Chapter 9 Imaging in Oncology
Disadvantages include exposure to ionizing radiation (approximately 0.04-Gy skin dose for a scan of the abdomen and pelvis) and the requirement of relatively large volumes (100 to 150 mL) of iodine-based intravenous contrast agents. Although these agents make tumors more visible and allow for vascular mapping for tumor resection, there is also the possibility of allergic-type reactions, ranging from urticaria (2 in 100 cases) to shock (1 in 60,0000 cases).1,2,3,4,5 These rates are similar to those for the antibiotic penicillin. Active asthma and/or a history of multiple allergies to food, drugs, or the environment increases the risk of an allergic reaction to iodine-based intravenous contrast medium by a factor of 20.1 These patients can be premedicated with steroids to decrease the risk of an adverse reaction. A second possible adverse outcome is that of contrast media-induced nephropathy. In general, patients with serum creatinine levels of less than 1.8 mg/dL can undergo intravenous contrast-enhanced CT without significant risk of nephropathy.5 For those patients with elevated serum creatinine levels, vigorous oral and intravenous hydration for several hours after the examination can significantly decrease the risk of renal damage. The now widespread use of F-18 fluorodeoxyglucose (FDG)–enhanced fused positron emission tomography/computed tomography (PET/CT) images allows for more exact localization of metabolically active sites of disease, particularly in primary lung and esophageal cancers and lymphoma.
Over the past 10 years, magnetic resonance imaging (MRI) has become more important in staging cancers and has become the preeminent tool for evaluation of the central nervous system. In certain centers it is also used extensively for head and neck cancers and detection of liver metastases. Sensitivity is similar to that of CT, although the spatial resolution of MRI is inferior, and motion artifacts (generally caused by breathing) can be problematic. Advantages of MRI include improved lesion characterization because of the superior contrast resolution and improved identification of normal anatomy, particularly within the central nervous system and bones. The lack of available protons in the air-filled lungs makes MRI of limited value in the detection of pulmonary nodules. Most oncology studies are done using an intravenous contrast material composed of a gadolinium chelate. The risk of anaphylaxis is very low, and in healthy patients the risk of nephrotoxicity is virtually nil. However, a recently described condition, nephrogenic systemic fibrosis, has been associated with the use of gadolinium-based contrast medium in patients with severe renal insufficiency (defined as an estimated glomerular filtration rate <30 mg/dL/1.73 m2). Contrast doses should be decreased or contrast should be withheld in these patients, as this is a systemic fibrosing disease that can be fatal, and no effective treatment has been identified.6
Intravenous contrast volumes used in MRI are in the range of 10 to 20 mL. Patients must be cooperative and be able to lie supine for 20 minutes. The presence of ferromagnetic implants, including cardiac pacemakers, ocular implants, and aneurysm clips, prohibits performance of MRI.7 Surgical clips, artificial joints, and heart valves can be safely imaged.
Imaging of the Brain, Head, and Neck
Primary Tumors of the Brain
Imaging is necessary in all patients with brain and head and neck cancer to evaluate the extent of disease, determine surgical and therapeutic options, and aid surgical planning to shorten procedure times.8 Primary brain tumors and metastatic disease can be imaged with CT or MRI. With CT, contrast enhancement (96%) and increasing volume (92%) are the most sensitive criteria for evaluating brain tumors, but these criteria lack specificity (83.3%).9 This is because of differential diagnoses, including inflammatory and infectious disorders, which can have a similar appearance. Therefore our preference is to use MRI for all staging of primary and metastatic disease of the brain because it provides increased visibility of lesions, better tumor grading, and improved accuracy of disease extent assessment compared with CT.10,11,12
