Image-Guided Biopsy of Nonpalpable Breast Lesions
Janice S. Sung
Christopher E. Comstock
IMAGE-GUIDED BIOPSY OF NONPALPABLE BREAST LESIONS
The increasing use of mammography, ultrasound, and breast MRI to screen asymptomatic women has resulted in the increased detection of clinically occult, nonpalpable breast lesions. Despite technological advances and improvements in image resolution, the imaging features of most breast lesions remain indeterminate, requiring tissue sampling for definitive diagnosis. Initially, surgical excision following image-guided needle localization was the gold standard for biopsy. However, because up to 70% to 80% of lesions for which biopsy is recommended represent benign etiologies, newer cost effective methods were investigated as alternatives to surgical biopsy (1).
Nonpalpable breast abnormalities were initially sampled using fine-needle aspiration (FNA). FNA is a fast, relatively inexpensive technique that patients tolerate well. However, significant limitations of FNA include the frequency of insufficient sampling, frequency of false positives, and limited accuracy compared to core biopsy or surgical excision. In the multicenter randomized Radiology Diagnosis Oncology Group V trial, there was a 35% insufficient sample rate with FNA of nonpalpable breast lesions, and the accuracy rate for ultrasound-guided FNA was 77% compared to 98% with ultrasound-guided core biopsy (2, 3). In addition, distinguishing between in situ or invasive carcinoma and determining receptor status may be difficult on cytology from FNA. For these reasons, FNA is primarily used to sample axillary nodes or lesions not amenable to core biopsy, including lesions that are superficial or abutting the chest wall.
Simple and complicated cysts are benign and do not require aspiration except when requested for symptomatic relief. In general, the fluid should be discarded due to the frequency of false positives unless the aspirate is not typical of cyst contents. Ciatto et al. examined the cytology following aspiration of 6,782 consecutive cysts and found that the 5 papillomas detected in this series all had bloody aspirates. Therefore, the fluid from a cyst aspiration is typically discarded and cytology obtained only with bloody aspirate (4). In cases when the ultrasound findings cannot distinguish between a complicated cyst versus a solid mass, initial aspiration is recommended for those masses that would require biopsy if solid (i.e., new or enlarging). Complete resolution with aspiration confirms that the lesion in question represented a complicated cyst. If a suspected cyst does not completely resolve with aspiration, the fluid should be discarded and the procedure converted to a core biopsy due to its increased accuracy.
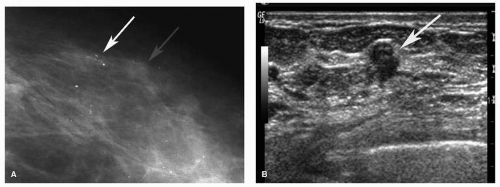
In the late 1980s and 1990s, automated large core needle biopsy using stereotactic or ultrasound guidance was demonstrated to be an accurate method to sample imaging-detected abnormalities with comparable results to surgical biopsies and decreased costs and patient morbidity (1). Stereotactic biopsy is most commonly used to sample mammographic microcalcifications. Less frequently, stereotactic biopsy is performed to sample a mammographic mass, asymmetry, or area of architectural distortion with no sonographic correlate. However, stereotactic biopsy of noncalcified lesions should be performed only after a thorough high-quality breast ultrasound has been performed, as these may be more difficult to target using stereotactic guidance. A focused ultrasound should also be performed if mammographic calcifications are associated with a mass or increased density as targeting the associated soft tissue density has a higher likelihood of diagnosing an invasive component (Fig. 15-1). Percutaneous biopsy using ultrasound guidance is the preferable method for sampling any lesion that is evident sonographically.
Compared to stereotactic or MRI-guided biopsy, ultrasound-guided biopsy is faster, more comfortable for the patient, and allows greater access to breast tissue, especially for far posterior and medial lesions that may not be amenable to either stereotactic or MRI-guided biopsy. In addition, adequate sampling is more consistently obtained because the needle can be seen traversing the target in real time. Parker et al. first described the accuracy of ultrasoundguided core biopsy using a 14-gauge automated needle. In this study, there was 100% concordance between the pathology obtained with ultrasound-guided core biopsy of 49 lesions and subsequent surgical excision. Of the 132 benign ultrasound-guided biopsies, no malignancies were identified at 12- to 36-month follow-ups (5).
Subsequently, vacuum-assisted devices were developed that improved diagnostic accuracy compared to automated devices and are now routinely used in all stereotactic percutaneous biopsies. Automated and vacuum-assisted devices were also modified to sample lesions detected only on MRI. Percutaneous biopsy using imaging guidance has been demonstrated to be a safe, accurate, less deforming, less invasive, and less expensive alternative to surgical biopsy and is the preferred method for sampling nonpalpable breast lesions. However, accurately performing percutaneous biopsies requires an understanding of all breast imaging modalities and an ability to correlate spatially between them despite differences in technique and patient positioning.
