are susceptible to some image degradation and magnetic field artifact, and most imaging can be reliably performed at 1.5 and 3 Tesla. With lower field strengths, the signal-to-noise ratio is considerably diminished. There are a variety of radiofrequency coils that are utilized as receivers for the radiofrequency energy emitted during proton relaxation and reorientation. Newer coil designs allow for rapid scanning techniques and for high-resolution imaging of specific body parts to be performed.
Multiple MR images of the same organ or tissue are typically generated; however, the imaging technique chosen results in different patterns of tissue contrast, allowing the unique features that characterize distinct components of an organ or tissue to be depicted. The tissue contrast that one observes is the result of the interplay between the externally applied magnetic field, the inherent properties of the tissue (i.e., freely mobile water protons or highly ordered soft-tissue protons), and the radiofrequency pulse-sequence parameters chosen to acquire the image. The tissue properties that have the greatest influence on image contrast are the proton density (PD) and the T1 and T2 proton relaxation times. Conventional MRI techniques are acquired with “T1- and T2- weighting,” in which the tissue contrast is weighted to reflect differences in the T1 and T2 relaxation times among the different tissues. This is accomplished by varying the interval between which the radiofrequency pulses are applied (the pulse repetition time, or TR) and the time that is allowed before the emitted signal is received (the echo time, or TE). T1-weighted images typically have a short TR (300 to 600 msec) and short TE (10 to 20 msec) and emphasize T1 characteristics of tissues. On T1-weighted images, fat is typically bright, fluid has low signal intensity, and complex or proteinaceous fluid is of intermediate to high signal intensity. Conventional contrast agents used in MRI result in T1 shortening and therefore produce increased signal on T1-weighted images.
T2-weighted images have longer TR (greater than 2000 msec) and longer TE (greater than 80 msec). T2-weighted images obtained using standard techniques display simple fluid such as cerebrospinal fluid (CSF) or urine as bright in signal intensity, whereas fat is low in signal intensity. Muscle and solid organs, depending on their tissue make-up, have variable signal intensity on T2-weighted images. Beyond these simple T1- and T2-weighted images, specific pulses sequences can be created that eliminate fluid signal (fluid-attenuated inversion recovery, FLAIR) or provide fat suppression either using chemically selective fat suppression techniques or so-called inversion recovery techniques, in which the TE is selected to minimize signal from fat and maximize signal from non–fat-containing structures.
Diffusion-weighted MR imaging (DWI) has also emerged as an important technique for evaluation of malignancy. DWI is sensitive to the molecular motion of water within the imaging volume and historically was developed for neuroradiologic applications, where it became routine for evaluation of CNS ischemia, identifying early areas of potentially reversible cellular damage by virtue of restricted water diffusion across ischemic cellular membranes. Tumors also commonly demonstrate restricted diffusion relative to surrounding tissue because of increased cellularity and reduced extracellular space. With improvements in gradient echo imaging techniques, DWI can help identify subtle sites of disease ( Fig. 66-5 ) and in some instances distinguish benign from malignant disease. DWI changes in response to therapy, with increasing free diffusion of water molecules in areas of cellular necrosis as opposed to the more restricted movement of water in cellular regions of a tumor, has also been shown to correlate with tumor necrosis induced by treatment, and is emerging as an important technique in assessing response to therapy.
MRI angiography and MR spectroscopy are all standard imaging techniques in common practice in the evaluation of the central nervous system (CNS). MR angiography takes advantage of rapidly flowing blood moving in and out of the imaging slice. Alternatively, gadolinium (Gd)-enhanced MR angiography relies on the use of Gd-based contrast agents to display the vasculature. The use of dynamic enhanced MRI allows multiple acquisitions in a specific region such as a tumor to be obtained, and there is evidence to suggest that the relative rates of contrast uptake and perfusion in tumors may be a reflection of tumor cellularity and/or necrosis. Contrast-enhanced angiography in particular may be useful as well for characterizing relationships of abdominal and mediastinal masses to critical adjacent vascular structures.
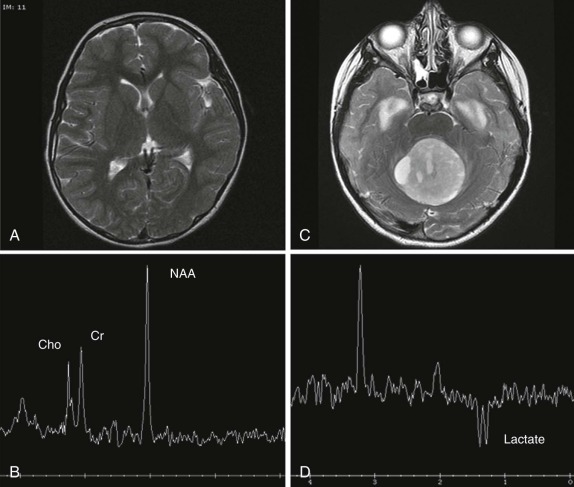
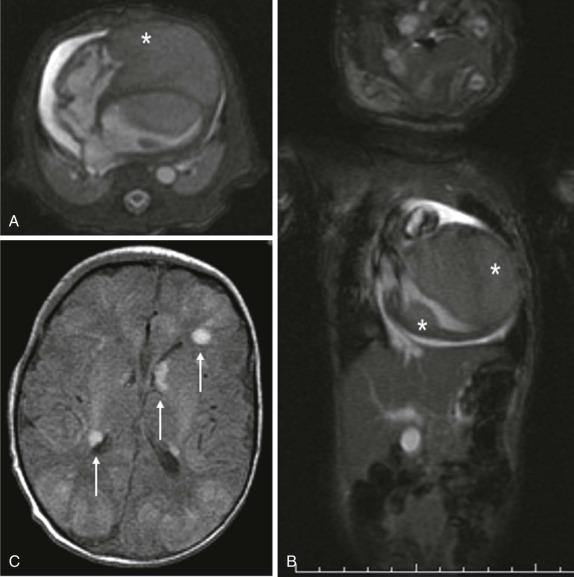
MR spectroscopy is used routinely in the evaluation of CNS tumors, where the metabolites N-acetylaspartate (NAA), creatine (Cr), and choline (Cho) have been defined and have specific and characteristic spectra. The use of spectroscopy, although not entirely diagnostic, adds additional information in evaluation of CNS tumors. For example NAA is generally thought to correlate with neuronal density, whereas increases in Cho levels usually signify increases in cell density and membrane turnover, reflecting the relatively rapid metabolic rate and division of actively dividing tumors. NAA peaks are typically decreased or absent in pediatric brain tumors, and a decreased NAA/Cho ratio is a characteristic finding. The presence of a prominent lactate peak, which is not usually present in the normal brain spectrum, signifies an increase in cellular hypoxia and necrosis ( Fig. 66-6 ) and has been described in more malignant CNS neoplasms. Because the obtainment of good spectral data relies on relatively static body parts, proton spectroscopy has not been used routinely outside the brain; however, investigations are underway to develop a better understanding of the spectra achieved in pediatric neoplasms outside the CNS.
In addition to the use of spectroscopy and diffusion-weighted imaging for characterization of brain tumors, diffusion MR tractography based on diffusion tensor imaging (DTI) has rapidly become an important clinical tool that can delineate functionally important white matter tracts for surgical planning ( Fig. 66-7 ). Tractography based on diffusion MR utilizes the correlation between water diffusion and brain structure to delineate the course of white matter pathways. White matter tracts in the brain are highly organized into fasciculi comprised of densely packed axons. Axonal membranes, myelin, and other structures affect the pattern of Brownian motion of water within white matter. Tractography involves following the trajectory of the white matter tract from voxel to voxel in three dimensions by assuming that the direction of least restricted diffusion corresponds with the axon orientation.
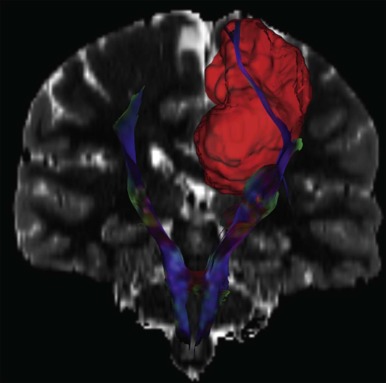
While quantitative assessments of tissue microstructure are essential for many applications of diffusion MR, these measurements are not directly used for presurgical tractography. Instead, the aim of presurgical tractography is to delineate the position of eloquent pathways such as the motor, sensory, visual, and language tracts. One of the goals of brain surgery is to avoid damage to eloquent cortex and subcortical white matter. DTI is the only noninvasive method available to segment the subcortical course of a white matter tract. Subcortical motor evoked potentials are often combined with tractography to preserve motor function.
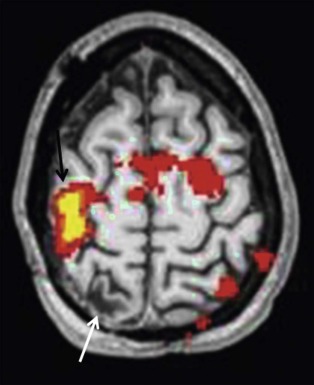
Functional MRI (fMRI) using the blood oxygenation level–dependent (BOLD) method allows mapping out of the eloquent areas and is a noninvasive, repeatable, and flexible technique for studying brain function in the clinical setting ( Fig. 66-8 ). It is often critical in surgical planning before resection of brain tumors and has become the standard of care for neurosurgical planning in centers where it is available. Although paradigms to measure eloquent cortices have not been standardized, simple tasks allow visualization of reliable maps for planning neurosurgical procedures. Current research indicates that patient-specific paradigm design can help refine the utility of fMRI for prognostication and recovery of function. It should be noted that certain pathologic conditions and technical issues limit the interpretation of fMRI maps in clinical use and should be considered carefully.
Nuclear Medicine
PET has emerged as an important imaging tool in the evaluation of both adult and pediatric patients with malignancy. A number of textbooks and reviews have been dedicated to this topic and should be consulted for a more in depth discussion of this exciting technology. The use of 18 F-fluorodeoxyglucose ( 18 F-FDG) has been routine in adult practice and is becoming increasingly commonplace in the evaluation of children with cancer. FDG-PET scanning relies on the differential uptake of glucose by metabolically active tumor cells relative to surrounding tissues. The use of FDG-PET has been shown to be feasible in the majority of pediatric tumors and is likely to play an important role in monitoring responses to therapy, particularly with agents that do not produce immediate tumor shrinkage or cellular necrosis but do block specific metabolic pathways. Although flourine-18 ( 18 F) is the most common PET radionuclide used clinically, other agents are being developed and are in preclinical testing, including copper-64 ( 64 Cu) and iodine-124 ( 124 I) and gallium-68 ( 68 Ga). The use of biomarkers other than fluorodeoxyglucose is starting to emerge clinically, with compounds such as fluorinated thymidine (FLT) allowing deoxyribonucleic acid (DNA) synthesis and cellular proliferation to be directly imaged.
Multiple studies have shown that the use of PET imaging alone, without the simultaneous review of anatomic imaging data acquired either by CT or MRI, results in high sensitivity of lesion detection but has an increased incidence of false positives and an overall decreased specificity. The use of correlative cross-sectional imaging improves the specificity of lesions detected by PET scanning and also increases the sensitivity with which lesions are detected by conventional imaging techniques. As a result, integrated PET/CT scanners have rapidly replaced stand-alone PET imaging equipment in most centers, allowing the PET scan and CT scan to be obtained on the same instrument with the patient in the same position and orientation. This has revolutionized the use of PET scanning in evaluation of oncology patients, and in many institutions it is considered standard of care for PET scans and CT scans to be reviewed simultaneously by skilled radiologists and nuclear medicine physicians, who use the complementary information available from both imaging modalities to achieve greater confidence in rendering a diagnosis. Current state-of-the art equipment utilizes high-sensitivity multirow PET detectors and multidetector-row CT scanners, allowing imaging to be performed more rapidly, with greater sensitivity, and, depending on the equipment, can incorporate respiratory and cardiac gating functions to improve image quality. Postprocessing techniques, relying on newer software, also allow the functional data from PET scanners to be fused to MRI images acquired separately, much in the same way that the PET images are fused to their correlative CT data.
In 2011 the first integrated PET/MRI scanners were approved by the FDA for clinical use and have now been installed at a number of major academic centers in the United States, Europe, and Asia. As with PET/CT, the PET/MRI scanners allow the PET scan and MRI scan to be simultaneously acquired. The advantages include lower radiation exposure, the ability to integrate quantitative MRI data with concurrently acquired functional PET data, and the increasing use of MRI for characterization of many pediatric malignancies. As additional PET tracers become available as well, PET/MRI is likely to have a significant impact on the imaging evaluation of pediatric oncology patients.
In addition to PET scanning, conventional γ-emission planar and SPECT imaging is still an important element of pediatric oncology imaging. In particular, 123 I- metaiodobenzylguanidine (MIBG) and technetium-99m ( 99m Tc)-methylene diphosphonate (MDP) bone scintigraphy are routinely used in the evaluation of neuroblastoma patients. Bone scintigraphy is also an important component of the sarcoma patient evaluation. As with PET scanning, SPECT images can be fused to anatomic data acquired by either CT or MRI in order to provide correlative information and to confirm sites of abnormality and clarify areas of equivocal disease. Integrated SPECT/CT systems are also available, which allows both SPECT and CT images to be acquired concurrently. Attenuation and scatter correction techniques can be used to further optimize SPECT image quality and potentially reduce tracer dose, as well as improve the accuracy of lesion colocalization. In many instances performing the SPECT and CT simultaneously may also obviate the need for two sedations, further enhancing patient safety and the overall imaging experience.
Staging Considerations
A detailed discussion of the different staging classifications used in pediatric oncology is beyond the scope of this chapter. Specific staging considerations are discussed in the disease-specific chapters elsewhere in this text. It is important to note, however, that there are unique staging systems in pediatric oncology, such as the International Neuroblastoma Staging System (INSS), the Wilms tumor staging system, the Hodgkin lymphoma staging system (Ann Arbor Staging), the non-Hodgkin lymphoma staging system (St. Jude Classification), and the Pretreatment Extent of Disease (PRETEXT) staging system for hepatoblastoma. Because these different staging systems do not simply rely on tumor size and nodal spread in determining disease stage (i.e., tumor-nodes-metastasis [TNM] staging), the imaging evaluation must be tailored to reflect the type of malignancy being evaluated. The radiologist should work closely with the oncologist during the review of the initial imaging data in order to accurately determine the patient’s disease stage and risk classification.
Central Nervous System Tumors
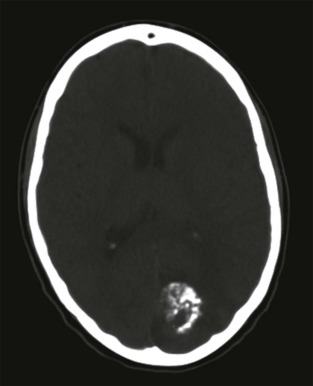
Primary CNS malignancies are the most common pediatric solid tumors, exceeded only by leukemia as a cause of pediatric cancer. Although many of the pediatric CNS tumors are also seen in adults, there are several tumors that are unique to infancy and childhood and have characteristic imaging features and intracranial locations. The imaging evaluation should be directed at characterizing the location of the lesion (supratentorial or infratentorial) and determining whether the lesion is intraaxial or extraaxial. CT is usually the first imaging study performed to investigate children with suspected CNS tumors. Although the role of CT has been diminished by the increased use of MRI for imaging the brain, CT is still widely available and readily accessible in nearly all institutions. CT can effectively identify foci of hemorrhage or necrosis and can identify critical abnormalities such as brain edema or impending brain herniation. CT may also provide clues as to the histologic nature of the tumor, for example showing either a cystic or solid mass or the presence of subtle calcifications. Bone invasion into the adjacent skull is typically better depicted by CT, and particularly lesions at the base of the skull should include an evaluation by CT.
MRI of the brain, as with other parts of the body, provides enhanced spatial resolution and, with the variety of newly developed pulse sequences and imaging coils, can give highly specific functional and anatomic information about the lesion in question and the condition of the surrounding brain. MR angiography can be performed during the same imaging evaluation and allows assessment of both arterial and venous systems. Obstruction to CSF flow can also be directly evaluated by MRI using specialized techniques aimed at monitoring CSF flow dynamics.
As described, advanced imaging techniques including MR spectroscopy, fMRI, and DTI allow biochemical and metabolic characterization of focal lesions, assessment of functional activity in the brain, and direct evaluation of the structural integrity of CNS white-matter tracts, respectively. FDG-PET and PET scanning with newer radiotracers such as 18 F-thymidine may also be of additional value in delineating postsurgical margins from residual tumor and in predicting response to therapy.
There are several classification systems for pediatric brain tumors. The most commonly used World Health Organization (WHO) classification of intracranial tumors is based on histopathologic characteristics and clinical course, with tumors further classified based on their intracranial location, specifically whether lesions are supratentorial or infratentorial. This classification system both allows a practical assignment of differential diagnoses to new CNS tumors based on their location and provides prognostic information related to histologic subtype and grade when this additional data becomes available.
Supratentorial Tumors
Astrocytomas
The most common supratentorial brain tumor is the astrocytoma, representing between 50% and 60% of all primary pediatric brain tumors. Astrocytomas are seen in all age groups, and presenting symptoms are typically of intracranial pressure, with headache, nausea, and vomiting commonly seen. Astrocytomas can be reliably diagnosed by CT and MRI ( Fig. 66-9 ). They range from benign to malignant in their histologic grade, with grade 1 lesions classified as benign, grade 2 as low-grade glioma, grade 3 as anaplastic glioma, and grade 4 as glioblastoma. The lesions are usually quite large with both solid and cystic components. Calcifications can occur but are unusual. Parietal, frontal, and temporal lobes are common sites of astrocytoma. On fluid-sensitive sequences, the cystic component of the lesion may show features of complex fluid, with decreased signal intensity relative to CSF on T2-weighted sequences. After contrast infusion, homogeneous enhancement of the solid component of the lesion is characteristic. Higher-grade, more malignant tumors often have less distinct margins, more heterogeneous enhancement, and increased peritumoral edema and mass effect. Newer techniques including perfusion-weighted MRI and MR spectroscopy have been evaluated as noninvasive techniques to determine tumor angiogenesis and capillary permeability. For example, in higher-grade gliomas increased blood flow to the tumor lesion has been demonstrated using this technique. Also, as the tumor grade increases to a more malignant variant, there is an increase in the Cho-to-NAA ratio, reflecting the increased metabolic activity present in higher grade lesions. Complete surgical resection is often not possible because of involvement of adjacent major neural structures. The 5-year survival rate for low-grade astrocytomas is between 40% and 50%, with less than 5% survival for the higher-grade malignancies. Because of a propensity to spread to the spine via the CSF, the staging evaluation must also include imaging of the entire spine.