The most common adult primary intra-axial brain tumors are, in descending frequency, glioblastoma multiforme (GBM) (WHO grades III to IV), astrocytomas other than GBM (WHO grades I to II), oligodendrogliomas (WHO grades II to III), lymphomas, gliomatosis cerebri (diffuse tumor with cortical involvement and prognosis equal to GBM), and hemangioblastomas.13,14,15 Lymphomas are most commonly seen in HIV-positive patients.16 GBM and lymphoma both have a tendency to involve the corpus callosum; metastatic disease is less likely to do so. In general, size, area of T2 signal abnormality, and contrast enhancement are the three MRI changes to focus on with regard to tumor response. Also important to assess are enhancement, subependymal spread, necrosis, and dural involvement on conventional MRI, which are suggestive of high-grade tumors and therefore are associated with a poor prognosis. However, contrast enhancement with both CT and MRI can be decreased following steroid administration, which can result in suboptimal assessment of tumor extent. Conventional MRI can be suboptimal for assessment of tumor extent and tumor grading, so additional, nonconventional MRI methods to assess tumor response are under extensive study. One of these methods is perfusion imaging. Perfusion is a technique for assessing the contrast bolus with four parameters. These parameters include time to peak (TTP), mean transit time (MTT), cerebral blood flow (CBF), and cerebral blood volume (CBV) or tumor blood volume. Because of the increased number of vessels supplying tumors as a result of angiogenesis, tumors typically have reduced MTT and TTP with increased CBF and CBV. Cerebral perfusion also appears to have potential as a prognostic indicator. In a study investigating oligodendrogliomas, perfusion imaging helped predict higher-grade tumors.17 Perfusion imaging has also been used in conjunction with a technique called diffusion imaging (DI) to grade gliomas. It demonstrated that both CBV and the apparent diffusion component of DI helped predict the glioma grade preoperatively.18 A third nonconventional method of assessment, MR spectroscopy, shows increased choline levels caused by increased cell turnover with decreased N-acetyl acetate (NAA), a neuronal marker in areas of active tumor. The NAA decreases because of destruction of neurons by the tumor. If a brain tumor is of a high grade, lactate and lipids may also be seen.19
Postirradiation changes can be seen following therapy both for brain tumors and head and neck carcinomas and can mimic other abnormalities and complicate radiologic assessment of residual disease.20,21 Within weeks of radiation therapy, an early reversible T2 white matter signal may be seen within the brain, and within 3 months of therapy, a delayed reversible increased T2 white matter signal is identified. Late changes, defined as those occurring more than 3 months following treatment, can demonstrate irreversible increased T2 white matter signal as well as atrophy, calcification, and contrast enhancement, and can mimic tumor.22 In these cases, MR spectroscopy, MR perfusion, and DI may help distinguish residual tumor from the changes that may follow therapy.23 For example, high apparent diffusion component values are seen within tumors, and low apparent diffusion component values are seen with radiation necrosis on DI. Likewise, CBV values will be decreased in regions of radiation necrosis but elevated in regions of tumor. MR spectroscopy is also useful for assessing changes that follow therapy because decreased NAA occurs with postirradiation changes, whereas decreased choline/lipid ratios help distinguish tumor recurrence from radiation necrosis.
Metastatic Disease to the Brain
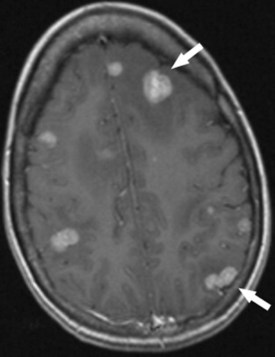
In adults, metastatic disease to the brain occurs as frequently as primary brain tumors. It is best demonstrated with MRI. The appearance of metastatic disease mimics that of primary brain tumors (Fig. 9-1). The most common cancers of origin for brain metastases are bronchial carcinoma (30%), breast carcinoma (15% to 30%), melanoma, prostate carcinoma, renal cell carcinoma, and thyroid carcinoma. In adults, metastases are more commonly found in the posterior fossa than are primary brain tumors. PET scanning can detect metastatic lesions to brain, but it is less sensitive than MRI.24 Dural-based metastases are more common with breast carcinoma and lymphoma, as well as carcinoma of the lung and prostate gland and melanoma. Metastases likely to hemorrhage include those of breast carcinoma, choriocarcinoma, lung carcinoma, melanoma, renal cell carcinoma, retinoblastoma, and thyroid carcinoma. Metastatic seeding of the central nervous system by primary brain tumors is uncommon in adults, but does occur with GBM, astrocytomas, and oligodendrogliomas. If the radiologist is uncertain about whether a lesion is a primary brain tumor or a metastatic lesion, MR spectroscopy may be used for differentiation because metastatic lesions are well circumscribed and noninfiltrating when seen with spectroscopy.25
Primary Tumors of the Head and Neck
Imaging for carcinomas of the sinuses, nasopharynx, and parotid glands is preferentially performed with MRI because of the propensity for these tumors to spread perineurally along cranial nerves V and VII.26 Nasopharyngeal carcinoma is also likely to spread via deep tissue infiltration into the pharyngobasilar fascia, sinus of Morgagni, cartilaginous eustachian tube, levator veli palatini muscles, and skull base. All of these areas are also better imaged with MRI than CT. In some cases, CT may still be useful for detection of skull base involvement and, therefore, must often be assessed for surgical planning.