This chapter will review the indications and techniques for performing biopsies using stereotactic, ultrasound, and MRI guidance as well the potential pitfalls in both performing these procedures and the management of the pathology obtained from percutaneous biopsy.
PATIENT SELECTION AND PREPARATION
Patient Selection
Mammography, ultrasound, and breast MRI examinations are classified using the American College of Radiology Breast Imaging Reporting and Data System (BI-RADS), which gives a final assessment category indicating the level of suspicion for malignancy for each study (6).
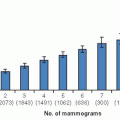
BI-RADS 3 lesions have imaging features that suggest a less than 2% chance of malignancy. The criteria of a BI-RADS 3 mammographic lesion are largely based on two studies that collectively include over 80,000 mammograms (7, 8). In these studies, a 6-month follow-up mammogram identified interval progression of those few BI-RADS 3 lesions that were actually malignant and diagnosed these cancers early enough to maintain a favorable prognosis (9). At the same time, close follow-up of BI-RADS 3 lesions reduces the false negative rate of biopsy and decreases health care costs. Examples of BI-RADS 3 mammographic lesions include a nonpalpable low-density solid mass with a round or oval shape and predominantly circumscribed margins on a baseline mammogram or clustered microcalcifications with a punctate morphology on a baseline study. New or enlarging solid masses should not be categorized as BI-RADS 3 lesions but should undergo biopsy.
The BI-RADS lexicon for lesions detected on ultrasound and MRI are less widely validated than for mammography. Stavros et al. initially developed a classification scheme to differentiate benign from malignant lesions on ultrasound with a 98.4% sensitivity and a 99.5% negative predictive value for malignancy (10). Subsequent studies have confirmed the low rate of subsequent malignancy in BI-RADS 3 masses (11, 12). An incidental homogeneously hypoechoic oval mass with circumscribed margins and parallel orientation is an example of an ultrasound BI-RADS 3 lesion.
The specific morphologic and/or kinetic features of lesions appropriate for a BI-RADS 3 recommendation on MRI have not been well established but are generally based on the principles used to characterize BI-RADS 3 lesions on mammography. The cancer yield of BI-RADS 3 lesions on MRI has varied between 0% and10% (13, 14). Eby et al. evaluated the characteristics of probably benign MRI lesions and found that foci, defined as lesions less than 5 mm in size that are too small to further characterize, comprise 46% of BI-RADS 3 lesions (14). Liberman et al. also found a 3% malignancy rate in suspicious lesions less than 5 mm in size (15). Studies have suggested that unique foci with a high T2 signal intensity correlate may safely be followed on a 6-month follow-up MRI given the low rate of malignancy of these lesions.
BI-RADS 4 lesions have between a 2% and 95% chance of representing malignancy and can be further subdivided
into BI-RADS 4a (low suspicion of malignancy), 4b (moderate suspicion), and 4c (high suspicion) categories. BI-RADS 5 lesions are highly suspicious with a greater than 95% chance of malignancy. Percutaneous biopsy is recommended for all examinations with BI-RADS 4 or 5 recommendations. Percutaneous biopsy is most useful for BI-RADS 4 lesions because surgery can be avoided in 70% to 80% of cases where biopsy yields benign and concordant pathology. Percutaneous biopsy of BI-RADS 5 lesions is recommended due to improved surgical outcomes at lumpectomy, including decreased positive margin rates (16).
into BI-RADS 4a (low suspicion of malignancy), 4b (moderate suspicion), and 4c (high suspicion) categories. BI-RADS 5 lesions are highly suspicious with a greater than 95% chance of malignancy. Percutaneous biopsy is recommended for all examinations with BI-RADS 4 or 5 recommendations. Percutaneous biopsy is most useful for BI-RADS 4 lesions because surgery can be avoided in 70% to 80% of cases where biopsy yields benign and concordant pathology. Percutaneous biopsy of BI-RADS 5 lesions is recommended due to improved surgical outcomes at lumpectomy, including decreased positive margin rates (16).
Patient Preparation
Patients are asked to discontinue aspirin, warfarin, nonsteroidal anti-inflammatory agents, vitamin E, or other anticoagulation or antiplatelet drugs for at least 5 days prior to the procedure. However, patients who are anticoagulated for a medical reason are advised to consult their ordering physician to determine whether the medication can be safely discontinued. Studies have demonstrated that core biopsy can safely be performed without clinically significant complications if anticoagulants cannot be discontinued or if urgent results are required (17). Premedication with antibiotics in patients with prosthetic valves or joint replacements is generally not necessary.