The infratentorial/cerebellar form of astrocytoma, also known as juvenile pilocytic astrocytoma, is distinct from the diffuse supratentorial astrocytoma discussed here and is described in further detail below.
Oligodendrogliomas
Oligodendrogliomas, although common in adults, are relatively rare in children and adolescents. The most common location is in the frontotemporal region, with less common involvement of the posterior fossa and spinal cord. Imaging evaluation shows a hypodense lesion on CT. Because the majority of these contain varying degrees of calcification, they are distinct as being the most common supratentorial tumor in childhood to calcify. The noncalcified solid component is typically isodense to the adjacent brain on CT. On MRI, the lesions are characterized by low signal intensity on T1-weighted images, hyperintensity on T2-weighted images, and minimal enhancement after infusion of gadolinium contrast ( Fig. 66-10 ). Although these tend to be slow growing lesions, depending on their size there may be edema evident in the surrounding brain.
Gangliogliomas
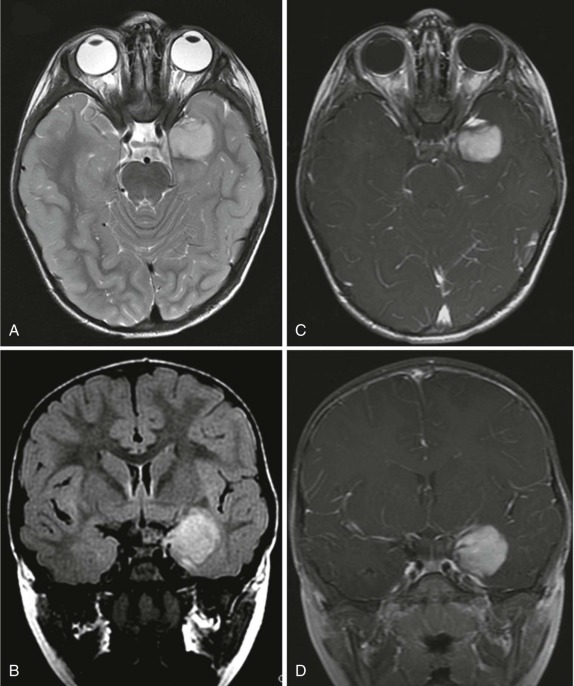
Gangliogliomas and ganglioneuromas comprise about 5% of the pediatric brain tumors. As with their counterparts in the peripheral nervous system, calcification is seen in up to 40% of the cases. The temporal lobes, frontoparietal lobes, and hypothalamic regions are the most common sites of involvement. The brainstem, posterior fossa, and spinal cord are less commonly involved. MRI demonstrates a low signal-intensity lesion on T1-weighted images with hyperintensity on T2-weighted images and hyperintense homogeneous enhancement after gadolinium infusion ( Fig. 66-11 ).
Tumors of the midline deep gray matter, including the basal ganglia, thalamus, hypothalamus, and chiasmatic/optic pathway region tend to be astrocytomas. As with the cerebral hemispheric astrocytomas, tumor grades range from benign to highly anaplastic. The imaging features are similar to the lesions located in the cerebral hemispheres with decreased signal intensity on T1-weighted images, hyperintensity on T2-weighted images, and variable contrast enhancement. Because of their location, behavioral changes, emotional and memory changes, movement disorders, visual abnormalities, and hydrocephalus are all common symptoms associated with these deep gray-matter tumors. Surgical resectability is often not possible, and the imaging evaluation is directed at identifying involvement of adjacent critical structures and monitoring posttherapy changes.
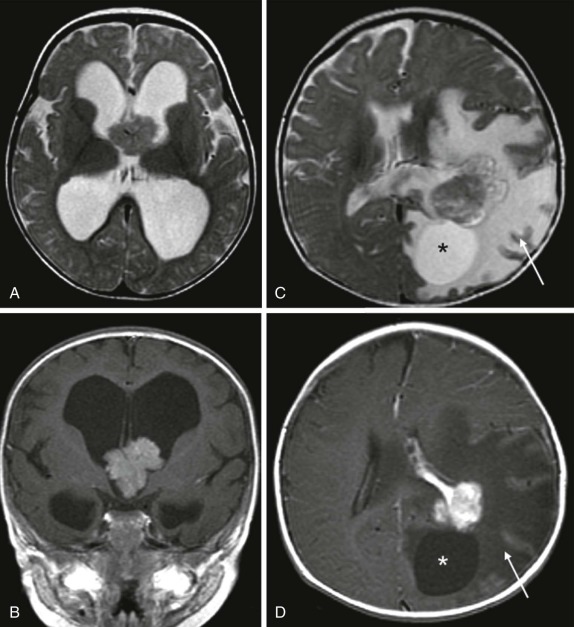
Intraventricular tumors include choroid plexus papillomas, neurocytomas, and dermoid/epidermoid tumors. Choroid plexus papillomas/carcinomas are relatively common, making up between 10% and 20% of the intraventricular tumors identified in the first year of life and comprising approximately 5% of all CNS neoplasms. The imaging features are characteristic and typically reveal large lobulated intraventricular lesions ( Fig. 66-12 ). These tumors are highly vascular and are often associated with calcification and occasionally hemorrhage. Depending on the size and location of the lesion, there may be obstructive hydrocephalus. MRI shows a low signal-intensity lesion on T1-weighted images with variable hyperintensity on T2-weighted images. Magnetic susceptibility is seen if hemorrhage is present, and after contrast infusion these lesions typically show marked contrast enhancement. The combination of a lobulated, intensely enhancing intraventricular lesion is nearly diagnostic of choroid plexus papilloma. Five percent to 10% of these lesions degenerate into choroid plexus carcinomas, and this latter diagnosis is suggested by accompanying necrosis within the lesion and metastatic spread into the adjacent brain, either by direct extension into the adjacent brain or by CSF dissemination. Because of their intraventricular location, metastatic spread throughout the CNS via CSF dissemination is unfortunately common for choroid plexus carcinomas, resulting in a uniformly poor prognosis.
Central Neurocytomas
Neurocytomas are rare in children and have imaging features that mimic oligodendroglioma, showing an intraventricular calcified mass with heterogeneous contrast enhancement. These tumors typically involve the lateral ventricle in the midline; however, surgical resection is necessary to distinguish this lesion from other intraventricular tumors such subependymal astrocytomas and ependymomas.
Midline Supratentorial Tumors
Tumors of the hypothalamic/suprasellar region include craniopharyngiomas, hypothalamic hamartomas, and pineal region tumors.
Craniopharyngiomas
Craniopharyngioma is the most common suprasellar tumor. It has fairly characteristic imaging features with lesions containing solid, cystic, and calcified elements. Symptoms usually result from increased intracranial pressure and local effects upon the hypothalamic pituitary region and optic chiasm. Because these are relatively slowly growing lesions, they may be quite large at diagnosis. The presence of a calcified suprasellar mass at CT scan is usually suggestive of the diagnosis; however, MRI is superior in evaluating the extent of involvement ( Fig. 66-13 ). Depending on the type of material present in the cystic component of the mass, variable signal intensity on T1- and T2-weighted images may be seen. It is common, for example, for the complex proteinaceous, cholesterol-laden fluid to be variably hyperintense on both T1- and T2-weighted images (see Fig. 66-13 ). After contrast infusion, the cyst wall commonly enhances. However, the solid components of the cyst often demonstrate inhomogeneous enhancement as well. Of note, craniopharyngiomas commonly cause T2 prolongation along the optic tracts. This is a nonspecific sign and has been reported in other tumors centered in this region. It is thought to signify optic tract edema caused by compression by the tumor.
Pineal-Region Tumors
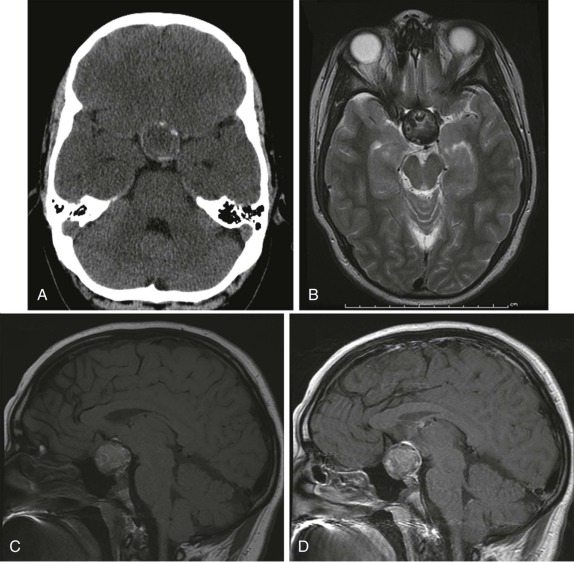
Pineal-region masses are characterized by their location in the quadrigeminal plate cistern. These tumors typically arise from primordial germ cells and include pineoblastomas, pineocytomas, and immature germ-cell tumors. It is important to distinguish these neoplastic lesions from benign pineal cysts, which are commonly seen within the pineal gland. Pineal cysts have a characteristic appearance on both CT and MRI and do not distort or compress the adjacent ventricles. Simple pineal region cysts typically do not achieve sizes greater than 1 cm. Also of note is normal calcification within the pineal gland. Calcification is unusual before the age of 10 but is commonly seen in adolescents and older children. Therefore the presence of calcification in the pineal region in an infant or young child should raise suspicion for a pineal region mass/germ-cell tumor and prompt further investigation.
Pineal-gland tumors, including pineocytomas and pineoblastomas, are relatively rare. Pineocytoma is benign and usually nonaggressive, whereas the pineoblastoma, representing an immature undifferentiated lesion, often metastasizes. Pinealoblastoma is also a component of the “trilateral retinoblastoma” and is seen with increased incidence in patients diagnosed with bilateral retinoblastoma. Pineocytomas are more often more calcified than pinealoblastomas. Pinealoblastomas are typically larger with low signal intensity on T1-weighted images, variable hyperintensity on T2-weighted images, and fairly homogeneous contrast enhancement ( Fig. 66-14 ). Very large pinealoblastomas frequently produce mass effect on the adjacent quadrigeminal cistern, and because of their large size often show areas of necrosis.
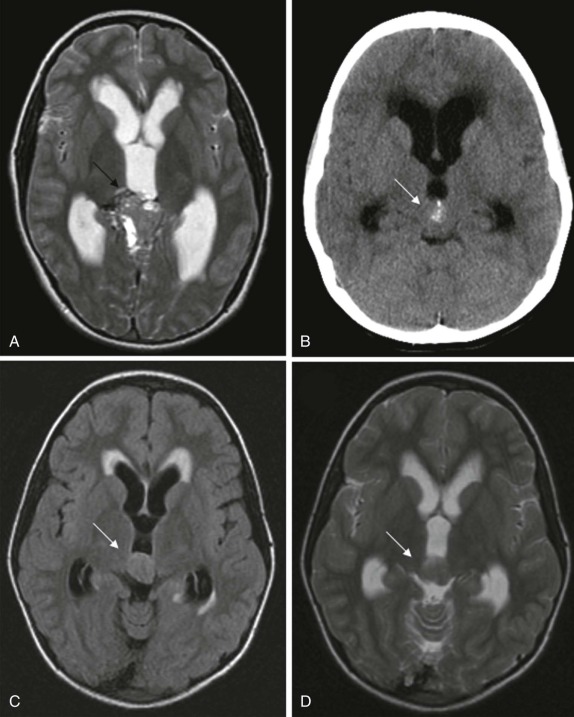
Germ-Cell Tumors
The pineal region is the most common location for intracranial germ-cell tumors. Histopathologically these are most often germinomas and are usually associated with calcification. This is well demonstrated by CT. MRI is indicated to better delineate the extent of the noncalcified solid portion of the mass as well as heterogeneous cystic portions that may also be present. On T2-weighted images, the solid portion is typically hyperintense with bright enhancement after contrast infusion. Other tumors of germ-cell origin are also seen, with choriocarcinomas more commonly showing hemorrhagic components and displaying more aggressive features. Benign teratomas, in contrast, display imaging features more in keeping with their well-differentiated dermoid/epidermoid components, including low attenuation fluid on CT, hyperdense fluid on T1-weighted sequences, and heterogeneous contrast enhancement ( Fig. 66-15 ).
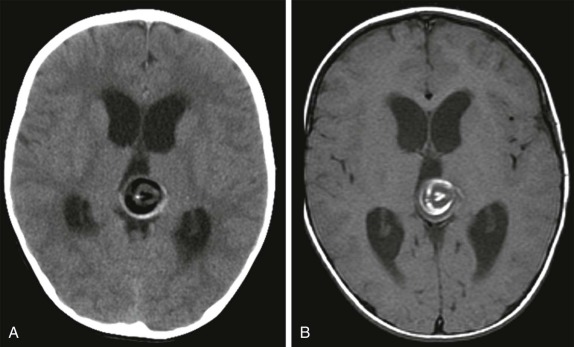
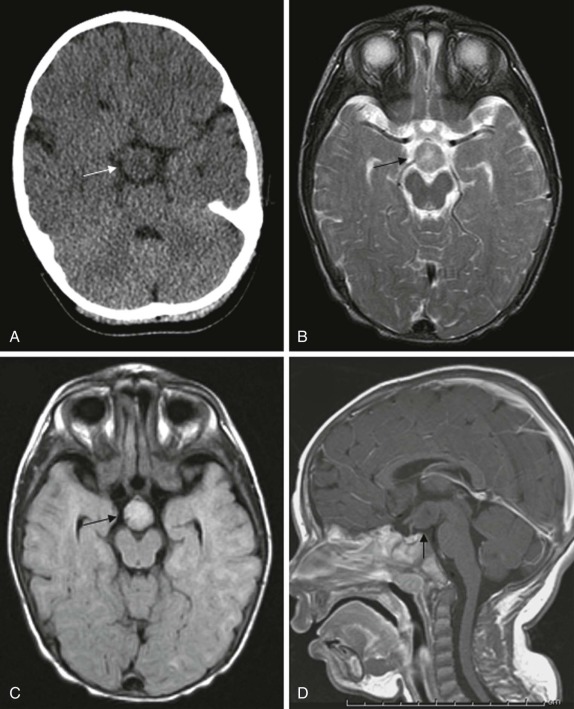
Hypothalamic Hamartoma
This is a rare congenital lesion, usually identified in infants under the age of two. Seizure disorders are common, and these patients often have the presenting symptoms of intractable gelastic seizures. The presence of precocious puberty or diabetes insipidus may provide clinical clues to suggest this diagnosis.
On imaging, CT scanning shows an isodense mass in the region of the hypothalamus. There is little to no contrast enhancement either on CT or MRI. On MRI, the lesion is usually isodense to the brain on T1-weighted images, minimally hyperintense on T2-weighted images, and interestingly may be hyperintense on FLAIR images ( Fig. 66-16 ). Because of the location of these tumors, surgical resection may be challenging, and MR- or CT-guided ablation techniques are now increasingly being used ( Fig. 66-17 ).
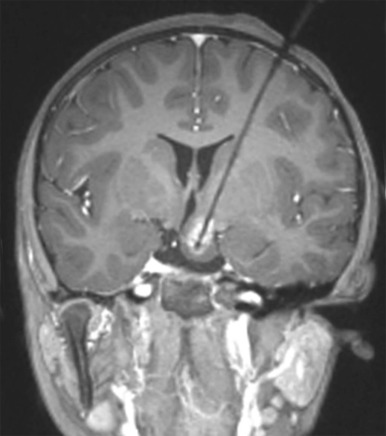
Infratentorial Tumors
The most common lesions of the posterior fossa/cerebellum are juvenile pilocytic astrocytomas (JPAs) and medulloblastomas.
Medulloblastomas
Medulloblastomas are slightly more common in incidence than cerebellar astrocytomas. They typically occur in the first decade of life. Presenting symptoms usually relate to increased intracranial pressure secondary to obstructive hydrocephalus, most often related to mass effect upon the fourth ventricle. Medulloblastomas are highly malignant and have a propensity to disseminate and seed the CNS via the CSF route. Hematogenous spread to other sites may also occur in medulloblastoma.
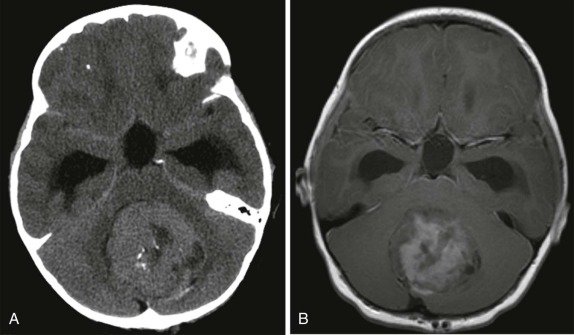
CT scanning is usually the first imaging study performed to evaluate new-onset nausea, vomiting, headache, or seizures. CT scanning usually demonstrates a hyperintense solid lesion within the cerebellum, often near the midline ( Fig. 66-18 ). MRI imaging is indicated to better characterize the lesions and to establish sites of local and metastatic spread. As with other highly cellular CNS tumors, on T1-weighted images medulloblastomas are usually hypointense relative to the adjacent cerebellum, with increased signal intensity on T2-weighted images (see Fig. 66-18 ) and homogeneous enhancement after gadolinium infusion. Calcification and cystic areas are unusual in medulloblastoma. Because of the propensity to disseminate to other sites via the CNS, the staging evaluation must include a contrast-enhanced evaluation of the entire spine.
Cerebellar Astrocytomas
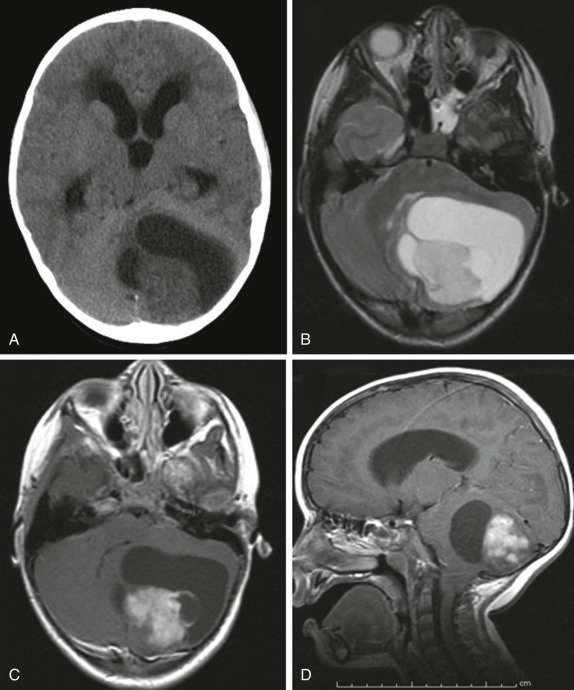
Astrocytomas are the next most common posterior fossa tumor. As with their intracerebral counterpart, cerebellar astrocytomas range in histologic grade from benign to anaplastic. The characteristic juvenile pilocytic form makes up the majority of the cerebellar astrocytomas. Both cystic and solid components are normally present in this type of astrocytoma ( Fig. 66-19 ). Calcification is unusual and occurs in 10% of tumors. After resection, survival is excellent with a 10-year overall survival rate greater than 90%. As with medulloblastoma, CT scanning is usually the first imaging modality used to evaluate initial symptoms. CT shows a heterogeneous, predominantly cystic lesion in the posterior fossa, typically with a small mural nodule eccentrically along the margin of the tumor. These lesions are often midline, near the vermis, but may also be eccentric in location. MRI imaging is indicated to better characterize the extent of cerebellar involvement. Although the presence of a predominantly cystic component suggests a lower-grade neoplasm, foci of local/metastatic spread may be present and will be manifest as a focus of increased enhancement after contrast administration. Therefore the imaging evaluation should include conventional T1- and T2-weighted imaging as well as postcontrast enhanced scanning in all three imaging planes. Because higher-grade neoplasms may also disseminate via the CSF, as with medulloblastoma, the initial staging evaluation should also include evaluation of the entire spine.
Brain-Stem Gliomas
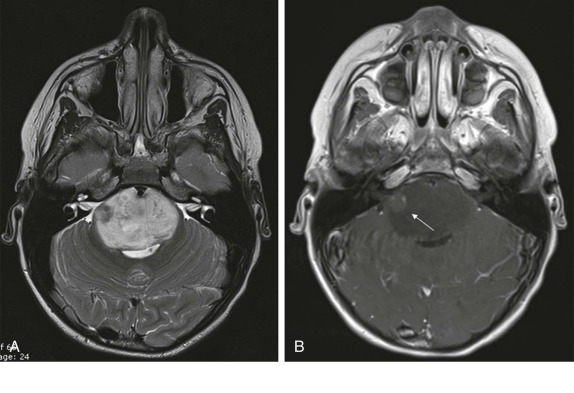
Brain-stem gliomas make up the majority of the neoplasms involving the brain stem. These tumors are of astrocytoma origin and because of their location result in nearly uniformly poor survival. These predominantly cellular solid lesions are best evaluated by MRI, and they typically appear as hypodense lesions on T1-weighted images, with increased signal intensity on T2-weighted images, and show heterogeneous enhancement. Lower-grade lesions, or lesions with larger cystic components may only show very minimal contrast enhancement ( Fig. 66-20 ). As with the other tumors in these locations, spread is most often via the CSF to other portions of the brain.
Ependymomas
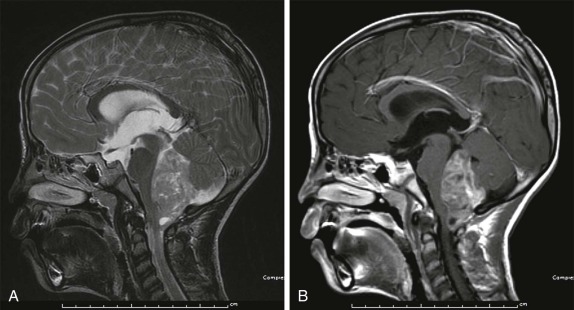
After medulloblastomas and juvenile pilocytic astrocytomas, the next most common posterior fossa tumor is the ependymoma, constituting approximately 8% to 15% of intracranial neoplasms. The majority of these tumors are benign and arise from the ependyma of the fourth ventricle. They typically present as a calcified intraventricular mass. Symptoms result from obstruction of CSF flow at the level of the fourth ventricle leading to obstructive hydrocephalus. There are two histologic types, with the benign undifferentiated type more common, typically less invasive locally, and with a lower likelihood of CSF dissemination. Malignant anaplastic ependymomas, in contrast, tend to spread via the CSF early in the disease course to other sites within the CNS. Because of their propensity to insinuate through neural foramina, resulting in a lobulated appearance, ependymomas have a fairly characteristic appearance on imaging ( Fig. 66-21 ). Depending on the degree of calcification, these lesions may be predominantly hypointense on T1- and T2-weighted images. In the absence of calcification, these are usually hyperintense lesions on T2-weighted images with heterogeneous intense enhancement after gadolinium infusion. Because of their propensity to seed throughout the CSF and ventricular system, the imaging evaluation must include examination of both the brain and spinal canal.
Hemangioblastomas
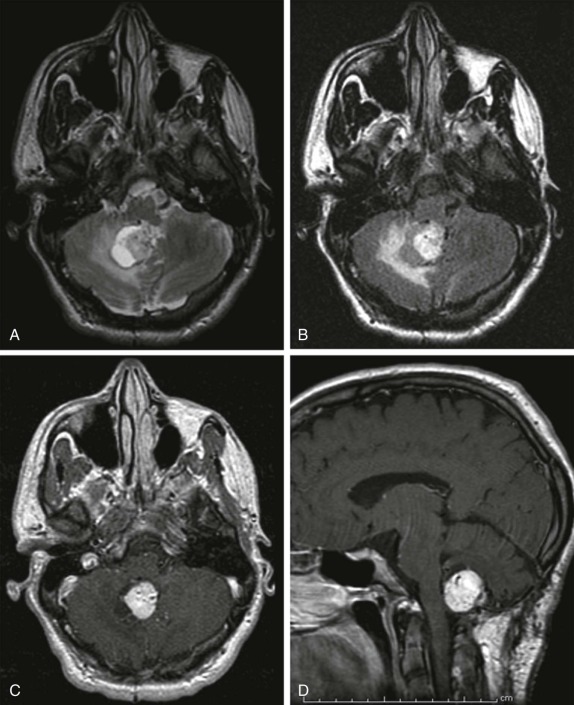
Hemangioblastomas are benign vascular lesions that are more common in adults than children. However, the cerebellum is still the most common location in children, and multiple hemangioblastomas may be encountered in familial predisposition syndromes such as the von Hippel-Lindau disease. The imaging features demonstrate a predominantly cystic lesion with an enhancing mural nodule that may be difficult to distinguish from JPAs. As with JPAs, these lesions show cystic characteristics on MRI with low signal intensity on T1-weighted images and T2 hyperintensity, with intense enhancement of the solid mural nodular component ( Fig. 66-22 ). Depending on the size of the mural nodule, the degree of enhancement, and its location, these lesions may also be difficult to discriminate from a benign posterior fossa arachnoid cyst.
Metastatic Disease
Metastatic disease to the CNS may result from leptomeningeal seeding, hematogenous dissemination, or direct extension. Leptomeningeal seeding results from a number of primary brain tumors, most notably medulloblastomas. Leptomeningeal seeding can also result from non-CNS primary tumors and systemic malignancies. The imaging appearance in this setting is similar to that of leptomeningeal dissemination occurring secondary to a primary CNS tumor.
Hematogenous metastases to calvarium or dura are most commonly seen in patients with neuroblastoma, leukemia, and lymphoma. These lesions present as lytic calvarial lesions or as nonspecific, enhancing dural masses. CNS involvement by leukemia is also common, although this is more often diagnosed by lumbar puncture and pathologic evaluation of the CSF rather than imaging.
Hematogenously disseminated metastases to the brain parenchyma are rare in childhood. When they do occur, primary tumors are sarcomas, particularly rhabdomyosarcoma, Ewing sarcoma, and osteosarcoma. A tumor with a particular propensity to metastasize to the brain is the rhabdoid tumor of the kidney, and imaging of the CNS is essential in children with this diagnosis. Parenchymal metastases are usually multiple and located at the interface between the gray and white matter. They are associated with marked vasogenic edema and demonstrate avid enhancement secondary to loss of the blood-brain barrier.
Although the imaging features may be variable depending on the primary malignancy, the presence of any focal area of signal abnormality or enhancement with a known primary tumor should raise suspicion for metastatic spread of disease to the CNS.
Spinal Cord Tumors
In comparison to pediatric brain tumors, spinal cord tumors are relatively rare in children. The majority of the intramedullary spinal tumors in children are low-grade astrocytomas, with the remainder being ependymomas. The imaging features of these lesions mimic their intracranial counterparts. The imaging assessment is usually prompted by neurologic symptoms and should include multiplanar T1, T2, and post–gadolinium-enhanced imaging. Because it may be difficult to discriminate a primary intraspinal tumor from metastatic disease, imaging of the brain must also be performed.
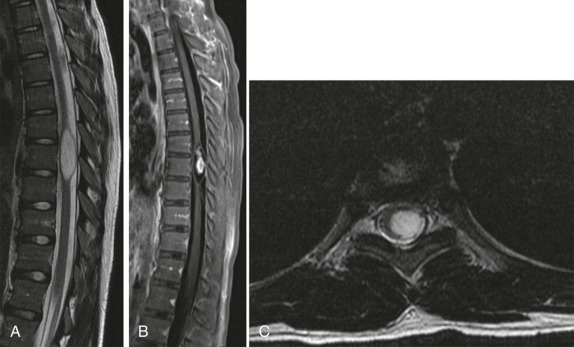
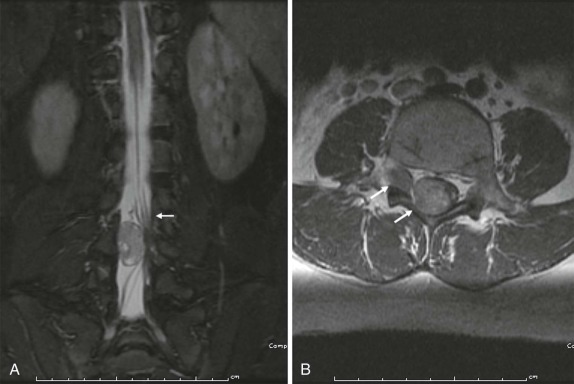
As in the brain, the imaging examination should be directed at identifying the site of origin of the tumor. It should be possible to make a distinction between primary intramedullary tumors, extramedullary intradural tumors, and extradural/epidural lesions. This is important, because the majority of the spinal tumors in children are extramedullary. Intradural extramedullary lesions are, by definition, located within the subarachnoid space and are typically the result of drop metastases, which as discussed earlier, spread via the CSF from primary brain tumors ( Fig. 66-23 ). Their appearance on imaging mimics their counterparts in the brain. Extradural extramedullary tumors may arise from local extension of other primary neoplasms such as neuroblastoma, lymphoma, or Ewing sarcoma ( Fig. 66-24 ). In evaluating these other primary pediatric neoplasms, when their location is adjacent to the vertebral column, it is essential that a thorough imaging evaluation be undertaken to evaluate the presence and extent of intraspinal involvement. In particular, with neuroblastoma, this will influence staging and surgical resectability. The presence of intraspinal extension may be suggested by CT, but will be typically better delineated by MRI ( Fig. 66-25 ).
Pulmonary and Intrathoracic Tumors
Thyroid and Parathyroid Tumors
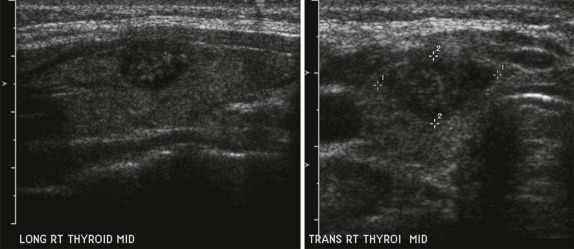
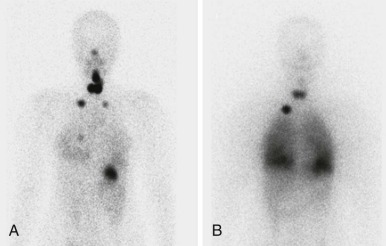
Thyroid carcinoma in children is rare. Three histologic subtypes are recognized in children, with the majority of patients (≈80%) having papillary carcinoma; follicular (≈15%) and medullary (≈5%) thyroid cancers occur less often. The risk is increased in patients with prior history or neck radiation or with predisposing familial syndromes (e.g., multiple endocrine neoplasia [MEN]). Presentation is typically a painless mass in the thyroid gland. Ultrasound is the imaging modality of choice in evaluating the thyroid gland and is usually sufficient to characterize a palpable thyroid abnormality. The goal of the ultrasound examination is to determine whether a cystic or solid mass is present ( Fig. 66-26 ). The presence of increased flow and size of the mass is not reliable in distinguishing benign from malignant thyroid nodules. Malignant nodules are nearly always cold on 123 I-radioiodine scintigraphy relative to the surrounding thyroid gland. Depending on the type of tumor, metastatic spread to adjacent lymph nodes and/or the lung may be present. In particular, papillary carcinoma of the thyroid often produces multiple small pulmonary nodules with a miliary distribution. These small pulmonary nodules may be challenging to detect by chest x-ray, and chest CT is indicated to provide increased sensitivity for lesion detection. Bone metastases and thoracic lymph node involvement are common sites of extrapulmonary metastatic spread. 123 I or 131 I scintigraphy may also detect lesions not seen by conventional imaging ( Fig. 66-27 ). The treatment of thyroid cancer in children includes total thyroidectomy and lymph-node dissection, followed by 131 I-radioiodine therapy for ablation of remnant thyroid tissue as well as to treat sites of micrometastatic disease not visualized by other imaging techniques or not amendable to surgical resection. Diagnostic imaging is initially performed with 123 I-radioiodine to establish whether significant remnant tissue, iodine-avid lymph nodes, and metastatic disease are present in order to determine the appropriate therapeutic dose of 131 I-radioiodine. Higher doses are used for patients with extensive lymph-node involvement or metastatic spread, as opposed to those with only remnant thyroid tissue in the surgical bed. As shown in Figure 66-27 , the higher doses of 131 I used for therapy often allow sites of disease to be detected at the time of the posttherapy follow-up 131 I scintigraphy examination that were not apparent on the initial diagnostic 123 I scan.
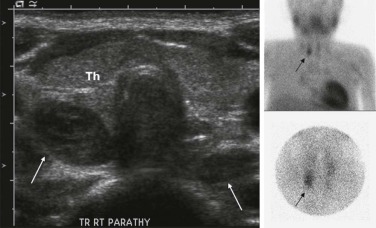
Abnormalities of the parathyroid gland usually come to clinical attention during evaluation of hypercalcemic states. Functional parathyroid hyperplasia and parathyroid adenomas have a similar imaging appearance and can be very well characterized by ultrasound. There is usually no need for additional imaging by CT. Because of the variable anatomic locations of the parathyroid glands, it may be difficult to identify all potential sites of abnormality by conventional anatomic imaging techniques. 99m Tc-Methoxyisobutylisonitrile (MIBI) scintigraphy is very sensitive and specific at showing focal accumulation of radiotracer in sites of functional parathyroid activity and can be used to identify sites of otherwise occult disease ( Fig. 66-28 ).
Mediastinal Masses
The mediastinum is located in the center of the thorax, between the two thoracic cavities, the diaphragm, and thoracic inlet. It is conventional to divide the mediastinum into anterior, middle, and posterior mediastinal compartments based on their location as seen on the lateral chest radiograph. Although there are no distinct tissue planes that delineate these compartments, this system of classification is useful in characterizing diseases based on their tissue of origin. Classifying mediastinal masses within a single mediastinal compartment helps narrow the differential diagnosis, calls attention to the potential effect of the mediastinal mass upon adjacent compartmental structures, and therefore commonly guides clinical decision making.
Anterior Mediastinal Masses
The anterior mediastinum is defined as the prevascular space situated between the sternum and the heart, pericardium, and great vessels. The anterior mediastinum extends superiorly from the thoracic inlet to the level of the diaphragm. Organs located in the anterior mediastinum include thymus, thyroid, and parathyroid glands and prevascular-space lymphoid tissue. With respect to mediastinal mass formation, the thymus and anterior mediastinal lymph nodes are the two most important structures to be considered.
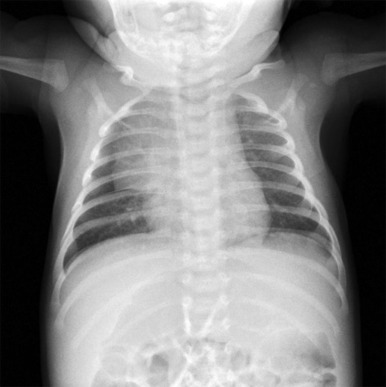
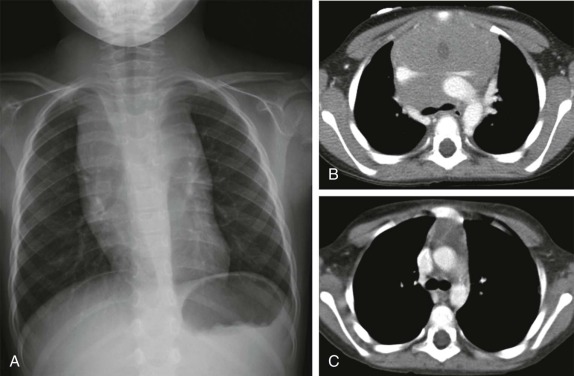
In infants, it is important to distinguish normal thymus from a mediastinal mass. The normal thymus typically has an undulating contour and on chest radiographs can be accurately identified based on subtle deformity by the adjacent anterior ribs’ costal cartilages ( Fig. 66-29 ). The normal thymus can be quite large and may extend posteriorly between the superior vena cava and aorta in the middle mediastinum or superiorly into the lower neck, making the distinction from neoplasm difficult. In the appropriate clinical setting thymic hyperplasia may also be seen and is characterized by a homogeneously enlarged thymus. The thymus also may rapidly involute in response to physiologic stress, and this can also provide an indirect means of confirming its identity as normal thymus.
Lymphoma.
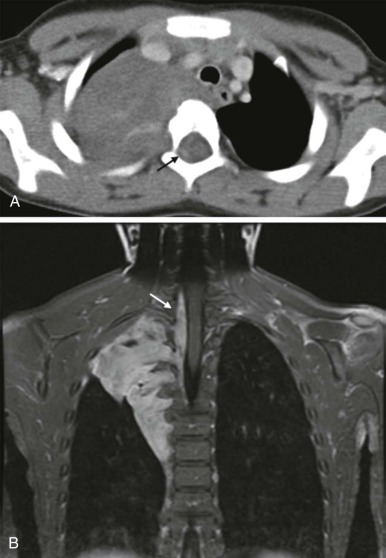
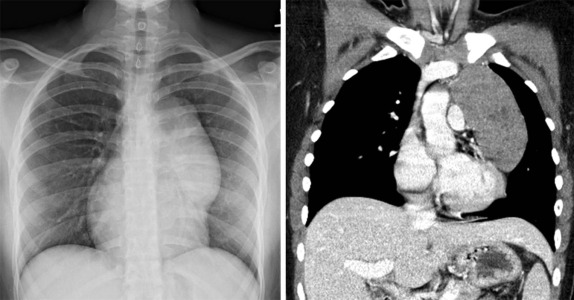
Lymphoma is the most common tumor arising in the mediastinum and most commonly arises in either the anterior mediastinum or middle mediastinum. Both Hodgkin and non-Hodgkin lymphomas can present as mediastinal masses. The imaging features of Hodgkin lymphoma characteristically reveal a bulky anterior mediastinal mass with a nodular appearance. In addition, Hodgkin lymphoma is characterized by contiguous lymph-node spread, which may aid in the distinction from other types of lymphoma. Approximately two thirds of pediatric patients with Hodgkin lymphoma show lymphadenopathy involving the mediastinum. Non-Hodgkin lymphomas include lymphoblastic lymphoma and other subtypes. Approximately one third of pediatric non-Hodgkin lymphomas have a mediastinal mass, and more than 50% of the lymphoblastic lymphomas have mediastinal involvement. Although the distinction between some subtypes of non-Hodgkin and Hodgkin lymphoma may be difficult, acute T-lymphoblastic lymphomas often demonstrate a homogeneous infiltration and enlargement of the thymus gland with encasement/compression of the vessels ( Fig. 66-30 ). All forms of lymphoma can result in significant tracheal compression/narrowing, vascular compression, and symptoms related to the superior mediastinal syndrome. Pleural and pericardial effusions are seen in both non-Hodgkin and Hodgkin lymphomas of the mediastinum and are not a distinguishing feature. Bone-marrow involvement is seen in patients with either Hodgkin or non-Hodgkin lymphoma. Recent studies have shown that FDG-PET is a sensitive method for detecting bone-marrow involvement in children with Hodgkin lymphoma and can safely replace routine bone marrow biopsy for establishing the presence of marrow involvement. Direct bone invasion by Hodgkin lymphoma is unusual; non-Hodgkin lymphomas often show more aggressive local involvement.
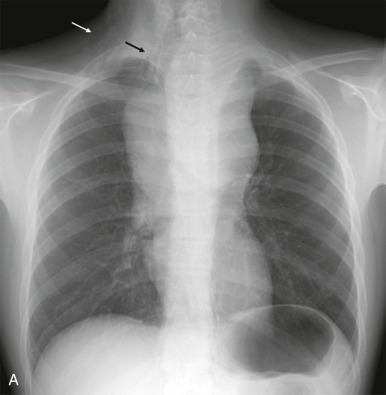
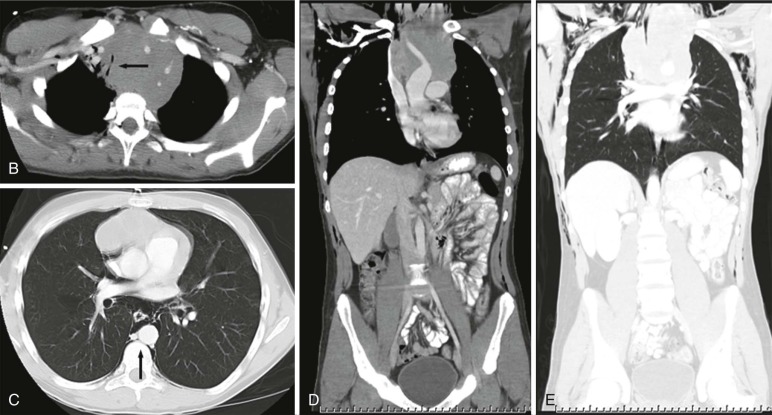
The imaging evaluation should always include a chest radiograph. In many instances this is the most important imaging study to guide the subsequent care of the patient. The presence of significant tracheal narrowing or tracheal displacement in the context of a large anterior mediastinal mass, particularly if accompanied by respiratory symptoms (see Figs. 66-2 and 66-30 ), should raise immediate concern for impending respiratory/cardiopulmonary compromise and prompt intensive care unit (ICU)–level monitoring. Pneumomediastinum and pneumothorax may also be identified on these initial imaging studies (see Fig. 66-30 ). The placement of the patient in a recumbent position for CT scanning may further exacerbate the patient’s already tenuous respiratory status, impair central venous return, and result in an acute cardiorespiratory event. In this setting it may be necessary to delay CT imaging, and the staging evaluation will have to be postponed until stabilization of the patient’s clinical status.
In a stable patient, staging of anterior mediastinal lymphomas must include imaging of the neck to evaluate the Waldeyer ring of lymphoid tissue and should also include the abdomen and pelvis. Intravenous contrast should be used to provide an accurate assessment of the mediastinal vascular structures. Oral contrast should be provided to opacify the bowel and aid in the distinction from mesenteric lymphadenopathy.
MRI, particularly with faster scanning techniques, may be effective in evaluating mediastinal masses and staging lymphoma but currently does not have an established role in the more acute stages of the evaluation. Gallium scintigraphy previously and currently FDG-PET imaging are recognized as standard of care in identifying sites of metabolically active lymphoma and in identifying sites of disease otherwise undetected by conventional imaging techniques ( Fig. 66-31 ). FDG-PET scanning, in particular, has also been shown to play an important role in the posttreatment evaluation, helping to stratify patients into early responder and nonresponder groups to allow appropriate modulation of therapy based on treatment response ( Fig. 66-32 ).
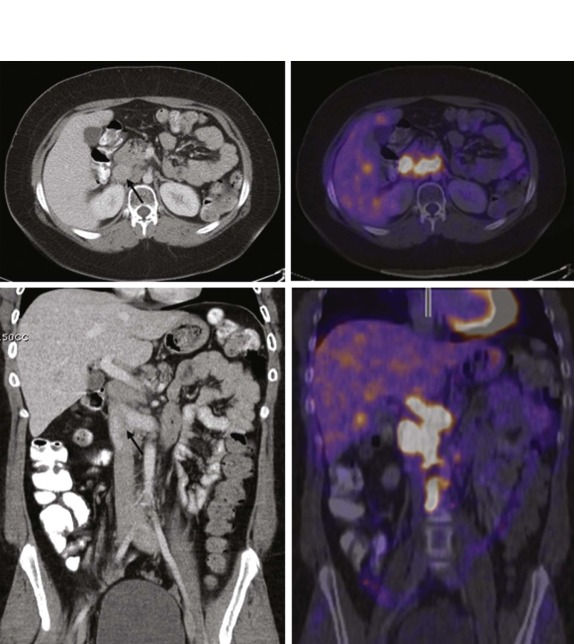
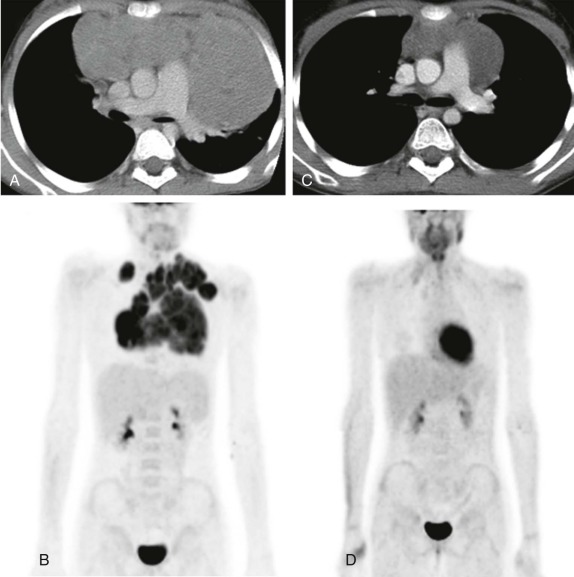
After treatment, particularly of Hodgkin disease, it is common for the mediastinal mass to slowly regress and for residual inflammatory tissue to persist even months after therapy. In contrast, lymphoblastotic lymphomas often respond quite rapidly to therapy with very little residual soft-tissue abnormality remaining in the anterior mediastinum, even after a relatively short duration of treatment ( Fig. 66-33 ). The use of FDG-PET for early response assessment in Hodgkin lymphoma is now considered standard of care, with images being simultaneously acquired and coregistered as fused PET-CT images to distinguish persistent metabolically active disease from residual posttreatment inflammatory tissue. There is a large body of literature from both pediatric and adult patients showing that the presence of residual metabolically active disease after two cycles of chemotherapy is prognostically significant, presumably reflecting chemosensitive versus chemoresistant disease. Patients with residual CT abnormalities that are FDG-avid have a worse overall outcome as compared with patients whose scans are FDG-negative, which has led to a paradigm shift in Hodgkin lymphoma therapy such that patients in whom early response assessment shows no metabolically active disease would be eligible for reduced chemotherapy and radiation in an effort to reduce treatment-related complications. Whether this approach will maintain the good outcomes (>90% overall survival) typically seen in Hodgkin lymphoma remains to be seen. There is currently no convincing data showing the prognostic value of FDG-PET imaging in non-Hodgkin lymphoma.
Germ-Cell Tumor.
Mediastinal germ-cell tumors are primarily located in the anterior mediastinum near the thymus gland and make up about 10% to 20% of all childhood mediastinal tumors. Germ-cell tumors are second only to lymphoma as the cause of a thymic/anterior mediastinal mass. When they present as predominately solid masses, they may be difficult to distinguish from lymphoma. Histologically, mature teratomas are the most common germ-cell tumor arising in the mediastinum. At diagnosis, calcification of lymphoma is unusual, and the calcifications that are often present in teratoma may suggest the diagnosis. Germ-cell tumors are often asymptomatic and may be identified incidentally as mediastinal masses on chest radiographs obtained for other indications. When symptoms are present, they are usually the result of compression of the tracheobronchial tree.
Either CT or MRI scanning are effective at further characterizing these masses. The presence of fluid attenuation, mixed attenuation fluids and gelatinous material, fat, and calcification all should suggest the diagnosis of mediastinal germ-cell tumor ( Fig. 66-34 ). Ten percent to 20% of all mediastinal germ-cell tumors are malignant and present with signs of local invasion into the pleura or pericardium. Malignant tumors include seminomatous and nonseminomatous histologic subtypes. Seminomas tend to be noncalcified, bulky solid masses that either remain localized or spread to local lymph nodes, whereas other malignant germ-cell tumors display greater heterogeneity with cystic and solid elements and more aggressive, locally invasive features. Germ-cell tumors may rupture into the pleural or pericardial spaces, the lung parenchyma, or the tracheobronchial tree. Imaging evaluation should be directed at determining the extent of rupture and sites of tissue involvement. Chemical pneumonitis can result from parenchymal rupture, and expectoration of blood, hair, and sebaceous material can result from rupture into the airways.
Thymic Masses
Thymoma.
Thymoma is rare in children, accounting for less than 4% of pediatric mediastinal tumors. As with adults, thymoma may present with symptoms related to associated autoimmune disorders, including myasthenia gravis, diabetes, and Hashimoto thyroiditis. Thymomas are typically solid, lobulated masses arising in or around the thymus ( Fig. 66-35 ). The imaging evaluation should be directed at assessing for aggressive features such as invasion into adjacent pericardial or pleural structures. MRI and contrast-enhanced CT reveal a heterogeneously enhanced low-density mass that is isointense on T1-weighted images and mildly T2 hyperintense relative to muscle. Both CT and MRI are excellent in delineating the relationship of these masses to the adjacent vascular structures (see Fig. 66-35 ). Nodular pleural or pericardial thickening should raise concern for invasive thymoma. Because complete surgical resection is the most effective treatment for ensuring long-term survival for patients with thymoma, postsurgical radiation therapy may be considered when invasive features are present.
Thymolipomas.
Thymolipoma, in contrast to thymoma, is relatively more common, making up about 3% to 9% of pediatric thymic tumors. These benign tumors are most often seen in older children, but they may present in infancy. These heterogeneous masses may contain calcification and cystic areas, making distinction from germ-cell tumors challenging. A predominantly fatty mass should, however, suggest the diagnosis of thymolipoma ( Fig. 66-36 ). The fat characterization can be sensitively and specifically evaluated by MRI, showing a high signal-intensity mass on T1-weighted imaging with loss of signal on fat-suppressed images. A low-attenuation mass with negative Hounsfield-unit density on either unenhanced or contrast-enhanced CT can also suggests the presence of a fat-containing mass (see Fig. 66-36 ).

Middle Mediastinum
Tracheobronchial Tree Masses.
The middle mediastinum is defined as the vascular space including the pericardium/heart, the great vessels, and trachea/proximal bronchi and associated lymph nodes. Although the most common middle mediastinal mass is lymphoma, lymphoma is rarely isolated to the middle mediastinum and usually occurs in conjunction with a large anterior mediastinal mass. The most common masses isolated to the middle mediastinum are benign bronchopulmonary foregut malformations, including bronchopulmonary sequestration, congenital cystic adenomatoid malformation (C-CAM), and foregut/bronchogenic cysts. These benign masses are not discussed further in this section except to emphasize that the distinction between cystic adenomatoid malformation and pleuropulmonary blastoma cannot be made on the basis of imaging alone ( Fig. 66-37 ). Surgical resection and histopathologic evaluation is required to distinguish the benign C-CAM from its neoplastic counterpart.
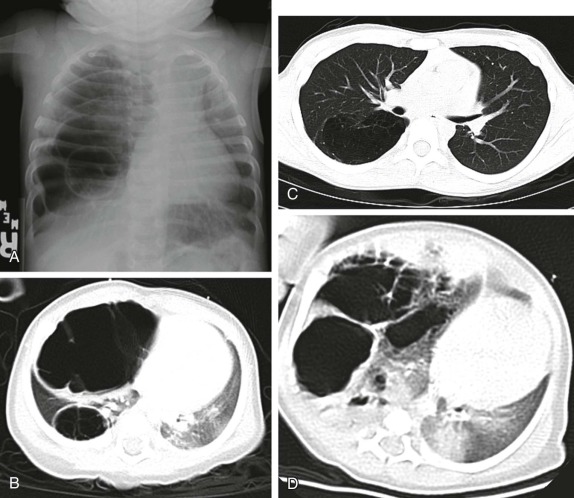
Masses of the endobronchial tree are also exceedingly rare in children. The most common endobronchial neoplasm in children is the bronchial carcinoid tumor, making up about 50% of all bronchial neoplasms. Although these tumors may secrete neuroendocrine peptides, the classic carcinoid syndrome is relatively unusual in children with bronchial carcinoids, and these patients are more likely to have presenting respiratory symptoms such as hemoptysis or lobar atelectasis/partial collapse caused by the obstructing endobronchial lesion ( Fig. 66-38 ). These lesions are commonly hilar in location and may be very difficult to identify on chest radiographs. The presence of a persistent area of segmental or lobar collapse that does not resolve after appropriate therapy should prompt further investigation by CT scanning to directly assess the airways. Multidetector-row CT scans can provide exquisite 3D reformations of the airways and allow accurate detection of endobronchial abnormalities. Chest CT of patients with suspected endobronchial carcinoid tumor may be performed without intravenous contrast for the purpose of identifying the endobronchial lesion; however, contrast infusion typically shows prominent enhancement of these highly vascular lesions. There may be intraluminal, mural, and extraluminal components of these lesions, and the extraluminal extent of the lesion may exceed its intraluminal component. Although the presence of calcification in an endobronchial mass should suggest the diagnosis of a carcinoid tumor, calcification is still relatively rare in childhood bronchial carcinoid tumors. Surgical resection is usually curative, and although the extent of local invasion around the primary lesion is variable, metastatic disease is relative rare (5% to 20%). Uptake of the neuroendocrine-specific radiotracer 111 I-octreotide provides highly specific scintigraphic imaging confirmation of the diagnosis (see Fig. 66-38, C and D ) and may aid in identifying occult sites of metastatic disease. FDG-PET imaging of metabolically active carcinoid tumors has also been performed and, although not specific for neuroendocrine tumors, may prove to be a sensitive means of identifying occult metastases.
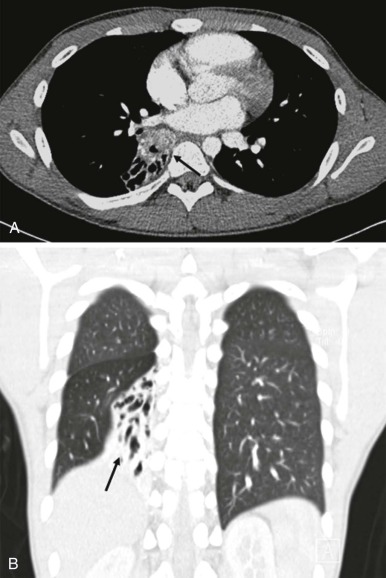
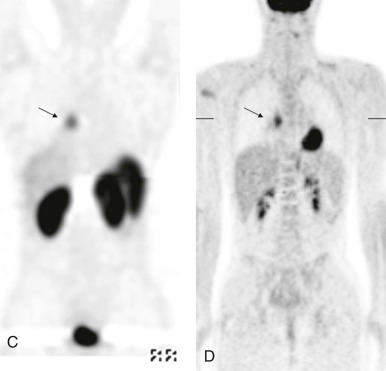
Mucoepidermoid carcinoma is the second most common primary bronchial neoplasm. These patients also have primarily respiratory presenting symptoms related to airway obstruction. Because primary airway neoplasia in children is rare and commonly characterized by nonspecific clinical symptoms, the diagnosis is often delayed and difficult to distinguish from infectious processes such as pneumonia. The most common finding on plain chest radiographs in patients with mucoepidermoid carcinoma is a central mass or nodule. These tumors range from low to high grade, with high-grade neoplasms tending to invade the adjacent pulmonary parenchyma. As with carcinoid tumors, multidetector-row CT with multiplanar and 3D reformats can be used to accurately identify an airway abnormality. However, in contrast to carcinoid tumors, mucoepidermoid carcinoma is relatively hypovascular and shows minimal enhancement. Recent studies have also suggested that FDG-PET imaging may be used to advantage in staging patients with mucoepidermoid carcinoma, with intense FDG uptake visualized ( Fig. 66-39 ).
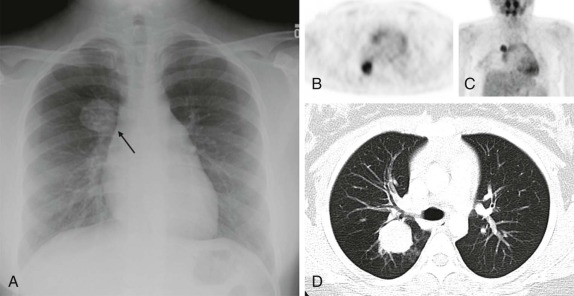
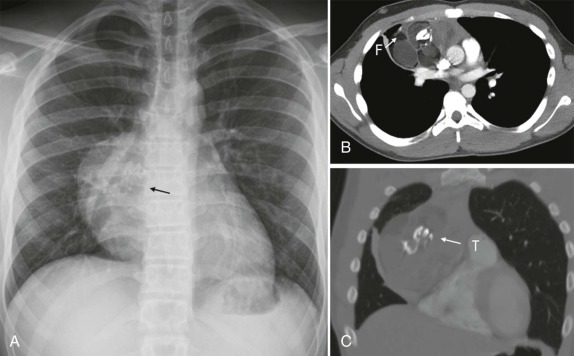
Tumors of the heart and pericardium are very rare in children. The majority of these are rhabdomyomas arising from the neoplastic transformation of cardiac myocytes. There is a well-known association between cardiac rhabdomyoma and tuberous sclerosis ( Fig. 66-40 ), with approximately half of the patients with this diagnosis having rhabdomyomas. Indeed the presence of a rhabdomyoma should prompt further evaluation, because rhabdomyomas may be the initial presenting sign of tuberous sclerosis.
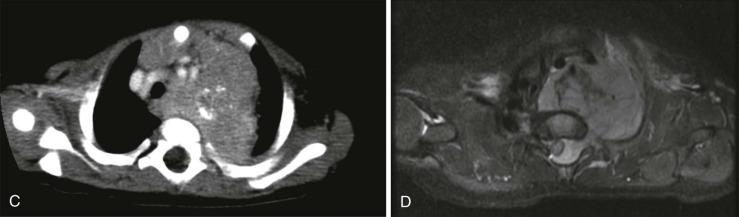
When symptoms are present, they are usually the result of tumor protruding into the cardiac lumen, resulting in outflow obstruction and congestive failure. Echocardiography is usually sufficient to suggest the diagnosis; however CT scanning and cardiac MRI are superior at defining the extent and attachments of these cardiac tumors and may be indicated for surgical planning. Cardiac fibromas and myxomas are much less common. Indeed it is more common to see secondary extension into the heart by other primary pediatric neoplasms such as neuroblastoma and Wilms tumor, both of which have a propensity to extend into the heart via the inferior vena cava (IVC); hepatoblastoma, which may show direct intracardiac extension ( Fig. 66-41 ); and mediastinal lymphoma, which may result in chest wall/pericardial involvement. Contrast-enhanced CT scanning usually allows an accurate delineation of sites of local extension in the thorax. Either abdominal ultrasound, multiphase contrast-enhanced CT, or MRI can be used to demonstrate intravascular/intracardiac extension from primary abdominal malignancies.

Posterior Mediastinal Masses
The posterior mediastinum is defined dorsally by the chest wall/vertebral column, ventrally by the pericardium and posterior wall of the great vessels, superiorly by the thoracic inlet, and inferiorly by the diaphragm. The primary components of the posterior mediastinum are the paravertebral sympathetic ganglia, azygos and hemiazygos veins, descending thoracic aorta, esophagus, and lymph nodes. The majority of the posterior mediastinal masses in children are neurogenic in origin and include ganglion-cell tumors, nerve-sheath tumors, and other nervous-tissue neoplasms such as paragangliomas. Ganglion-cell tumors arise from sympathetic chain ganglia and range from well-differentiated and benign ganglioneuromas to malignant neuroblastomas. Ganglioneuroblastomas contain elements of both benign and malignant tissue types. The distinction between these different histologic subtypes cannot be made reliably based on imaging features, although age at presentation is important in narrowing the differential diagnosis. Neuroblastoma, the most common non-CNS solid tumor in children, typically occurs in young children with a median age at presentation of younger than 2 years and more than 95% of cases occurring by the age of 10 years. In contrast, the median age at presentation of ganglioneuroblastoma is approximately 5.5 years, whereas mature ganglioneuromas occur in later childhood/early adolescence, typically after the age of 10. Whereas the majority of neuroblastomas occur in or around the adrenal gland, approximately 15% arise in the posterior mediastinum.
It is important to emphasize that these lesions may be present in a child who is otherwise asymptomatic, and a thorough inspection of the chest radiograph using multiple windows and levels is necessary to identify small foci of disease. This is of particular importance, as shown in Figure 66-42 , because a localized low-stage favorable histology neuroblastoma identified in a child under the age of 1 year may make the difference between being treated on low-risk protocols with higher likelihood of overall and disease-free survival versus more advanced stages of disease, when the patient’s risk of relapse and treatment failure increases. Primary intraabdominal neurogenic tumors may also have a significant posterior mediastinal extent and often have an associated posterior mediastinal/paravertebral component near the thoracic inlet (Virchow node). For this reason, it may be prudent to include imaging of the chest, in addition to the abdomen and pelvis, in the staging evaluation of patients with suspected neuroblastoma.
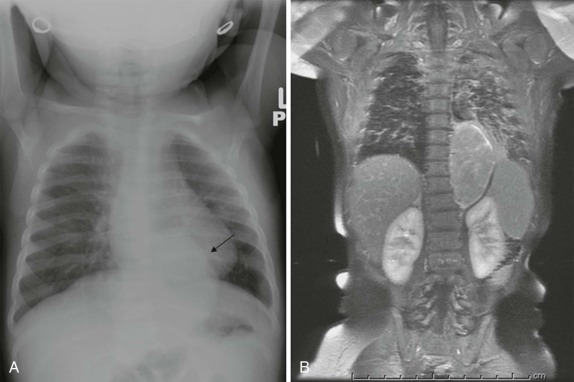
Radiographically, ganglion-cell tumors appear as paraspinal soft-tissue masses. Calcifications may be present. The presence of chest-wall invasion or destructive bony changes are more commonly seen with neuroblastoma, although benign ganglioneuromas can produce a considerable neural foraminal widening. As with primary adrenal neuroblastomas, staging should include 123 I-MIBG scintigraphy to assess for other sites of disease, including marrow involvement. 99m Tc-MDP bone scintigraphy is also commonly performed to identify sites of cortical bone involvement, although recent data suggests that bone scintigraphy rarely affects that clinical stage of the patient. The presence of a paraspinal mass can usually be adequately assessed by CT scanning, particularly with sagittal and coronal reconstructions. However, the extent of intraspinal extension is better depicted by MRI ( Fig. 66-43 ). MRI also provides a more accurate assessment of chest-wall involvement, nerve-root compression, and impingement upon the spinal cord. MRI of ganglion-cell tumors typically reveals a low signal-intensity lesion on T1-weighted images and intermediate to high signal intensity on T2-weighted and fast spin echo inversion recovery (FSEIR) images, with variable patterns of enhancement. Bone-marrow metastases are well demonstrated by MRI, with replacement of normal bone-marrow signal by areas of low signal intensity on T1-weighted images and high signal intensity on T2-weighted images. Indeed, several studies have suggested that MRI may be sufficient to identify sites of bone-marrow involvement in neuroblastoma, although is has not yet become common in clinical practice to rely on the MRI findings of bone-marrow involvement for staging of the patient. The use of FDG-PET in neuroblastoma has received attention as an alternative to conventional imaging techniques. However, a number of studies have shown variable patterns of FDG avidity in neuroblastoma, and FDG-PET has not yet been shown to offer a clear advantage over MIBG scintigraphy in either the staging or response assessment of patients with thoracic neuroblastoma.
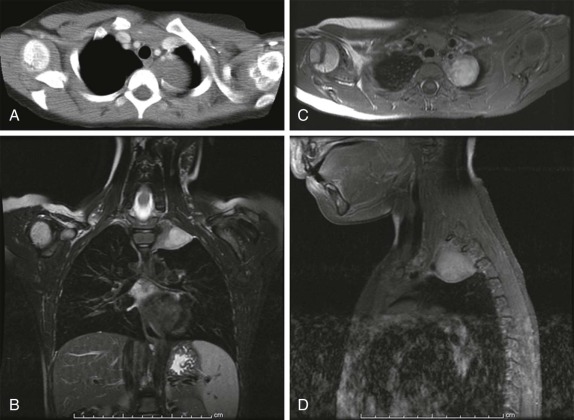
Nerve-sheath tumors, including schwannomas and neurofibromas may be similar in appearance to mature ganglion cell tumors, presenting as relatively sharply marginated, lobulated paraspinal masses. The presence of rib erosions and neural foraminal widening is commonly seen with nerve-sheath tumors, although intraspinal extension is less common. Paraspinal paragangliomas are catecholamine-secreting tumors arising from extra-adrenal chromaffin cells present in sympathetic chain ganglia and occur much less often than either primary ganglion-cell or nerve-sheath neoplasms. Distinguishing between these tissue types is difficult on the basis of imaging, and surgical resection is the mainstay of therapy. Local recurrence can occur, however, and close imaging follow-up is necessary in these patients.
Chest-Wall/Pleural-Based Neoplasms
Most of these tumors are of mesenchymal origin and are primary neoplasms arising from the chest wall. They are relatively rare in infants and children, with a reported incidence of approximately 2% of all pediatric tumors. The most common chest-wall neoplasms are the Ewing sarcoma family of tumors and primitive neuroectodermal tumors (PNETs), collectively known as Askin tumors. These tumors have a peak incidence between 10 and 15 years of age and are more common in males. Because of their origin within the chest wall and propensity to invade the adjacent bone, symptoms are commonly of a palpable mass or pain secondary to local extension. Chest radiography should be the first imaging study in the evaluation of these patients and is usually accurate at depicting sites of bone destruction, pleural effusion, and pleural thickening. CT and/or MRI are almost always performed to better delineate the extent of local disease. CT scanning of the chest is essential to assess for the presence of pulmonary metastatic disease ( Fig. 66-44 ). For paravertebral and intercostal masses, MRI may also be necessary to identify and accurately delineate the extent of intraspinal involvement. FDG uptake is usually seen with these tumors, and as with Ewing sarcomas of the appendicular skeleton, FDG-PET imaging should be considered both for identifying sites of occult disease that would otherwise go undetected and for monitoring responses to therapy when complete surgical resection cannot be achieved.
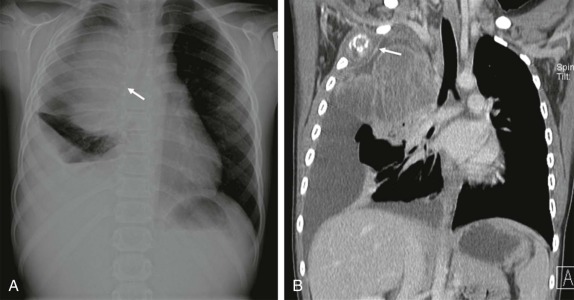
Wide excision of the primary tumor is critical for local disease control, with variable response to radiation therapy and chemotherapy. These chest-wall neoplasms are extremely malignant and can be quite destructive, demonstrating extensive invasion into the airways, local vascular structures, and the lung parenchyma. There is a high rate of both metastatic spread and of local recurrence, and cure requires intensive therapy to control both distant and local disease. The presence of disseminated disease invariably results in a poor long-term outcome in patients with these aggressive poorly responsive tumors.
Rhabdomyosarcoma is the most common soft-tissue sarcoma of childhood and is the second most common malignancy of the chest wall in children. Occurring in a younger population of children than thoracic Ewing sarcomas/PNETs, approximately 7% of pediatric rhabdomyosarcomas involve the chest wall and should be included in the differential diagnosis for a chest-wall mass occurring in children younger than the age of 10 years. Biopsy is necessary to make the diagnosis, and the imaging features are nonspecific, with considerable overlap between chest-wall Ewing sarcoma and rhabdomyosarcoma. Chest radiographs reveal a large unilateral chest mass, often with accompanying pleural thickening or effusion. As with Ewing sarcoma, there may be associated destructive rib changes. Cross-sectional imaging by either CT or MRI better delineates the extent of local disease spread. These tumors are heterogeneous in density on CT with variably increased signal on both T1- and T2-weighted MRI images. Contrast enhancement is heterogeneous, and these features likely reflect varying degrees of tumor necrosis. Tumor size, nodal status, and gross total tumor resection (upfront or delayed) have been shown to be significant predictors of event-free and overall survival. Tumors 5 cm or smaller were shown to be amenable to upfront surgical resection. Tumor size and the ability to achieve gross total resection were the strongest predictors of overall survival, with local recurrence after resection resulting in poor overall outcome. As with other malignant sarcomas, FDG-PET has been used effectively in staging and response assessment in patients with rhabdomyosarcoma and should be considered as an essential component of the imaging evaluation for these patients.
Mesenchymal Hamartomas
Mesenchymal hamartomas of the chest wall are rare, benign neoplasms occurring during infancy. The presence of a large chest-wall mass, often containing calcifications with associated rib destruction and chest-wall deformity should prompt this diagnosis. Because these lesions may contain a significant necrotic/cystic component, the distinction between Langerhans cell histiocytosis, lymphoma, and metastatic neuroblastoma may be difficult to make. These masses often become quite large, and the radiographic finding may be sufficient to suggest the diagnosis, showing a large calcified extrapleural mass with expansion/destruction of multiple ribs. CT or MRI allows more accurate measure of size, characterization of the soft-tissue component of the lesion, and mass effect on the lung and adjacent structures ( Fig. 66-45 ). In particular, the presence of hemorrhagic cystic components has been described as characteristic of these benign neoplasms. There is some data to suggest that spontaneous resolution of these masses may also occur, although the extent of local invasion and tissue destruction may prompt surgical resection, which if complete, is usually curative. The importance of the imaging evaluation is to suggest this diagnosis in order to guide appropriate management of these benign lesions.
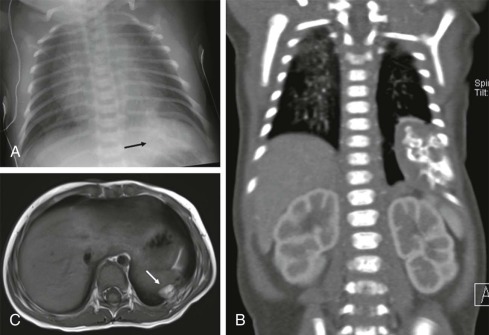
Pulmonary Metastases
Metastatic disease to the lung represents the most common pulmonary neoplasm encountered in childhood. The lung is a common site for metastatic disease spread but is an uncommon site in childhood for primary malignancy.
Extrathoracic pediatric tumors that are commonly associated with pulmonary metastases include Wilms tumor, rhabdomyosarcoma, hepatoblastoma, Ewing sarcoma, and osteosarcoma. Neuroblastoma does not typically result in hematogenously spread pulmonary metastases, although in its disseminated form, pulmonary metastases may be seen.
The imaging evaluation in a patient with a known primary malignancy with a propensity to metastasize to the lung should include chest CT. CT is more sensitive than conventional radiographs in detecting pulmonary metastatic disease, although the increased sensitivity of nodule detection has led to difficulty in distinguishing benign lesions from malignant pulmonary nodules ( Fig. 66-46 ). Once the staging evaluation has been completed and the absence of pulmonary metastatic disease and other sites of metastatic disease have been established, it may be reasonable to proceed with routine chest radiographs for subsequent follow-up care, reserving CT for patients with suspected relapse or at high risk for relapse. In particular, in osteosarcoma, which has a high frequency of developing pulmonary metastases and for whom resection of these metastases may be curative, routine follow up chest CT scanning is justifiable.
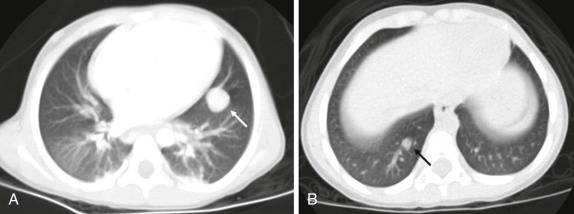
 -year-old child with metastatic Wilms tumor.
-year-old child with metastatic Wilms tumor. It is often difficult, particularly with small pulmonary nodules, to distinguish benign causes from malignancy. Calcification within a pulmonary nodule is usually associated with benign causes (except in the case of osteosarcoma); however, there are no specific imaging findings that can be used to reliably make this distinction. Benign pulmonary nodules may result from postinflammatory change or represent small benign intrapulmonary lymph nodes. Short interval follow-up care and documentation of stability in areas of low suspicion is usually adequate to confirm the benign nature of these findings. For more suspicious or enlarging lesions, biopsy or surgical resection is necessary to arrive at a definitive diagnosis. The use of FDG-PET scanning has been advocated in an attempt to distinguish benign from malignant causes of pulmonary metastatic disease. However, while CT can accurately identify lesions of only 1 to 2 mm in size, even the newest generation PET scanners have inherently lower limits of resolution, on the order of 5 mm, because of both the intrinsic spatial resolution of PET detectors and the distance traveled by positrons in the tissue before undergoing annihilation and emission of 511 keV coincident photons.
Controversy still exists as to the significance of pulmonary nodules detected by chest CT in patients with Wilms tumor. There are conflicting data as to the importance of pulmonary nodules not visualized by chest radiography but detectable by chest CT in predicting relapse-free and overall survival in patients with Wilms tumor, with the most recent data from the National Wilms Tumor Study Group (NWTSG) 4 and 5 showing that patients with lung lesions visible only on CT have improved event-free, but not overall, survival and do not appear to benefit from pulmonary radiation. Nonetheless it has been shown that the sensitivity of malignant nodule detection is enhanced by CT, and current treatment protocols routinely advocate the use of chest CT in staging these patients. In an effort to develop a better understanding of CT features that might distinguish benign from malignant pulmonary nodules, current Children’s Oncology Group (COG) Wilms tumor protocols call for surgical resection or biopsy of persistent, suspicious lung nodules detected by CT in patients with intermediate and high-risk disease.
CT scanning of the chest for the purposes of identifying pulmonary metastatic disease need not include intravenous contrast, and the presence of hyperdense contrast material in small pulmonary vessels may confound the interpretation of a subtle parenchymal nodules. In addition, multidetector-row CT scanners allow the acquisition imaging data to be reviewed in 1-mm thick increments or less, as well as in sagittal and coronal planes. This allows the radiologist to make an accurate judgment as to the presence or absence of a suspicious pulmonary nodule and distinguish it from branching vessels. There is no data to suggest that additional high-resolution CT (HRCT) imaging, which is performed using a different image acquisition technique, provides any additional benefit or higher resolution over the 1-mm thick slices obtained during the multidetector helical acquisition. MRI for the detection of pulmonary nodules has been shown to be feasible, but there are no studies showing that MRI can replace CT for the routine identification of pulmonary metastatic disease. Similarly low-dose attenuation-correction CT images of the lungs obtained as part of combined PET/CT examinations are not sufficient to exclude small pulmonary nodules, and a diagnostic chest CT obtained during suspended inspiration is still recommended for evaluation of patients at risk and in whom pulmonary metastases will impact clinical management.
Although the imaging features of small pulmonary nodules may be nonspecific and the difficulty in distinguishing benign from nonneoplastic etiologies is challenging, the presence and persistence of lung nodules in a child with a solid tumor that has a propensity to metastasize to the lung is usually indicative of metastatic disease. The use of CT in detecting these sites of disease spread remains an essential component of the staging of most pediatric solid tumors.
Gastrointestinal Tumors
Tumors of the gastrointestinal tract, including the visceral abdominal organs, are relatively uncommon in childhood. In neonates, the majority of intraabdominal masses are not malignant. Children commonly have nonspecific complaints such as abdominal pain, weight loss, or failure to thrive. The imaging evaluation should begin with abdominal radiographs. These often provide valuable initial information, such as the presence of calcifications, mass effect on adjacent visceral organs, and the presence of bowel obstruction or perforation. Abdominal ultrasound is often used to gain further information about a suspicious mass. Doppler assessment may help distinguish cystic from solid masses and delineate patterns of blood flow in and around a tumor. These initial imaging studies are usually sufficient to narrow the initial differential diagnosis and to guide the subsequent imaging evaluation by CT, MRI, and/or PET.
Liver
Although rare in infants and young children, tumors of the hepatobiliary tract are the most common of the gastrointestinal tract tumors seen in childhood. Usually a combination of diagnostic studies is obtained, including imaging findings, clinical findings, and serum markers, in order to arrive at a definitive diagnosis. Both benign and malignant tumors may be encountered. Hepatoblastomas are the most common primary malignant tumors in children under the age of 5. Hepatoblastoma is usually encountered in infants and young children under the age of 3, with an average age at presentation of 16 months. At diagnosis the mass is typically quite large (greater than 10 cm).
Metastatic disease is encountered in approximately 20% of the patients, with pulmonary metastases most common. The imaging evaluation should be directed at determining surgical resectability, establishing sites of metastatic disease, and monitoring response to chemotherapy. More than 90% of the children have elevated α-fetoprotein (AFP) levels at diagnosis; the presence of metastatic disease, high AFP levels, and a high PRETEXT stage of liver involvement by tumor are all associated with poor outcome. Surgical resection alone is insufficient to achieve cure in most cases, and the combination of surgery and chemotherapy has resulted in 5-year overall survival rates of over 70%.
Abdominal radiographs typically show an abdominal mass. Calcifications may be present, and pulmonary masses may be visible at the time of diagnosis at the lung bases ( Fig. 66-47 ). Ultrasound should be the initial imaging test in a child with an abdominal mass and in hepatoblastoma reveals a large echogenic, intrahepatic mass. It may be difficult with very large masses to define a margin between the hepatoblastoma and normal hepatic parenchyma (see Fig. 66-35 ). Hypoechoic areas may relate to necrosis or hemorrhage. Color Doppler imaging and pulsed-wave Doppler should be used routinely to assess for intravascular extension of tumor, IVC invasion, and portal vein thrombosis, both of which may affect eligibility for liver transplantation.
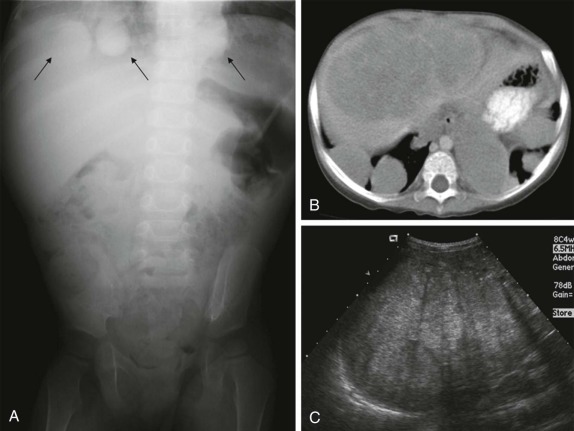
CT and or MRI are important to better define the relationship of the intra-hepatic mass to adjacent vascular structures, adjacent organs, and to determine the extent of hepatic involvement and surgical respectability. This is best accomplished with biphasic CT or MR angiographic techniques and imaging should include scanning after early arterial and portal venous phases of contrast opacification, since small satellite lesions may only be visible on the early arterial phase imaging (see Fig. 66-2 ). Typically, hepatoblastomas show a heterogeneous pattern of enhancement. Multiplanar reconstructions are also crucial, both in aiding surgical planning and in better delineating invasion into adjacent vascular structures (i.e., IVC invasion; portal venous thrombosis). MRI imaging typically shows heterogeneously T2-hyperintense lesions with variable patterns of enhancement after gadolinium contrast administration ( Fig. 66-48 ). MRI may be of particular value in delineating subtle lesions that are inconspicuous on contrast enhanced CT scanning. The use of hepatobiliary contrast agents, which are taken up by normal functioning liver tissue but not by malignant liver lesions, as well as diffusion-weighted imaging, can further enhance lesion detection and characterization. As shown in Fig. 66-49 , the hepatobiliary agent gadoxetate (Eovist) identifies a poorly enhancing lesion on a background of normally enhancing hepatic parenchyma in a patent with unresectable hepatoblastoma.
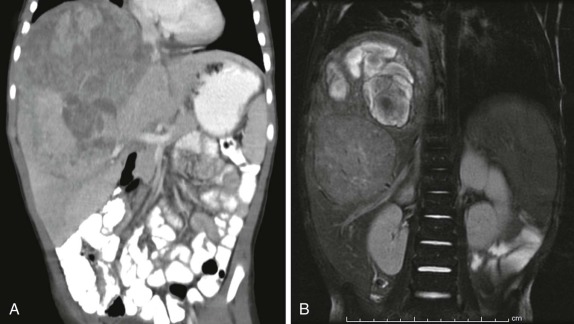
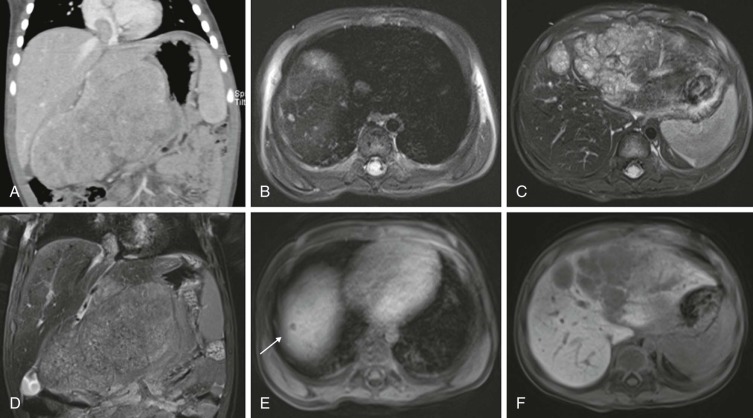
The PRETEXT staging system was developed by the International Childhood Liver Tumors Strategy Group (SIOPEL) in an effort to develop a pretreatment imaging-based measurement of disease burden and surgical resectability as a basis for staging and risk stratification of children with hepatoblastoma. In the PRETEXT system of staging the liver is divided into sections, based in part on Couinaud’s system of segmental anatomy. PRETEXT staging is based on the number of adjacent liver sections involved with tumor ( Table 66-1 ) and has been shown to be effective in predicting resectability and overall survival in patients who complete neoadjuvant chemotherapy. Because the PRETEXT staging system relies almost entirely on the pretreatment imaging assessment, it is essential that MRI and/or CT scanning be performed using optimal scanning parameters (i.e., multiphasic technique, properly timed contrast bolus, sedation to minimize motion artifact) in order to provide and accurate assessment of the extent of liver involvement. The COG staging system, used widely throughout North America, historically had relied on intraoperative and postoperative assessment, with less dependence on preoperative imaging. However, current protocols have adopted the SIOPEL recommendations for PRETEXT characterization to monitor response to neoadjuvant chemotherapy and determine surgical resectability.
| Tumor Stage | Description |
|---|---|
| 1 | Tumor involves only one quadrant. Three adjoining liver quadrants are free of tumor. |
| 2 | Tumor involves two adjoining quadrants. Two adjoining quadrants are free of tumor. |
| 3 | Tumor involves three adjoining quadrants or two nonadjoining quadrants. One quadrant or two nonadjoining quadrants are free of tumor. |
| 4 | Tumor involves all four quadrants. There is no quadrant free of tumor. |
| Extrahepatic Considerations | |
| V+: Involvement of the inferior vena cava and/or all three hepatic veins | |
| P+: Involvement of the main portal vein and/or both left and right branches | |
| E+: Extrahepatic disease in the abdomen | |
| M+: Distant metastases | |
The presence of a rising AFP level in a patient previously free of detectable disease should prompt a thorough investigation to identify sites of recurrent disease. The detection of recurrent hepatoblastoma after chemotherapy and surgical resection may be challenging with conventional cross-sectional imaging techniques. Although there has been relatively little published experience using FDG-PET scanning to monitor early sites of disease recurrence, there are reports suggesting FDG-PET and/or CT can improve both the sensitivity and specificity of identifying early foci of disease recurrence. Characterization by FDG-PET of unusual sites of disease relapse or equivocal abnormalities on CT and/or MRI may confirm the presence of metabolically active disease and guide subsequent treatment decisions ( Fig. 66-50 ).
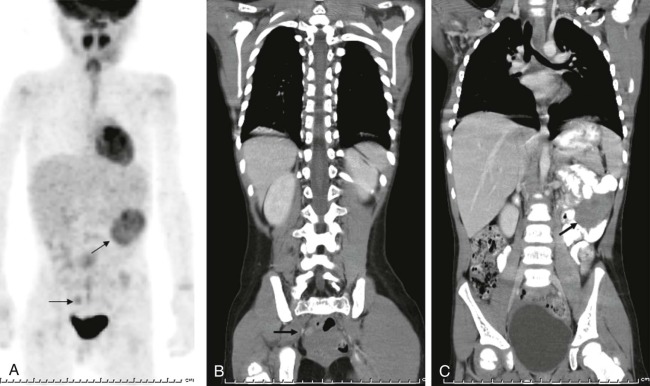
Hepatocellular Carcinomas
Hepatocellular carcinoma, although rare in childhood, is the second most common primary hepatic malignancy. It typically occurs in older children, with a mean age at diagnosis of approximately 12 years. There is a slight male predominance and an association between development of hepatocellular carcinoma and preexisting liver disease, such as hepatitis B, biliary atresia, and Fanconi syndrome, as well as certain metabolic diseases such as hereditary tyrosinemia and glycogen storage disease. The imaging features of hepatocellular carcinoma are not unique, and as for hepatoblastoma, ultrasound examination is often the first imaging study performed. Multiphase CT scanning with multiplanar reconstruction affords the greatest likelihood of detecting subtle lesions and in assessing relationships to adjacent structures and the vasculature, although the use of diffusion-weighted MR imaging and hepatobiliary MR contrast agents has added to the appeal of MRI for evaluating these patients. The CT appearance of hepatocellular carcinoma depends on tumor size and phase of contrast administration. Lesions are typically isointense or mildly hypointense relative to liver and show hyperattenuation on early arterial phase scanning, becoming isointense during later venous phases of imaging ( Fig. 66-51 ). Early arterial phase, portal venous phase, and delayed equilibration venous phase images have all been shown to contribute to enhancing lesion conspicuity, providing the greatest sensitivity and specificity in lesion detection. MR imaging, with opposed-phase imaging to detect microscopic fat and hepatobiliary agents to identify lesions with hepatocyte function can help in distinguishing hepatocellular carcinoma from benign liver lesions such as hepatic adenoma and focal nodular hyperplasia (FNH) ; however, biopsy may still be necessary to confirm the diagnosis ( Fig. 66-52 ). Gadolinium-enhanced MRI, like CT, shows hyperenhancing lesions during early arterial phase imaging, with larger tumors showing variable signal intensity and patterns of enhancement and absent enhancement on delayed hepatocyte phase imaging when hepatobiliary agents are used. Superparamagnetic iron-oxide particles, which are taken up by Kupffer cells present in normal liver and in FNH, have been used to help differentiate hepatocellular carcinoma, which contains few if any Kupffer cells, from benign liver masses and to enhance lesion conspicuity relative to the surrounding liver tissue.
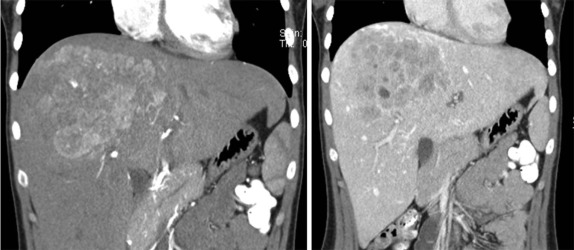
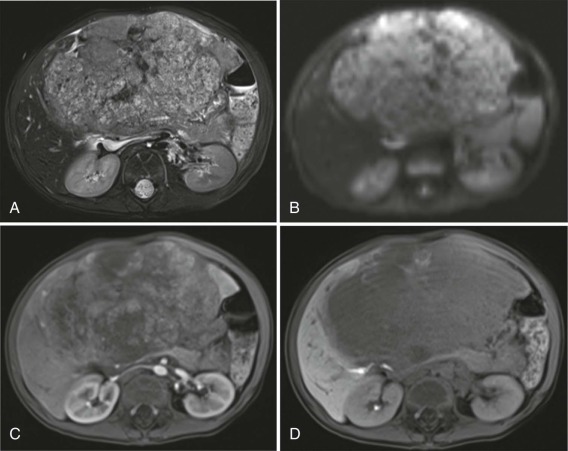
The fibrolamellar form of hepatocellular carcinoma occurs in adolescents and has imaging features that are distinctive, showing a central scar and often associated with calcification ( Fig. 66-53 ). Fibrolamellar hepatocellular carcinoma is not associated with elevated AFP, and overall survival is higher than in other forms of hepatocellular carcinoma, in part because of its presentation as a solitary localized mass amenable to complete surgical resection. Ultrasound reveals a hypoechoic mass, often with a focal central hyperechoic scar. After the initial characterization by ultrasound, CT or MRI is indicated for further staging. On CT, fibrolamellar hepatocellular carcinoma is usually relatively low attenuation, well-circumscribed mass with heterogeneous enhancement, initially becoming hyperintense relative to the surrounding liver parenchyma and ultimately becoming isointense to the liver during the equilibration phase of scanning. A nonenhancing central scar is seen in the majority of the cases (see Fig. 66-53 ). On MRI, lesions are isointense to hypointense relative to the liver on T1-weighted images and isointense to hyperintense on T2-weighted sequences. The pattern on gadolinium enhancement is similar to that observed on CT, with lesions becoming hyperintense early after contrast infusion and ultimately isointense to liver on delayed scanning. The distinction between fibrolamellar hepatocellular carcinoma and FNH, both of which have similar imaging characteristics and patterns of early contrast enhancement, may be difficult to make (compare Figs. 66-53 and 66-54 ). However, as shown in Figure 66-54 , the uptake and retention of a hepatobiliary contrast agent by the lesion on delayed hepatocyte phase imaging is nearly diagnostic of FNH.
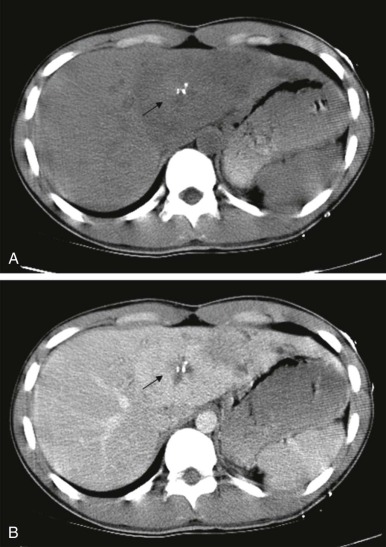
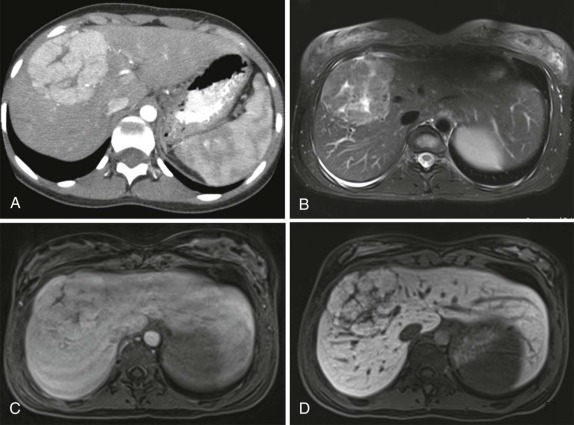
Embryonal Sarcomas (Hepatic Mesenchymomas)
Embryonal sarcomas of the liver are the fourth most common hepatic neoplasm in children after hepatoblastoma, hepatocellular carcinoma, and infantile hepatic hemangioma. These tumors are highly malignant, usually present in later childhood (between the age of 6 and 10), and are typically accompanied by symptoms of abdominal pain and an abdominal mass. Embryonal sarcomas may be difficult to distinguish from mesenchymal hamartomas, although the latter tumors are more commonly diagnosed before the age of 2 years. At presentation embryonal sarcomas are often large and contain both cystic and solid areas with areas of necrosis. Ultrasound reveals a heterogeneous echogenic mass with many features overlapping with mesenchymal hamartoma and infantile hemangioma. Calcifications are rare. CT reveals a large hypodense mass, often with septations and areas of internal high attenuation; MRI shows a high signal-intensity lesion on T2-weighted images ( Fig. 66-55 ), with predominantly low signal on T1-weighted images. After contrast enhancement, imaging by either CT or MRI demonstrates peripheral enhancement and central areas of heterogeneous signal intensity that likely reflect solid tumor elements, cystic spaces, necrosis, and foci of hemorrhage. Despite being highly malignant, complete surgical resection and adjuvant chemotherapy can be curative in the majority of these patients.
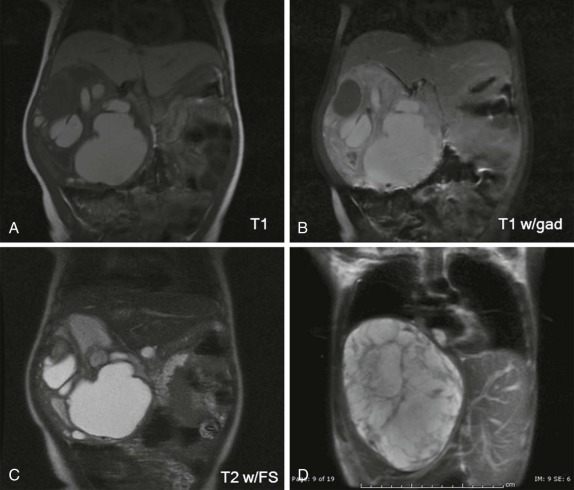
Mesenchymal Hamartomas
Mesenchymal hamartoma, in contrast to hepatoblastoma, is a predominantly cystic mass most often occurring in infants under the age of 2 years. It commonly presents as a painless, palpable abdominal mass comprised of mesenchymal tissue, bile ducts, hepatocytes, and hematopoietic cells. In contrast to hepatoblastoma, which is the primary differential consideration in an infant, the AFP levels are normal. On imaging, radiographs reveal a large right upper quadrant mass. Calcifications, which are seen in hepatoblastoma, are unusual in mesenchymal hamartoma. Ultrasound examination typically shows a complex, predominantly cystic mass. MRI and CT may be of value in further characterizing the lesion and typically show large multilocular cystic masses with septations. Only minimal enhancement is seen. Some of the masses may have a predominantly solid component yielding a so-called Swiss cheese appearance to the mass, and contributing to some difficulty in the differential diagnosis. MRI may also demonstrate the complex nature of the cystic fluid, with gelatinous, more complex fluids showing high signal on T1-weighted images instead of the more common T2 bright/ T1 dark serous fluid contained in the majority of the cysts (see Fig. 66-55 ). Treatment is surgical resection, and recurrence is uncommon.
Infantile Hepatic Hemangiomas
Infantile hepatic hemangioma are proliferative endothelial cell neoplasms that may be solitary or may diffusely involve the liver. Infantile hepatic hemangiomas have characteristic phases of cellular proliferation followed by spontaneous involution. They are often referred to as hepatic hemangioendothelioma, creating some confusion in nomenclature and must be distinguished from epithelioid hemangioendothelioma. The latter is a proliferative tumor with malignant potential that does not involute in contrast to the benign hepatic hemangioma. Infantile hepatic hemangiomas share the same growth characteristics as the more common cutaneous infantile hemangiomas. The majority present by 6 months of age, and about half are associated with cutaneous lesions. The majority of the infantile hepatic hemangiomas involute spontaneously, and adverse outcomes are only observed when there is massive hepatic involvement and arteriovenous (AV) shunting with accompanying high-output cardiac failure. The presence of extensive hepatic involvement and near complete replacement of the liver parenchyma occurs in the diffuse infantile hemangioma variant and is associated with profound hypothyroidism secondary to overproduction of type III iodothyronine deiodinase.
Ultrasound is usually the first step in the evaluation and typically shows either focal or multiple hepatic masses with heterogeneous echotexture. It may be possible to identify a large draining varix or varices. Both arterial and venous waveforms are often detected, and calcifications may be present. More characteristic findings are seen on CT and MRI. MRI typically shows homogeneously hypointense lesions on T1-weighted imaging with heterogeneous hyperintense masses on T2-weighted images. After contrast enhancement, imaging by either CT or MRI reveals a typical pattern of peripheral contrast enhancement with gradual filling in centrally (centripetal enhancement) ( Fig. 66-56, A and B ). Diffuse infantile hepatic hemangiomas may nearly completely occupy the entire liver parenchyma (see Fig. 66-56, C and D ). The associated hypothyroidism is the result of the elaboration of deiodinase enzymes affecting thyroid hormone synthesis, which can in turn result in cardiac failure and neurologic impairment. These endocrinologic findings are important in securing the diagnosis, because the multiple hepatic masses may share overlapping imaging features with malignant hepatic lesions such as hepatoblastoma or metastatic neuroblastoma.
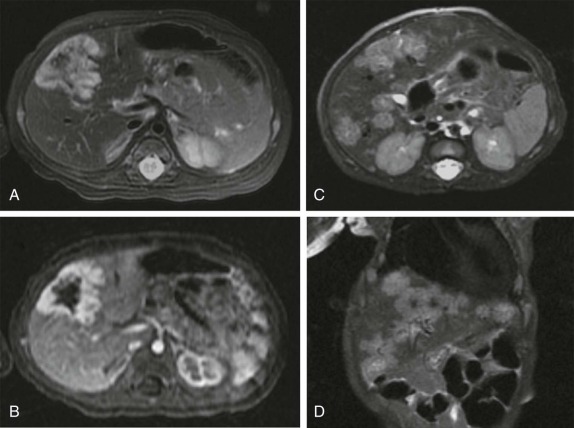
High-output cardiac failure associated with large varices and significant high-volume AV shunting can be effectively treated using interventional radiologic techniques, including coil and particle embolization. This may be essential in stabilizing the patient in order to initiate treatment to accelerate the involution of these benign proliferative lesions.
Hepatic Adenoma
Other benign hepatic lesions include solitary hepatic adenomas. Hepatic adenomas are unusual in children in the absence of a predisposing condition, such as glycogen storage disease, galactosemia, or tyrosinemia. The imaging features of hepatic adenomas are not specific, and imaging is usually directed at monitoring these lesions for increases in size and number. Rapid increase in size is generally considered to be an indication of degeneration of benign adenoma to hepatocellular carcinoma, particularly in patients with glycogen storage disease. Regular surveillance, typically by ultrasound, is indicated in these patients. MRI should be considered to further aid in characterizing new or multiple lesions.
Focal Nodular Hyperplasia
FNH is rare in children, most commonly occurring in teenage and adolescent years. It is usually found incidentally during imaging evaluation for other reasons. This benign lesion typically shows a central fibrous scar with surrounding hyperplastic hepatocytes and small bile ducts. As discussed, these imaging features are also seen in the fibrolamellar variant of hepatocellular carcinoma, and the distinction between FNH and fibrolamellar hepatocellular carcinoma may be difficult. CT and MRI scans usually show rapid homogeneous contrast enhancement during early arterial-phase imaging (see Figs. 66-53 and 66-54 ). Lesions then characteristically become isodense to the liver during later phases of enhancement, with the central scar remaining hypodense even during the delayed imaging period. With newer MRI imaging techniques performed either with iron-oxide particles that accumulate in reticuloendothelial cells (Kupffer cells) present in the hyperplastic FNH lesions or with hepatocyte selective gadolinium based agents such as gadoxetate (Eovist), the diagnosis of FNH can generally be made with confidence. For equivocal cases, scintigraphy with 99m Tc-sulfur colloid can also be performed, although in practice this is rarely necessary. These masses may be monitored by ultrasound to ensure stability and do not typically require surgical resection unless they become symptomatic or undergo progressive enlargement. FNH has also been observed in patients who have completed therapy for nonhepatic primary tumors. Whereas larger masses may be by detected by ultrasound or CT, it has been found that dynamic contrast-enhanced MRI is a highly sensitive technique for identifying these lesions. Occasionally these lesions can be characterized by ultrasound using high-resolution, high-frequency transducers; however, they are unequivocally shown by contrast-enhanced MRI ( Figs. 66-57 and 66-58 ). These FNH lesions are also isointense to the liver on conventional imaging sequences and display rapid early arterial enhancement, after which they become nearly isointense to the liver and indistinguishable from the surrounding parenchyma. Hepatobiliary contrast agents are now used routinely to further characterize these lesions as benign FNH (see Fig. 66-53 ) and distinguish them from hepatic metastatic deposits. It is important recognize these characteristic patterns of enhancement so as to not mistake this benign entity for recurrent disease or a new site of disease relapse.
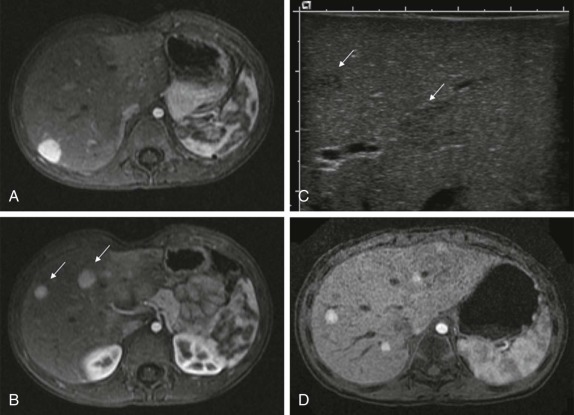

Other lesions occurring in the liver include sarcomatous tumors such as malignant angiosarcomas and rhabdomyosarcomas of the biliary tree, malignant vascular tumors, and metastatic disease. The most common metastatic lesions occurring in the liver include neuroblastomas, locally invasive and hematogenously-disseminated Wilms tumors, rhabdomyosarcomas and Ewing sarcomas. The imaging characteristics of these metastatic hepatic lesions are nonspecific with lesions characteristically hypoechoic on ultrasound relative to normal liver parenchyma, decreased attenuation on CT scan, low T1 and increased T2 signal intensity on T1- and T2-weighted MR images, respectively, and variable patterns of contrast enhancement with both CT and MRI.
Spleen
Primary tumors of the spleen are exceedingly rare. Angiosarcomas have been reported but are not common in childhood. Involvement of the spleen by lymphoma and lymphoproliferative disease is the most common cause of malignant infiltration of the spleen. Imaging evaluation by ultrasound often demonstrates multiple or solitary hypoechoic masses ( Fig. 66-59 ). CT scanning may be equivocal because of variable phases of splenic parenchymal enhancement. MRI, in contrast, can be very sensitive at detecting focal lesions that are occult by a CT and/or ultrasound. In the setting of lymphoma and lymphoproliferative disease, FDG-PET imaging has been shown to be an effective adjunctive imaging modality for detecting splenic involvement. This is of importance both in determining the patient’s stage of disease at diagnosis and in identifying sites of disease to be watched and assessed for response after treatment. In addition, the negative predictive value of PET/CT in discriminating between benign and malignant lesions has been emphasized both in patients with coexistent malignant disease and in patients without history of malignancy in whom a solid splenic mass is identified by other imaging techniques.
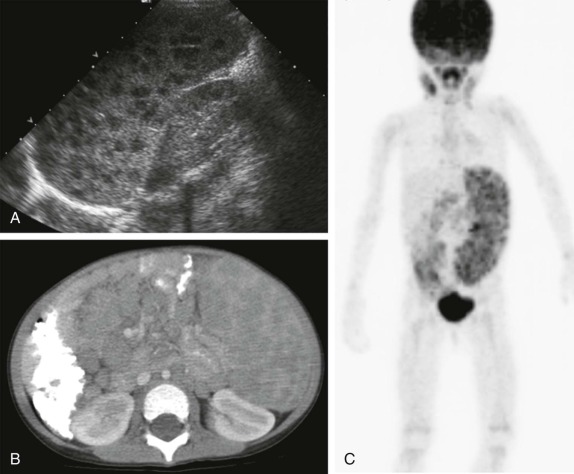
Biliary Tract
Tumors of the gall bladder and bile ducts are rare in children, and include biliary rhabdomyosarcomas and cholangiocarcinomas, the latter of which occur in the setting of chronic ulcerative colitis. The initial imaging evaluation is usually by ultrasound, often for symptoms of biliary-tract obstruction such as pain, jaundice, and weight loss. Ultrasound may show evidence of bile-duct dilation and biliary-tract obstruction; however, identification of a focal mass usually requires additional evaluation by MRI/magnetic resonance cholangiopancreatography (MRCP), with diagnostic confirmation by endoscopic retrograde cholangiopancreatography (ERCP). Particularly in the setting of rhabdomyosarcoma, FDG-PET is of value in accurately determining the stage of disease. Biliary rhabdomyosarcoma can arise from the intrahepatic or extrahepatic biliary bile ducts, gallbladder, cystic duct, or ampulla of Vater. At the time of presentation with obstructive jaundice and abdominal pain, these neoplasms are usually quite large, and as a result the distinction between biliary rhabdomyosarcoma, primary hepatic tumors arising in the hepatic hilum, pancreatic neoplasms, and renal/suprarenal masses may be challenging ( Fig. 66-60 ).
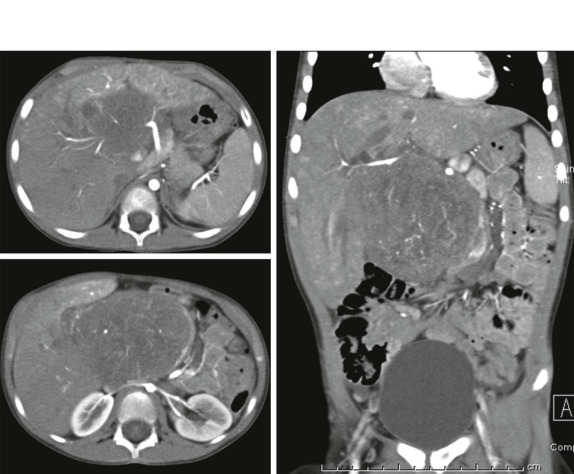
Pancreas
Primary tumors of the pancreas are rare in children, in contrast to adults. In addition, the types of tumors found in children differ from the adult tumors, in whom adenocarcinomas predominate. Pancreatic tumors can be classified into nonfunctional tumors of the exocrine pancreas and functional tumors of the endocrine pancreas, as well as secondary metastatic pancreatic neoplasia.
Malignant epithelial neoplasms arising from the exocrine pancreas include pancreatoblastomas, and solid papillary epithelial neoplasms of the pancreas. Primary nonepithelial tumors of the pancreas are very rare and include lymphomas, rhabdomyosarcomas and primitive neuroectodermal tumors. Pancreatic endocrine neoplasms are rare in children, and they are usually associated with the multiple endocrine neoplasia syndrome. These include hormonally active tumors such as insulinomas, gastrinomas, VIPomas and glucagonomas.
Pancreatoblastomas
Pancreatoblastomas can occur at any age but are most common in children between the ages of 1 and 8 years. Pancreatoblastomas are reported as arising in or around the head of the pancreas in roughly half of the cases. Because they are nonfunctional, they are typically quite large at the time of diagnosis. Presenting symptoms include abdominal pain, nausea, and weight loss. Obstructive symptoms such as jaundice or pancreatitis are less common despite the large size of these masses, which has been attributed to the soft, gelatinous, and compliant consistency of these tumors. These tumors are typically well encapsulated, have heterogeneous patterns of echogenicity on ultrasound, and show variable patterns of contrast enhancement on CT ( Fig. 66-61 ). Calcifications are commonly seen and are probably the sequelae of hemorrhage and necrosis known to occur within these tumors. MRI shows a similar pattern of heterogeneous signal intensity and enhancement, with masses usually hypointense on T1-weighted images and variably hyperintense on T2-weighted and post–gadolinium-enhanced images. As with hepatoblastoma, pulmonary metastatic disease is often present, and the staging evaluation must include a CT of the chest. Because of their large size, the tissue of origin may be difficult to clearly delineate, and differential considerations includes exophytic hepatoblastoma, renal and suprarenal masses, and retroperitoneal neoplasms.
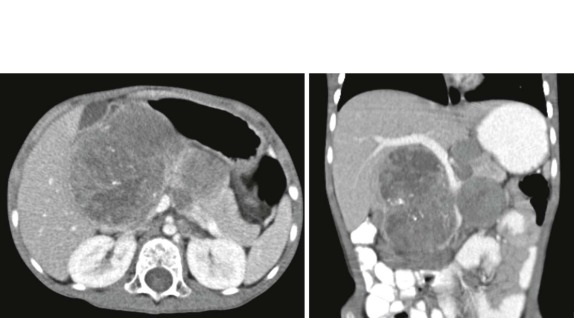
Solid Pseudopapillary Neoplasms
Solid pseudopapillary neoplasms of the pancreas (also known as solid and papillary epithelial neoplasms ) are a rare primary pediatric pancreatic tumor. In contrast to other pancreatic tumors occurring in children, however, the prognosis after complete surgical resection is favorable. Studies have reported up to a 90% survival rate after complete resection. In one small study, median age at presentation was 13 years, with abdominal pain, a palpable mass, dyspepsia, and pancreatitis the most common presenting symptoms. On imaging, usually by ultrasound and/or CT, solid pseudopapillary tumors appear as solid, well-demarcated masses. On ultrasound there is heterogeneous echotexture, occasionally with fluid-filled cystic spaces ( Fig. 66-62 ). CT shows a heterogeneous mass with peripheral contrast enhancement. Although these tumors are slow-growing tumors with low-grade malignant behavior, FDG uptake on PET imaging has been shown in solid papillary tumors of the pancreas. FDG-PET scanning may therefore be of value in follow-up imaging to assess for recurrence. Despite high rates of overall 5-year survival, if metastases are present and not resectable, survival is poor with no clear, beneficial role reported for adjuvant chemotherapy or radiation therapy.
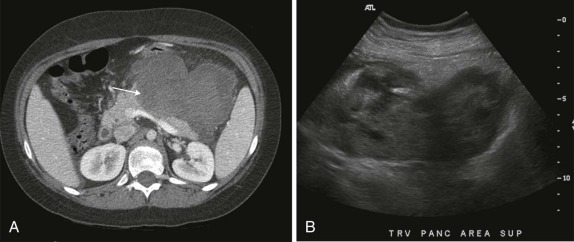
Pancreatic Sarcomas
Mesenchymal tumors of the pancreas include rare synovial-cell sarcomas, myofibroblastic tumors, and metastatic rhabdomyosarcomas. Extremity rhabdomyosarcomas seem to have an unusual predilection for metastases to unusual cites such as the breast, ovary, testicle, and pancreas. Two patients shown in Figure 66-63 had metastatic rhabdomyosarcoma to the pancreas. Local presentation may include pancreatitis and abdominal pain or may be incidental (see Fig. 66-48, B ). A recent report from three major pediatric centers also noted an association of alveolar rhabdomyosarcoma with pancreatic metastasis, emphasizing the importance of follow-up imaging in these patients and including an increasing role for routine FDG-PET/CT imaging to identify unusual sites of metastatic spread in patients with rhabdomyosarcoma.
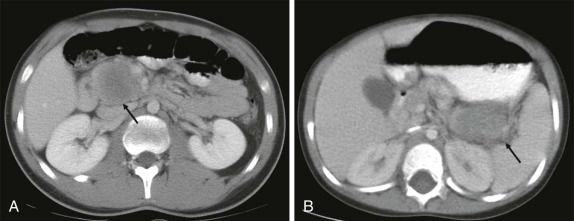
Pancreatic Lymphomas
Burkitt lymphoma is well known to involve organs of the gastrointestinal tract, although isolated pancreatic involvement is unusual. Typically pancreatic involvement is accompanied by other sites of visceral and mesenteric disease. Diffuse pancreatic infiltration in the setting of Burkitt lymphoma is shown in Figure 66-64 in a child who had symptoms of pancreatitis. On ultrasound the pancreas was diffusely enlarged and heterogeneous in echogenicity. CT scan showed diffuse pancreatic enlargement, poor enhancement, and a homogeneous low attenuation. As is common for the rapidly dividing Burkitt lymphoma, a prompt response to chemotherapy was observed, with near complete resolution of pancreatic abnormalities 10 days after onset of chemotherapy.
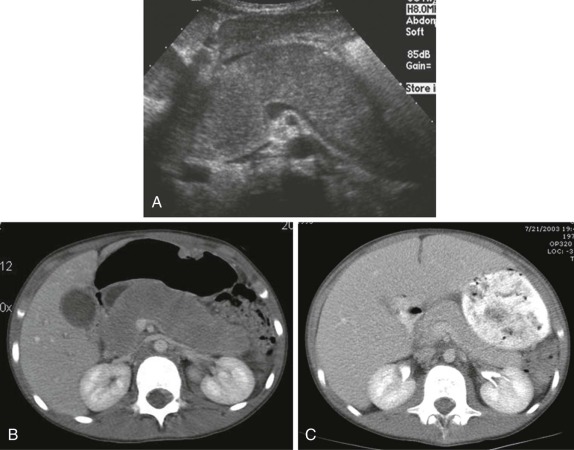
Pancreatic Islet-Cell Tumors
Functioning islet-cell tumors of the pancreas are rare but important clinically because of symptomatic endocrine abnormalities that result from constitutive secretion of hormones normally tightly regulated by the endocrine pancreas. As shown in Figure 66-65 , in which the patient had symptomatic hyperinsulinemic hypoglycemia, these tumors are often quite small and may be difficult to diagnose and characterize. As with other neuroendocrine neoplasms, optimal scanning techniques for detection of pancreatic islet-cell tumors include early arterial-phase scanning as part of a multiphase imaging technique. Thin sections (≈1 mm) are now routinely acquired by most multidetector-row CT scanners and the evaluation should include a careful review of sagittal and coronal reconstructed images. Although there are no controlled studies comparing CT and MRI in evaluating these tumors, a well-administered MRI examination with respiratory triggering/breath-holding, fast gradient-echo imaging, diffusion imaging, and dynamic-contrast administration is likely to yield comparable, if not superior results to CT. These small lesions are often difficult to see by conventional ultrasound; however, intraoperative ultrasound is invaluable for localizing small, nonpalpable lesions (see Fig. 66-65, B ).
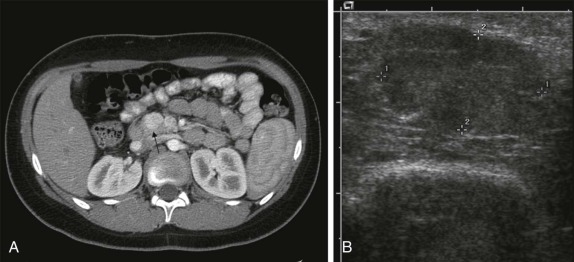
Alimentary Tract Tumors
Tumors of the gastrointestinal tract including the esophagus, stomach, and large and small bowel are rare in children. In the stomach, lymphomas, leiomyomas, lyomysarcomas, and adenocarcinomas are all rare as compared with adults. Gastrointestinal stromal tumors (GISTs) also have a low incidence in the pediatric population. Since early 2000, however, these tumors have attracted increased attention because of molecularly-targeted therapies (e.g., imatinib [Gleevec]) specifically inhibiting cell-surface receptors expressed on these cells. Although the majority of pediatric GISTs express a different cell-surface receptor mutation ( KIT or PDGFRA ) and thus do not respond to imatinib like adult GISTs, the radiographic features are similar, with patients having a large mass closely apposed to the gastric wall. Depending on the size of the mass it may be difficult to distinguish between a pancreatic tumor invading the posterior gastric wall and a primary gastric tumor. On CT these tumors are often heterogeneous in attenuation with modest enhancement relative to the adjacent pancreatic tissue. For patients with localized disease, complete surgical resection is still the mainstay of treatment for this tumor, with targeted therapies being reserved for those patients with advanced or metastatic disease. FDG-PET scanning has been shown to be important for staging and in follow-up evaluation, both as a measure of metabolic response to molecularly targeted therapy and for early detection of small, local sites of relapse within the gastric wall that may be difficult to detect by conventional cross-sectional imaging techniques ( Fig. 66-66 ).
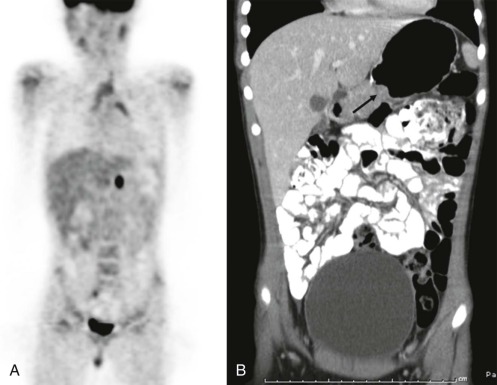
The most common primary bowel malignancy in childhood is lymphoma. Small bowel is more often involved than large bowel, and this is most often non-Hodgkin lymphoma. Presentation is typically with abdominal pain, weight loss, anemia, gastrointestinal bleeding, or constipation. Intussusception or a palpable abdominal mass may bring the patient to attention sooner. In an older child (older than age 6 years) with intussusception, lymphoma should be the leading diagnostic consideration ( Fig. 66-67 ). The majority of the small-bowel non-Hodgkin lymphomas are Burkitt lymphomas. These rapidly dividing tumors involve the bowel or small bowel/colonic mesentery and can become quite large and lead to intestinal obstruction. Burkitt lymphoma responds rapidly to chemotherapy and can be effectively treated with no residual imaging abnormalities seen.
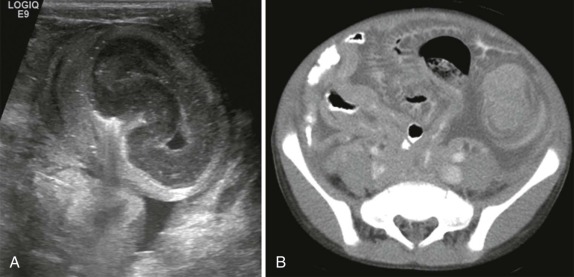
The imaging evaluation may initially involve fluoroscopic/barium studies to assess nonspecific symptoms of abdominal pain. In the setting of suspected intussusception, conventional radiographs and ultrasound are usually sufficient to confirm the diagnosis. Attempts at hydrostatic or pneumatic enema reduction of intussusceptions occurring around pathologic lead points in the bowel because of lymphomatous infiltration of the bowel or mesentery should be undertaken with caution, because there is increased risk of perforation at the site of intussusception and greater risk of failure at reducing the intussusception in these patients. Once the diagnosis of lymphoma has been made, a complete cross-sectional imaging (CT) staging examination, including the neck and chest, should be undertaken. Both intravenous and oral contrast are necessary for this evaluation. CT reveals bowel-wall thickening, mesenteric adenopathy, and often a large intraperitoneal/mesenteric mass. FDG-PET scanning has been shown to be effective in adult patients in staging and response assessment for non-Hodgkin lymphoma, and small case series have confirmed this finding in children, suggesting FDG-PET should probably be included in the diagnostic staging and posttreatment assessment of these patients.
Adenocarcinomas of the colon and rectum are fortunately rare in children. As in adults, colonoscopy may provide the best early diagnosis for a suspected carcinomatous lesions. The staging evaluation for metastatic spread should involve imaging of the chest, abdomen, and pelvis, again with both intravenous and oral contrast. There is no clear role established for routine use of FDG-PET scanning in the staging of children with adenocarcinomas of the gastrointestinal tract.
Carcinoid tumors occurring in the gastrointestinal tract are rare and usually slow-growing but have malignant potential. The appendix is the most common location for carcinoid tumors. It is not uncommon for patients to show symptoms of acute appendicitis, with carcinoid tumors discovered at the time of appendectomy or incidentally during evaluation of the pathologic specimen. When large, carcinoid tumors may also produce obstruction of the bowel or intussusception. Because of their propensity to metastasize to the liver, work-up should include imaging of the entire abdomen and pelvis with intravenous and oral contrast. Somatostatin receptor analogues (octreotide) are useful for occult sites of metastatic disease spread. These hepatic metastases are typically best demonstrated during the early arterial phase of contrast injection, which nicely delineates these hypervascular tumors relative to the surrounding hepatic parenchyma. Complete surgical resection remains the mainstay of therapy for disease localized to the appendix and is usually curative.
Genitourinary Tumors
Renal Masses
Wilms Tumors
Wilms tumors are the most common renal malignancy in children, representing 6% to 10% of all pediatric malignancies and making up greater than 80% of pediatric renal masses. The peak age of incidence is between 3 and 4 years, and bilateral disease is seen in between 5% and 10% of patients. Wilms tumor is often associated with syndromes and nephroblastomatosis and typically presents as a painless, palpable abdominal mass. Occasionally, Wilms tumor is an incidental finding during a radiologic assessment for other indications such as trauma. Hypertension and hematuria only occur in about 25% of the cases.
Tumor staging based on the NWTSG classification depends on surgical, pathological, and radiologic findings, and the imaging evaluation should be directed at determining spread to adjacent organs such as the pancreas, spleen, and liver and for intravascular extension into the renal vein, IVC, and right atrium ( Table 66-2 ). In addition, the imaging examination should assess for the presence of distant metastatic disease (lung and liver) and for the presence of contralateral kidney involvement. Wilms tumor is a rapidly growing malignancy, and intraabdominal rupture at diagnosis, during chemotherapy, or at the time of surgical resection is a well-appreciated complication and a major risk factor for abdominal recurrence, increasing the recurrence rate to as much as 20%. Accurately identifying tumor rupture can be a challenge. In a recent review of 70 patients treated through the COG, preoperative CT imaging was found to have moderate specificity (88%) but relatively low sensitivity (62%) in the detection of Wilms tumor rupture, although ascites seen outside of the cul-de-sac, irrespective of fluid density, was most predictive of rupture. When tumor rupture is identified, these patients are upstaged and treated as having stage 3 disease.
| Tumor Stage | Description |
|---|---|
| 1 | Tumor is limited to the kidney and is completely resected. |
| The renal capsule is intact and tumor is not ruptured or biopsied before removal. | |
| No involvement of renal sinus vessels or evidence of the tumor at or beyond the margins of resection. | |
| 2 | Tumor is completely resected, with no evidence of tumor at or beyond the margins of resection. |
| Tumor extends beyond the kidney as evidenced by any one of the following: | |
| Regional extension of the tumor (i.e., penetration of the renal sinus capsule, or extensive invasion of the soft tissue of the renal sinus) | |
| Blood vessels within the nephrectomy specimen outside the renal parenchyma contain tumor. | |
| 3 | Residual nonhematogenous intraabdominal tumor after surgery. |
| Any one of the following may occur: | |
| Lymph nodes within the abdomen or pelvis are involved by tumor. | |
| Tumor implants are found on or penetrated through the peritoneal surface. | |
| Gross or microscopic tumor remains. | |
| Tumor is not completely resectable because of local infiltration. | |
| Tumor spillage occurs either before or during surgery, or tumor was biopsied before removal. | |
| Tumor is removed in more than one piece (e.g., tumor thrombus within the renal vein is removed separately from the nephrectomy specimen). | |
| 4 | Hematogenous metastases (e.g., lung, liver, bone, brain) or lymph node metastases outside the abdominopelvic region are present. |
| 5 | Bilateral involvement by tumor is present at diagnosis. |
The initial examination is often a conventional abdominal x-ray obtained for symptoms of abdominal pain or a palpable mass. The typical findings include displacement of bowel and a masslike opacity in the abdomen. An ultrasound should serve as the initial evaluation and usually confirms the renal origin of the suspected mass. Sonographically Wilms tumors are often quite large with relatively homogeneous echogenicity. There may be focal areas of hypoechogenicity or cystic change. Calcifications are uncommon. Ultrasound is also accurate and sensitive at detecting intravascular invasion, and both color and Doppler imaging are effective at establishing vascular patency ( Fig. 66-68 ). Intravascular extension occurs in 4% to 10% of patients with Wilms tumor but is rare in neuroblastoma, helping to differentiate between these two tumors. In a recent study evaluating the diagnostic performance of CT versus Doppler ultrasound in identifying intravascular extension of Wilms tumor, investigators from the COG found CT to be superior to ultrasound for detection of intravascular and cavoatrial thrombus. Nonetheless because ultrasound is often the initial examination performed in these patients, early identification of intravascular extension into the right atrium is essential and may increase the sedation/anesthesia risk for a child for whom subsequent CT or MRI is planned.
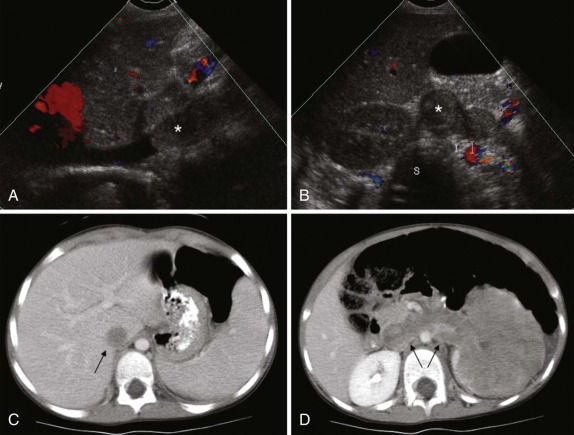
Once the diagnosis has been suggested by ultrasound, further imaging of suspected Wilms tumor requires either CT or MRI to accurately stage the disease. Multidetector-row CT scanning allows for rapid scanning and in some instances may allow sedation to be avoided. High-resolution imaging techniques with multiplanar reconstructions are essential in determining the extent of tumor spillage, local invasion, invasion into vascular structures, and presence of pulmonary metastatic disease. Early studies suggested that pulmonary metastases detected only by CT should not result in upstaging of the patient, relying on the chest x-ray to identify pulmonary metastases. Subsequent work by European investigators in the International Society of Paediatric Oncology (SIOP) and in North America with the NWTSG identified a small cohort of patients with pulmonary lesions visualized only by CT scanning in whom disease stage would be advanced based on the CT results. One study showed an increased risk of relapse in patients with positive CT findings. Others, however, reported either no difference in prognosis between patients with positive or negative chest CT scans or improvement in event-free survival rates, but with no impact on the overall survival rate and with no benefit from whole-lung radiation for patients with CT-only pulmonary nodules. These studies are limited by their retrospective nature, and it remains controversial whether patients would have benefited from increased therapy. Furthermore other investigators have argued that CXR and CT data are concordant in the majority of cases, and it as been shown that not all pulmonary lesions detected by CT represent metastases, even in the hands of experienced radiologists, suggesting that surgical confirmation may be necessary to establish the malignant nature of suspicious lesions detected by CT in patients with Wilms tumor. These considerations aside, it is current practice to obtain a chest CT for staging of all patients with Wilms tumor, with ongoing studies investigating the positive predictive value of this practice and its impact on overall clinical outcome.
Because of the need for chest CT in the staging evaluation, MRI is usually obtained for follow-up evaluation, either after resection or chemotherapy. In addition, MRI is the imaging modality of choice in monitoring nephrogenic rests that are seen in approximately 40% of patients with unilateral Wilms tumor and nearly all patients with bilateral Wilms tumors ( Fig. 66-69 ). Nephrogenic rests are remnants of primitive blastemal tissue that persist after birth and have the potential to transform into Wilms tumor. Nephroblastomatosis is the presence of multifocal or diffuse nephrogenic rests and is prevalent in patients with hemihypertrophy. The distinction between nephrogenic rests and Wilms tumor may be difficult; however, there are certain distinguishing MRI features that may help discriminate between the two. On T1-weighted MRI images, Wilms tumors appear as well-defined heterogeneous masses with slightly lower signal intensity as compared with the adjacent renal cortex. On T2-weighted images Wilms tumors appear isointense to slightly hyperintense relative to the normal renal cortex. After intravenous gadolinium-contrast administration, Wilms tumors typically enhance heterogeneously but less than the surrounding renal parenchyma. Nephrogenic rests also exhibit decreased signal intensity on T1-weighted images, but in contrast to Wilms tumors maintain low signal on T2-weighted images. Furthermore, nephrogenic rests show minimal enhancement after contrast infusion relative to the normal renal parenchyma, whereas foci of Wilms tumors show heterogeneous enhancement. A role for FDG-PET imaging in the routine management of patients with Wilms tumor has not been established. However, studies documenting increased FDG accumulation in metabolically active Wilms tumors suggest there may be role for FDG-PET imaging in Wilms tumor staging, posttherapy response evaluation, and possibly in helping discriminate benign nephrogenic rests from foci of transformed malignant tumor.