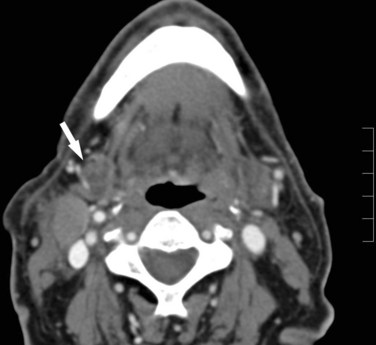
For tumors arising in and around the vocal cords, CT is preferred because of less motion artifact, faster imaging, and extension of imaging into the chest. Imaging to the level of the hila allows assessment for synchronous lung carcinomas as well as enlarged hilar and mediastinal nodes27,28 (Fig. 9-2). This inferior extent of imaging also allows for assessment of the course of the entire recurrent laryngeal nerve. MRI may be preferable if multidetector CT with coronal imaging is not available.
PET imaging of the head and neck is being performed more frequently.29,30 Performing PET without CT or at CT registration leads to errors in location of the abnormality and is therefore limited. PET shows increased uptake based on metabolism of glucose and is therefore positive with both tumor and infection. Negative PET findings can occur in necrotic nodes that are positive at histology, but this appears to be less of a problem with fused PET/CT.31 Fused PET/CT is expensive and time-consuming and so is not the first-line study for tumor detection at our institution, but we currently use it when CT or MR results are indeterminate. In patients with neck nodes positive for cancer in whom the primary site is unknown, fused PET/CT has higher sensitivity than CT alone for detecting primary tumors (87.5% vs. 43.7%), but there is no increase in the specificity of PET used in conjunction with CT compared with CT alone.32 PET imaging also demonstrates improved sensitivity in the detection of lymph node metastases in head and neck cancer compared with CT alone; therefore it is used to guide radiotherapy33 and detect distant metastases and is particularly beneficial in stage III and IV disease.29 Recently, PET has been assessed with regard to prognosis, with decreased uptake being used as a prognostic indicator for response to therapy.34,35 Another area showing great promise is CT perfusion monitoring, which may be of predictive value for response to chemotherapy.36
Metastatic Disease to the Neck
Ultrasound detection of metastatic nodes, particularly color-flow Doppler ultrasonography, may be more sensitive for differentiating benign and malignant cervical adenopathy than CT or MRI.37,38 When ultrasound reveals no increased blood flow in neck nodes contralateral to the known primary tumor, a unilateral neck dissection is performed. Iron oxide particles, MR spectroscopy, sentinel node mapping, and CT perfusion are all newer investigational techniques for improved lesion detection. Quantification of CT perfusion accurately differentiates normal adjacent tissue from tumor. This is important because poorly perfused tumors respond poorly to radiotherapy and have increased rates of local recurrence.
Imaging of the Chest
Primary Tumors of the Lung
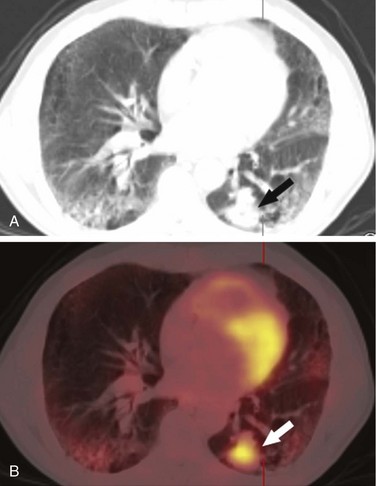
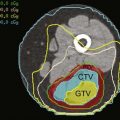
Lung cancer is probably most commonly discovered on chest radiographs, where it appears as a central or peripheral mass, often with deformation of the mediastinum caused by enlarged lymph nodes. A pleural effusion veiling the lucency of the lung may also be present. When there is a suspicion of lung cancer either clinically or on a chest radiograph, contrast-enhanced CT scanning is the technique of choice for characterization of the primary mass, identification of additional nodules, and assessment of size and location of lymphadenopathy. Lymph nodes larger than 1 cm in the short-axis view are considered pathologic.39 Fused PET/CT images can be useful in determining the avidity of mediastinal and hilar nodes. In most cases, CT or fused PET/CT is used to follow response to therapy (Fig. 9-3).
Histologically, primary lung cancers can be divided into two broad categories: non–small cell lung cancer (NSCLC) and small cell lung cancer. NSCLC is further divided into several histologic subtypes, including adenocarcinoma, squamous cell carcinoma, and large cell carcinoma. Most primary lung cancers (80%) prove to be NSCLC.40 For imaging purposes, lung cancer can be divided based on predilection for the peripheral or central location.
Peripherally Located Primary Lung Tumors
Adenocarcinoma accounts for 40% of lung cancers.40 These tumors are typically small, round, smoothly marginated, and peripherally located. Calcifications are present in only 6% of primary lung adenocarcinomas. However, central location does not exclude the diagnosis of adenocarcinoma.41 When the primary mass is located peripherally, hilar and mediastinal lymph node metastases are present at diagnosis in 18% and 2% of cases, respectively. When the primary mass is located more centrally, hilar and mediastinal lymphadenopathy is present in 40% and 27% of cases, respectively.41
Broncheoalveolar carcinoma (BAC) is considered a subtype of adenocarcinoma. Approximately 60% of BACs present as a solitary peripheral pulmonary nodule.42 Other common presentations include multiple nodules (~15%) and an alveolar filling pattern that mimics pneumonia (~10%).43 These opacities may present a diagnostic challenge, as they can remain unchanged in size for several years and are generally not hypermetabolic on PET/CT imaging. Mediastinal lymphadenopathy is present at diagnosis in 18% of patients.43
Approximately 10% of lung cancers are large cell carcinomas.40 These typically present as large, peripherally located masses. Cavitation and calcification are uncommon findings. Hilar and mediastinal lymphadenopathy is found in 30% and 10% of cases, respectively.42 Large cell carcinomas are characterized by a rapid growth pattern with early lymphatic and hematogenous metastases.44 Large cell carcinomas can be histologically difficult to differentiate from poorly differentiated adenocarcinomas and squamous cell carcinomas.40
Centrally Located Primary Lung tumors
Squamous cell carcinoma (SCC), also known as epidermoid carcinoma, makes up 30% of lung cancers.40 Although SCC often presents as a centrally located mass, it is peripherally located in a third of cases and may occur as an endobronchial mass.42 Cavitation of the primary mass occurs in 10% to 20% of patients. Most apical tumors, or Pancoast tumors, are SCC.43 Pancoast tumors may extend superiorly and invade the subclavian artery and brachial plexus. Brachial plexus involvement is best examined with MRI.
Small cell lung cancer accounts for 20% of all primary lung cancers.40 Also known as oat cell carcinoma, this histologic subtype is composed of neoplasms arising from neuroendocrine cells. Small cell lung neoplasms are commonly central lesions and may encase mediastinal structures at diagnosis. Metastases to the bone marrow, liver, adrenals, and brain occur early.42
Nodal Involvement of Lung Cancer
Assessing the mediastinum for nodal metastases is important for cancer staging. Contrast-enhanced CT scanning is routinely used for the detection of abnormal lymph nodes. Differentiation between benign and malignant lymph nodes is made using size criteria alone. Lymph nodes with a short-axis diameter of greater than 1 cm are defined as pathologically enlarged and concerning for metastatic involvement.39 Unfortunately, morphologic criteria on MRI and CT are not effective in characterizing lymph nodes. Using the 1-cm short-axis criteria, CT has been reported to be 41% to 67% sensitive and 79% to 86% specific for detection of nodal metastases.45 Oftentimes, enlarged nodes are hyperplastic because of infection or inflammation, particularly in the setting of postobstructive pneumonia.
Positron emission tomography is useful in differentiating benign from malignant lymphadenopathy. If an enlarged node discovered by CT does not show FDG avidity, it will be free of metastases with nearly 100% certainty.46 PET has a reported sensitivity of 76% to 100% and a specificity of 82% to 100% for the detection of lymph node metastases.35 Problems remain with detection of lung nodules less than 5 mm in diameter and superimposed or concurrent infection, which also shows increased uptake.
Lymphoma
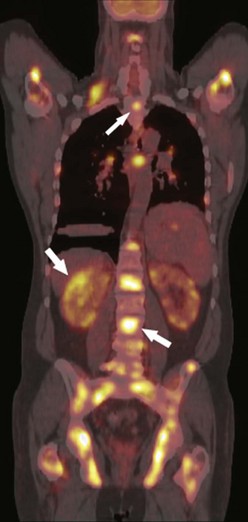
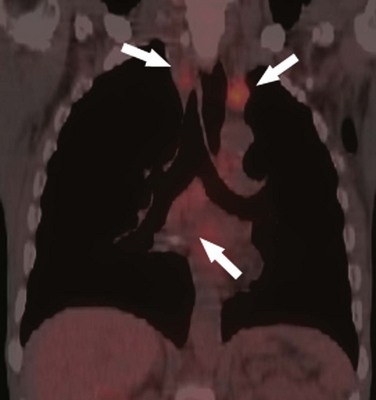
Although lymphoma is primarily a disease of white blood cells, more than 80% of patients with Hodgkin’s disease have intrathoracic involvement at presentation consisting of enlarged anterior mediastinal and paratracheal lymph nodes47 (Fig. 9-4). Non-Hodgkin’s lymphoma is a more heterogeneous group of diseases, and thoracic lymph node involvement is present in 45% of cases.48 The paratracheal and anterior mediastinal lymph nodes remain the most commonly involved sites. Large mediastinal lymphadenopathy (>10 cm) is associated with an increased risk of relapse and usually requires aggressive combination therapy.49 Recurrence is common in the pericardial and internal mammary lymph nodes as these are not included in the radiation field. Treated lymph nodes tend to calcify, either in a rimlike fashion or as a coarse, dense mass. Lung nodules are uncommon in both Hodgkin’s and non-Hodgkin’s disease. Non-Hodgkin’s lymphoma occurs in 2% to 6% of patients following heart transplantation.50 It is the third most common cause of death beyond the perioperative period. There is a strong association with the antirejection drug cyclosporine and Epstein-Barr virus. This is the only variety of lymphoma to present primarily as a pulmonary mass or nodules.51,52
Cancers of the Abdomen and Pelvis
Primary Tumors of the Gastrointestinal Tract
Primary Esophageal Tumors
In North America there has been a shift of esophageal cancers toward the gastroesophageal junction over the last few decades. In addition, the cell type is now most often adenocarcinoma, likely caused by reflux disease.53,54 Although a fluoroscopic examination such as a barium swallow performed in a patient with dysphagia can identify an advanced cancer, most smaller tumors are identified at endoscopy. Enlarged lymph nodes along the course of the esophagus are subdivided into 20 stations. Enlarged nodes are sampled during endoscopy to determine involvement and prognosis. Involvement of the chest and liver is best assessed using contrast-enhanced CT. Staging accuracy using combined endoscopic ultrasound and contrast-enhanced CT ranges from 62% to 90%.45 Fused PET/CT images are now used routinely in many centers to follow therapy, with diminished uptake indicative of successful cell kill55,56 (Fig. 9-5).
Primary Stomach Tumors
Gastric adenocarcinoma, similar to distal esophageal cancer, is usually identified at endoscopy and staged using contrast-enhanced CT scan. Endoscopy is superior to CT at depicting the depth of invasion into the gastric wall, whereas CT is superior in the identification of lymph nodes increased in size and number, ascites, and distant disease.57,58 Gas-releasing crystals are given orally before the scan to distend the stomach. The gastric wall may be focally or diffusely thickened. Ulceration is common. Differential diagnosis includes gastrointestinal stromal tumor (benign or malignant), metastatic disease, and lymphoma. For tumors located in the gastric cardia or fundus, lymph node patterns are similar to those of distal esophageal tumors and include the gastrohepatic ligament and periaortic spaces. Distal cancers often drain along the lesser curvature of the stomach. This can be a particularly difficult area to survey with axial images alone, so coronal reformatted images are often helpful.59