SELECTION OF BIOPSY DEVICE
Both the gauge of the biopsy needle and the type of biopsy device are important factors to consider when performing percutaneous core biopsies. Percutaneous biopsies should be performed using a 14-gauge or larger bore needle due to increased accuracy compared to 16- or 18-gauge needles (18).
Two types of biopsy devices are available: automated spring-loaded devices or vacuum-assisted biopsy devices. Vacuum-assisted devices are standard for stereotactic or MRI-guided biopsies. Vacuum-assisted devices are faster and more accurate due to the larger size of specimens and the ability to obtain contiguous samples. The median specimen weight is approximately 17 mg with a 14-gauge automated needle, 35 mg with a 14-gauge vacuum-assisted biopsy probe, and 100 mg using an 11-gauge vacuum-assisted needle (1). Successful calcification retrieval rate and the rate of histologic underestimation are improved using a vacuumassisted device compared to a 14-gauge automated large core biopsy device.
Although vacuum-assisted devices are routinely used during stereotactic and MRI-guided biopsies, most ultrasound-guided biopsies can be accurately performed using an automated spring-loaded device (19). A vacuum device may be preferred when sampling complex masses when lesion conspicuity may decrease due to loss of the fluid component once the needle is inserted or in cases of a suspected fibroepithelial lesion or papilloma when larger specimens may improve pathologic accuracy.
BIOPSY METHODS
Stereotactic Biopsy
Stereotactic biopsy may be performed on either dedicated prone tables or an upright add-on unit. Add-on units convert a standard diagnostic mammography unit, and the biopsy is performed with the patient in a sitting or decubitus position. Add-on units require less space, are less expensive, and allow the room to be used for routine mammography when a biopsy is not being performed, but they are associated with an increased risk of vasovagal reactions. Advantages of prone tables include that the biopsy is performed out of the patient’s view, that vasovagal reactions are uncommon, and that more working space between the biopsy gun and the patient is permitted (1). The cost and space requirement for a dedicated room make prone tables impractical for centers that perform a low volume of stereotactic biopsies, and both types of units are considered acceptable and commonly used.
The technique for performing stereotactic biopsy is similar regardless of the type of unit used. The direction of approach is selected based on lesion location and/or visibility. A scout image is taken with the target centered in a cutout compression plate. A stereotactic pair in which images are taken at +15 and -15 degrees from a center line is obtained. Stereotactic biopsy is based on the concept that a lesion can be localized in three dimensions—the x, y, and z axes—based on the apparent change in position on the stereotactic pair (parallax). Once the target is selected, the housing unit is moved to the x and y coordinates. The skin is cleansed and anesthetized, and the biopsy needle inserted into the breast to the predetermined depth. Another stereotactic pair is obtained with the needle in the prefire position to confirm accurate targeting, and then the needle is typically fired (Fig. 15-2). A postfire stereotactic pair may be obtained to confirm needle position prior to sampling. If the target is calcifications, a specimen radiograph is taken to confirm retrieval of some of the targeted calcifications. If the target is a mass or asymmetry, a postfire image should be obtained to confirm that the needle trough is within the mammographic abnormality. Finally, a localizing clip is placed at the biopsy site and an image obtained to confirm clip deployment. After completion of the biopsy, a 2-view mammogram is performed to confirm clip placement and position relative to the biopsy site. This postbiopsy mammogram is also useful to confirm sampling of a mammographic mass or asymmetry, although these may be obscured by postbiopsy change.
Pitfalls of Stereotactic Biopsy
Several factors may complicate successful stereotactic biopsy, including the following:
Error in targeting: Localization requires targeting the same lesion on both stereotactic pairs. Targeting two different lesions on the stereotactic pairs will miscalculate the depth (z axis), resulting in unsuccessful retrieval of the target.
Skin calcifications: Skin calcifications mistakenly may be thought to be within the breast parenchyma. The possibility that the target represents skin calcifications should be considered when the calculated Z value (depth) is approximately 5 mm (Fig. 15-3).
Negative stroke margin: Stroke margin is the distance between the postfire needle position and image receptor. A negative stroke margin indicates that the breast thickness is insufficient and that the needle will strike the image receptor when fired (Fig. 15-4). Standard biopsy needles typically have a needle trough of 18 to 20 mm, and the calculated depth (Z value) centers the target within the needle trough. With thin breasts, devices with a shorter cutting chamber of 12 mm and a blunt tip may be employed. However, these petite devices decrease the sample size and require more precise targeting. Minimizing compression to ensure maximal breast thickness may also permit sampling in thin breasts. If the stroke margin error is only a few millimeters because the calculated Z value
centers the target in the middle of the needle trough, which is typically 18 to 20 mm, the needle can be pulled back to prevent the tip from striking the image receptor while keeping the target within, but not centered in, the trough. A reversed compression paddle, which has an aperture allowing tissue to push through, also may be placed on the far side of the breast to increase breast thickness.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree