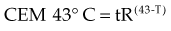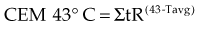Chapter 21 Hyperthermia
The Biology of Hyperthermia
The Arrhenius Relationship and Thermal Isoeffect Dose
Arrhenius found a temperature dependence of sucrose hydrolysis in the presence of various acids that was a logarithmic function of the absolute temperatures at which the reactions were conducted.1 These observations were physiologically relevant in that the rate of cellular metabolism increases as the temperature rises in cells or tissues, up to a point at which thermal damage is created. The principles discovered by Arrhenius extend to cell killing by HT as well. The temperature dependence of the rate of cell killing is referred to as the Arrhenius relationship.
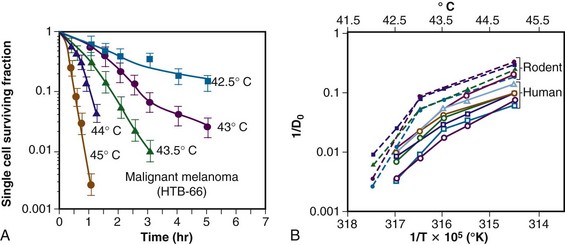
The temperature dependence of the rate of cell killing has been used as a method for thermal dosimetry. This is done by plotting temperature versus log of the slope of the survival curve (1/Do). Do is analogous to a Do in a radiation survival curve (the dose to reduce survival to 37% of a starting value on the exponential portion of the survival curve). In the case of HT, “dose” is the number of minutes at a specified temperature (Fig. 21-1). Typically, Arrhenius plots have a biphasic curve and the point at which there is a change in slope is referred to as a breakpoint. Above the breakpoint for nearly all cell types, a change in temperature of 1° C doubles the rate of cell killing. Acquired resistance to HT killing (thermotolerance) that occurs during HT is responsible for the change in slope of the heat cell survival curve at longer heating times for temperatures below 43° C (see Fig. 21-1A). Arrhenius plots for human cell and rodent lines are well described. The breakpoint for human cells is near 43.5° C, and the slope below the breakpoint is between 2 and 4. The change in slope below the breakpoint is thought to be due to thermotolerance induction at lower temperatures. The breakpoint for rodent cell lines is lower than that of humans, at about 43° C. The slopes of Arrhenius plots derived from in vivo studies are virtually identical to those derived from in vitro studies.
Sapareto and Dewey2 proposed using the Arrhenius relationship to normalize thermal data from HT treatments. The rationale came from the observations that time-temperature histories are not stable and that they vary from patient to patient, and that temperatures within tumors are almost always nonuniform. Using the Arrhenius relationship, it would be possible to convert all time-temperature data to an equivalent number of minutes at a standard temperature. The formulation takes the following form:
The CEM 43° C (thermal isoeffect dose) formulation has been used extensively and successfully in clinical trials to assess the efficacy of heating. This is in spite of the fact that the R values and breakpoints have historically been derived from studies done in rodents, which are not exactly equivalent to human cells.*,3
Mechanisms of Hyperthermic Cytotoxicity
Cellular and Tissue Responses to Hyperthermia
Protein is the primary target for hyperthermic cytotoxicity. When cells are exposed to elevated temperatures (≥41° C), damage is inflicted in multiple sites, but the predominant molecular target appears to be protein. The heat of inactivation for cell killing and thermal damage to tissues is in the range of that necessary for protein denaturation (130 to 170 kcal/mol). Additional evidence for protein being the primary target for cell killing is the importance of heat shock proteins in protecting thermotolerant cells from thermal damage. One of the primary functions of heat shock proteins is to refold other proteins that have been damaged.4
Some organelles are especially important in controlling thermal response. For example, modification of membrane lipid content or use of membrane active agents, such as alcohols, can sensitize cells to heat killing, but the sensitization is probably related to destabilization of the membrane as it relates to lipid-protein interactions.5 The cytoskeleton of cells is particularly heat sensitive.6 When it is collapsed by heat, there is disruption of cytoskeletal-dependent signal transduction pathways as well as inhibition of cell motility.7,8 Enzymes in the respiratory chain are more heat sensitive than enzymes in the glycolytic pathway.9 The heat sensitivity of the centriole leads to chromosomal aberrations following thermal injury.10 Finally, the DNA repair process is heat sensitive, and this may be one of the mechanisms that leads to heat-induced radiosensitization and chemosensitization.11
Physiologic Response to Hyperthermia
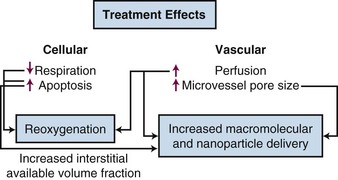
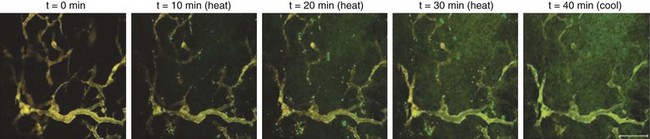
As temperatures increase, there is an increase in blood flow. The temperature threshold for this change is 41° to 41.5° C in skin.12 Changes in vascular permeability also occur, leading to edema formation in the heated volume. At higher thermal doses, vascular stasis and hemorrhage develop. The change in normal tissue perfusion upon heating is much greater (10 times) than one sees in tumors (1.5 to 2 times).13 Mechanisms underlying vascular stasis may include arteriovenous shunting, thrombus formation, and leukocyte plugging.14 Importantly, physiologic changes occur in tumors at lower temperatures that are potentially beneficial (Fig. 21-2).
Taking Advantage of the Physiologic Response to Hyperthermia
Improvement in Macromolecular and Liposomal Drug Delivery
A liposome is a small lipid vesicle (≈100 nm in diameter) that contains water or saline in the center. Drugs can be loaded into liposomes at high concentration. It has been recently shown that HT increases microvascular pore sizes preferentially in tumor microvessels, which leads to enhanced liposomal accumulation in tumor.15 The threshold for increased liposomal extravasation is 40° C, and it increases by a factor of 2 for every degree of temperature rise until vascular damage occurs.16 The effects of HT on liposomal extravasation have been studied extensively in preclinical models, but a twofold to thirteenfold enhancement in accumulation occurs in spontaneous soft tissue sarcomas of pet cats, an encouraging result that may translate to human tumors.17 HT also increases the available volume fraction (fraction of tissue not occupied by cells or stromal fibers) that may develop as cells undergo either necrosis or apoptosis following heat treatment.18 This increases the tissue space available for nanoparticles to be deposited. The increase in liposomal drug delivery achieved with HT has been shown to result in increased antitumor effect in many preclinical studies.19 HT has been used in conjunction with liposomal doxorubicin and irradiation in one small clinical series of patients with chest wall recurrences of breast cancer. Encouraging results were obtained, although drug concentrations were not measured in this series.20
For relatively small agents, such as most chemotherapeutic drugs, there is no advantage gained via increased vascular permeability to macromolecules or nanoparticles unless the drugs are protein-bound.21 For drugs with a molecular weight of less than 1000 MW, the primary mechanism that governs drug transport is diffusion,22 which is not highly temperature dependent. However, for molecules larger than 1000 MW, the primary driving force for transport is convection, which is controlled by the pressure gradient across the vessel wall. Accordingly, HT can augment the transvascular delivery of agents such as monoclonal antibodies23 and polymeric peptides that carry drugs or radioisotopes.24 Dreher and colleagues used a novel peptide polymer that undergoes an inverse-phase transition at 41° C to “pump” polymer into tumors. The inverse-phase transition refers to a physical feature of the polymer that renders it to be water soluble below 41° C; above this temperature, the polymer comes out of solution in aggregates. The aggregation occurs primarily intravascularly but is reversible. Therefore, when the tissue is cooled down, the particles rapidly disaggregate and diffuse into the tissue down their concentration gradient25 (Fig. 21-3). This same type of peptide polymer could be used for noninvasive thermometry with magnetic resonance imaging (MRI) or electron paramagnetic resonance (EPR) imaging because the interaction of the polymer with water changes when it aggregates. Proof of principle for this method was shown using EPR imaging.26
Effects of HT on Tumor Metabolism and Oxygenation.
Enzymes for aerobic metabolism are more heat sensitive than those involved in anaerobic metabolism.9 Decreases in adenosine triphosphate (ATP) concentration after HT are inversely related to the temperatures achieved during HT in preclinical models and canine sarcomas27,28 and are related to the pathologic complete response (pCR) rate in human sarcomas.27 Decreases in ATP and increases in lactate concentration occur following tumor blood flow reduction after HT.29 In human patients with soft tissue sarcomas who were treated preoperatively with HT and radiation therapy, reduction in the ratio of ATP to inorganic phosphate (Pi) (measured using phosphorus-31 [31P] magnetic resonance spectroscopy) was significantly correlated with a higher probability for pCR.27 These results are consistent with the theory that tumor respiration can decrease after HT treatment.
A shift toward anaerobic metabolism would decrease oxygen consumption rates, which could lead to improvement in tumor oxygenation. Some of the benefits of HT may therefore result from improvements in oxygenation.30 Several preclinical studies have shown that mild temperature heating (between 40° and 42° C for an hour) can lead to improvements in the oxygen partial pressure (pO2) in the tumor up to 24 hours after heating.31 However, in some models, the extent of reoxygenation is quite heterogeneous between and even within individual tumors.32 It has been reported that HT improves tumor oxygenation in canine and human soft tissue sarcomas, with reoxygenation being associated with a greater probability for pCR in human tumors.33,34 In a canine study, median temperatures of less than 44° C improved oxygenation, whereas median temperatures higher than 44° C for 60 minutes led to decreased oxygenation.33 In patients with locally advanced breast cancer who were treated in a phase II study with preoperative HT plus radiotherapy and taxol, there was a significantly increased likelihood for achieving pCR if the tumors reoxygenated 24 hours after the first HT treatment. The probability for achieving a pCR was greater when the median temperatures were below 41.5° C.35 Improvement in oxygenation does not occur in all tumors, however, and currently there is no method for predicting which tumors will show this effect.36 In summary, mild temperature elevations during HT will benefit some patients if HT induces reoxygenation.31 To take full advantage of this effect, it will be necessary to use methods to enhance the reoxygenation effect, such that it occurs in the majority of patients. Song and Griffin have suggested that the addition of hyperoxic gas breathing after HT but during radiotherapy may achieve this goal.37,38
Physiologic Approaches to Enhance Thermal Cytotoxicity
pH Modification
It is well established that acidification can enhance sensitivity to HT. Changes in intracellular pH are responsible for increased thermal sensitivity.39,40 The most widely studied method to achieve acidification has been induction of hyperglycemia. The logic is that excess glucose load will push tumors toward glycolysis and lactic acid production.41,42 However, hyperglycemia alone is insufficient to reliably achieve adequate acidification.43,44 The addition of agents that can selectively acidify tumor intracellular pH, such as glucose combined with the respiratory inhibitor meta-iodobenzylguanadine (MIBG), have the potential to further enhance hyperthermic cytotoxicity selectively in tumor tissues45,46 because the acidification appears to be selective for tumors. Quercetin, a naturally occurring flavinoid, inhibits thermotolerance induction, particularly at a low pH, and can accordingly increase the thermal sensitivity of cells.47–49 An alternative approach is to block the extrusion of hydrogen ions from cells, which is normally accomplished via membrane-bound pumps. Use of such agents, in combination with acidification of the extracellular space, can lead to enhanced hyperthermic cell killing both in vitro and in vivo.50
Hyperthermia and Metastases
HT causes abrupt changes in tumor microvascular function, as described above, which could enhance tumor cell shedding from the heated site. Preclinical studies are mixed on this issue. One report showed that local HT alone enhanced the metastatic rate of B16 melanoma.51,52 When curative doses of radiotherapy were added to HT in the B16 melanoma model, however, the incidence of lymph node and lung metastases was decreased, compared with controls.52 The combination of curative doses of radiation and HT has generally been reported in other tumor models to reduce the incidence of metastases,53,54 although in a few models, simultaneous administration of HT with irradiation has been reported to increase metastases.53,54
The question of whether local HT increases the risk for metastasis is difficult to answer in clinical trials unless the primary therapy has a high probability for local control. This is because of the problem of competing risks. In a phase III trial of canine patients with primary malignant melanomas treated with the combination of HT and irradiation or irradiation alone, no difference in the likelihood for metastasis between the two groups was seen.55 However, local recurrence was a common event, and its onset was frequently followed by the appearance of distant metastases. In a phase III randomized trial of human melanomas treated with thermoradiotherapy versus radiotherapy alone, there was significant improvement in the likelihood for survival when the local tumor was controlled. Because the use of HT in that trial resulted in improved local control, the implication is that the combination therapy reduced the probability for metastases.56,57 In a study comparing graded doses of irradiation with and without HT for the treatment of canine soft tissue sarcomas, higher normal tissue temperatures in the region of the tumor were correlated with lower likelihood for distant metastases.58 A large series of patients (n = 95) with previously untreated high-grade soft tissue sarcomas who were treated preoperatively with HT and irradiation achieved a local control rate of nearly 90%, but 50% developed distant metastases.59 This rate of metastasis is essentially identical to that seen with preoperative radiotherapy alone, however, suggesting that HT had an undetectable influence on metastases.60
Normal Tissue Damage from Hyperthermia
Thresholds for thermal damage depend on the tissue type being heated and the severity of the injury. Mild damage can merely lead to edema, whereas more severe injury can lead to massive necrosis and organ failure. This subject has been reviewed in detail.3 The ranking of tissue thermal sensitivities does not follow classical principles derived from other cytotoxic agents, such as irradiation or chemotherapy. With such agents, the most sensitive tissues are those with the highest proliferative potential or activity. For HT, the most sensitive tissue classification includes brain tissue, which is composed of cells with almost no proliferative potential, as well as testicular tissue, which has high proliferative potential. There has been speculation as to whether tumor tissues might be more sensitive to thermal damage than normal tissues. Many studies have compared tumor tissue with normal cells tissue in vitro. There is no inherent difference in the thermal sensitivity of the two types of tissue cells. However, the microenvironment of tumors, which is often acidotic and nutritionally deprived, leads to an increase in thermal sensitivity that has been reported in a clinical series.61
Radiotherapy and Hyperthermia
Rationale for Combining Hyperthermia with Radiotherapy
When irradiation is combined with HT, complementary effects occur. Cells in S phase are radioresistant but are sensitive to HT. Hypoxic cells are three times more resistant to radiation compared with aerobic cells, but there is no difference in thermal sensitivity between aerobic and hypoxic cells. As discussed above, HT can lead to reoxygenation, which will further improve radiotherapy response.31,33,34 Finally, HT inhibits the repair of both sublethal and potentially lethal damage by inactivating crucial DNA repair pathways.62–64
Factors to Consider when Combining Hyperthermia with Radiotherapy
The interaction between radiation and HT is described by the thermal enhancement ratio (TER), which is defined as the ratio of doses of radiation to achieve an isoeffect for radiotherapy alone compared with radiotherapy plus HT. TERs for local tumor control have been estimated for a number of human tumors using historical control data for radiation alone.65 In most tumor types examined, these ratios were higher than 1. Assessment of normal tissue TER has not been attempted except in a few cases. For those examples, TER values for normal tissue damage have been less than those for tumor tissue damage in the same patient population, suggesting that there is potential for therapeutic gain with the use of radiotherapy combined with HT compared with radiotherapy alone.65 Prospective randomized trials in dogs with spontaneous tumors have also shown evidence for improved local tumor control with radiotherapy plus HT compared with radiotherapy alone,58,66,67,68 with no clinically observable increase in the frequency of clinically relevant late normal tissue complications. In one canine trial, enhancement of late radiation damage (as assessed histologically) was reported, and the duration of acute radiation complications was prolonged.68 There is clinical evidence, however, that excessively high intratumoral temperatures (i.e., >45° C for 60 min) can lead to damage to surrounding normal tissues, an effect that is often caused by rapid tumor regression.66,69 Such damage is not easily repaired and can lead to chronic tissue consequences, such as fibrosis, fistula formation, and bone necrosis.
Two cases of fatal pelvic necrosis have been reported as a late complication in patients with locally advanced cervical cancer treated with thermoradiotherapy,70 and there was speculation as to whether the complication was the result of HT. More recently, however, this rare complication has been reported following chemoradiotherapy treatment of cervical cancer in two small series.71,72 Thermal doses achieved in the HT series were several degrees lower than the radiotherapy doses reported to be associated with enhancement of normal tissue complications. This finding strongly suggests that these two cases were not the result of HT but more likely resulted from the radiotherapy treatment; An additional complication may have been the heavy smoking history of both patients.70
Hyperthermia and Chemotherapy
Rationale for Using Hyperthermia with Chemotherapy
Many chemotherapeutic agents have been shown to exhibit synergism with HT, including cisplatin and related compounds, melphalan, cyclophosphamide, nitrogen mustards, anthracyclines, nitrosoureas, bleomycin, mitomycin C, and hypoxic cell sensitizers.21 The mechanisms that underlie the synergy may include (1) increased cellular uptake of drug, (2) increased oxygen radical production, (3) increased DNA damage and inhibition of repair, and (4) reversal of drug resistance mechanisms.73,74–76 Hypoxia and pH are also important in the thermochemotherapeutic response.77–81 There are some classes of drugs, such as etoposide and vinca alkaloids, that do not synergistically interact with HT.21
Rationale for Using Hyperthermia with Targeted Agents
Very little is known about whether HT affects the cytotoxicity of newer targeted agents, such as those that block epidermal growth factor receptor (EGFR), mitogen-activated protein (MAP) kinase, mammalian target of rapamycin (mTOR), or other signal transduction pathways. Identification of targets can be accomplished by examining changes in gene expression brought on by HT treatment. Such studies have been conducted in vitro and in vivo. Genomic analysis of cells from U937, a human myelomonocytic tumor, exhibited differential expression in approximately 1000 genes, following a noncytotoxic treatment of 41° C for 30 minutes.82 A higher thermal exposure of 42° C for 90 minutes led to more significant change in proapoptotic signaling pathways,83 in addition to the expected heat shock response in both cases. Genome-wide analysis has also been done on one rat tumor line in vivo, following local heating (43° C for 60 min).84,85 The effects of HT were rather pleiotropic, involving over 1200 genes and a host of cellular responses, including apoptosis regulation, cell cycle control, MAP kinase- and calcium-regulated cell signaling, and genes involved in angiogenesis and metabolism regulation. There was also substantial down-regulation of a number of genes involved in immune function at 3 hours after treatment, which recovered to baseline by 24 hours. These results suggested that HT does not adversely affect immune function. The fact that HT appears to augment proapoptotic signaling pathways suggests that it could work additively with targeted agents that promote apoptosis. To date, such strategies have not been examined, however.
It has been reported recently that HT can induce autophagy via activation of nuclear factor kappa B (NFκB) as a protective mechanism against cell death.86 Therefore it would make sense to consider combining agents that inhibit NFKB activation with HT as a means of enhancing thermal cell killing. Alternatively, the combination of HT at 43° C with increased oxidative stress can increase cell killing via inhibition of the Akt pathway and induction of irreversible autophagy.87 Pajonk and coworkers88 reported that inhibition of proteasome activity in combination with HT at 44° C for 60 minutes was particularly effective in augmenting apoptosis, and, in addition, caused substantial radiosensitization.
There has been some effort, however, related to the idea of using HER2-targeted thermosensitive liposomes to enhance specificity of drug delivery to HER2-positive tumors.89 Alternatively, magnetic nanoparticles have been coated with HER2 antibodies to enhance delivery to tumor cells, where they can induce HT when placed in a high-powered magnetic field.90,91 Targeted nanorods have also been investigated for therapeutic potential with HT.92
Factors to Consider when Combining Hyperthermia with Chemotherapy
Temperature Dependence
The degree of enhancement of cytotoxicity has been shown to be temperature dependent for many drugs.93 Combinations of camptothecins with HT have not shown consistent results in vitro. The interaction between these agents and HT is schedule and temperature dependent.94,95 In one report, temperatures up to 41.8° C increased the activity of topoisomerase II; this finding may explain the increased activity of these drugs at elevated temperatures.94
In Vitro Results May Not Predict In Vivo Activity
Tubulin-binding agents, such as taxol, show no evidence for interaction in vitro,96 but studies of these agents in combination with radiation therapy in vivo are more encouraging.97 There may be physiologic consequences of the combination of taxol and HT that make the combination work better than predicted from in vitro studies.
Sequencing
For most drugs (excluding 5-fluorouracil [5-FU] and perhaps other antimetabolites), the optimal sequence for heating and drug therapy is to administer both simultaneously or to give the drug immediately before the onset of heating. For platinum-containing drugs, the tissue extraction rate is increased with HT, further substantiating the rationale for use of this sequence.98 Most antimetabolites do not interact with HT when given concomitantly.21 However, 5-FU has been shown to interact supra-additively with HT by maintaining temperatures between 39° and 41° C. Temperatures in this range lead to enhanced conversion to active metabolites, thereby increasing drug cytotoxicity. In addition, continuous infusion protocols with this drug may lead to cell cycle block in S phase, a part of the cell cycle that is relatively sensitive to HT.99
Immunologic Effects of Hyperthermia
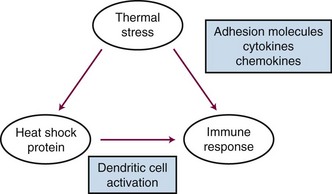
Heat shock proteins have been recently recognized for their potential role in regulating immune responses. There are several recognized functions of these proteins: (1) They are known to bind, in a noncovalent fashion, to immunogenic peptides. (2) When tumor cells are exposed to HT, heat shock protein-peptide complexes are presented on the cell surface. These complexes can be recognized by antigen-presenting cells (dendritic cells) via major histocompatibility complex (MHC) class I molecules. Once dendritic cells have received this type of stimulus, they migrate to lymph nodes, where they prime T-cell lymphocytes to be cytotoxic toward cells that express the peptide-heat shock protein complex. HT has been shown to enhance the rate of dendritic cell migration.100 (3) Heat shock proteins also induce dendritic cell maturation and proinflammatory cytokine release.101,102 (4) Cell membrane localization of heat shock proteins also activates the innate immune system by activating natural killer (NK) cells.103 Other sources of cellular stress such as viral infection, fever, hypoxia, and radiation exposure have been shown to up-regulate heat shock proteins as well. Because this process appears to occur naturally, there have been efforts to exploit the use of HT to produce tumor-derived vaccines and to augment the in vivo response to such vaccines.102 HT has also been reported to up-regulate a number of proinflammatory cytokines and adhesion molecules that facilitate immune cell trafficking across endothelial cells to gain access to the tumor interstitium.104 Additionally, shed heat shock proteins, with or without associated peptides, may act as chemokines to attract immune cells (particularly, macrophages) toward a region of tissue that has undergone heat stress.105 A summary of some of the key immunologic effects of HT is shown in Figure 21-4.
Hyperthermia Physics and Engineering
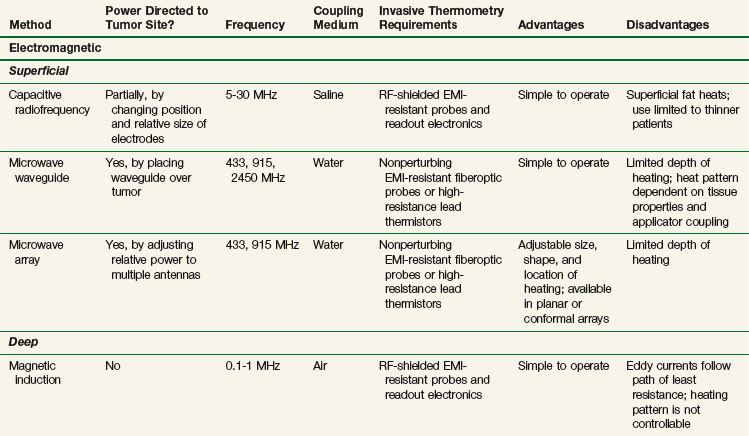
Clinical HT is usually achieved by exposing tissues to nonionizing radiation (e.g., electromagnetic [EM] or ultrasonic [US] fields) or by conducting heat into tissue from a heated source (e.g., hot pad or needle). Although these modalities deposit energy in tissue by different physical mechanisms, they have general similarities. Uniformity of heating is sensitive to the heterogeneity of tissue properties, geometry of blood flow, and practical problems of coupling the energy source into tissue. HT can be delivered noninvasively with externally applied power sources or invasively by placing heat sources either interstitially or inside natural body cavities. A brief presentation of noninvasive methods is provided below and is summarized in Table 21-1. Invasive methods have been developed extensively and include radiofrequency electrodes, microwave antennas, hot water tubes, ferromagnetic implant rods or seeds, and ultrasound transducers. Further details on all methods are available elsewhere.*
Electromagnetic Heating
Electromagnetic Heating Devices
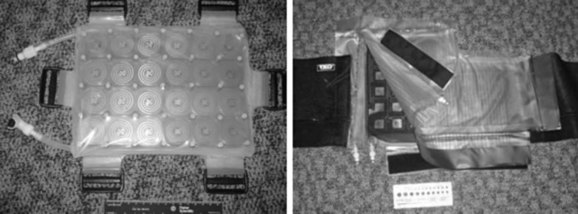
Electromagnetic heating devices can be separated into two categories: superficial applicators with effective penetration into tissue of less than 4 cm, and deep heating devices that have effective penetration of more than 4 cm. Superficial devices include waveguides (Fig. 21-5) and microstrip or patch antennas (Fig. 21-6) operating at microwave frequencies of 433, 915, or 2450 MHz.111,112,113,114 Microwave energy is usually coupled into tissue through a temperature-controlled de-ionized water bolus to maintain the skin temperature below 44° C.
Capacitive Heating Technique
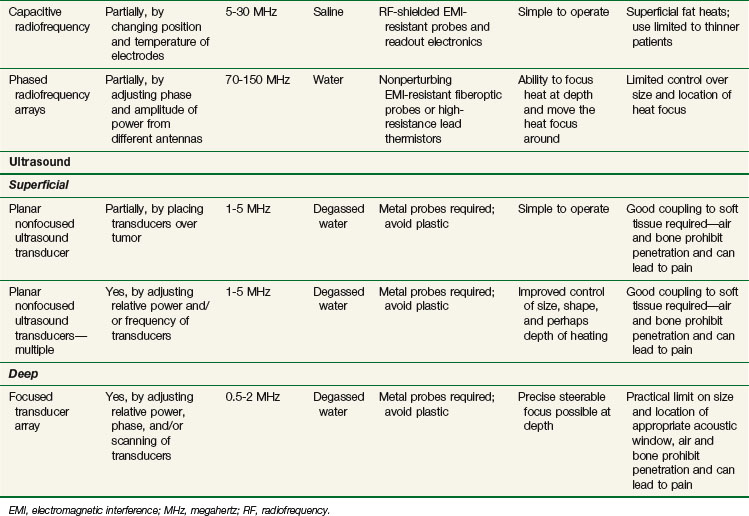
The capacitive heating technique uses radiofrequency fields of between 5 and 30 MHz to generate electric current between two or more conductive electrodes. Heating tends to be concentrated nearest the electrodes, but temperature-controlled saline boluses are used to reduce hot spots on the skin surface and help cool superficial fat.115 Using different sizes of electrodes shifts the maximum power deposition toward the smaller electrode. This technique can be used with large electrodes on the skin116,117 and one or more small intracavitary electrodes such as a balloon electrode for esophageal tumors.118 The thickness of the superficial fat layer is a significant factor for this method because of extra heating in high-resistance fat tissue relative to deeper-lying, high-conductivity soft tissues. To date, radiofrequency capacitive heating has been used most widely in Asia, where patients tend to be thinner.
Radiofrequency Phased Array
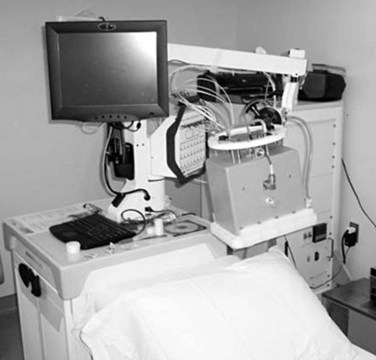
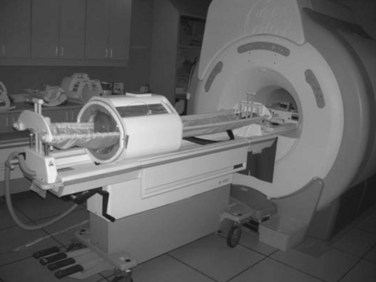
The third option for heating with electromagnetic fields is the radiofrequency phased array.119,120,121,122 These devices consist of an array of radiofrequency antennas arranged in a geometric pattern surrounding the target body region.123 Antennas are driven with multiple independently controllable power amplifiers but use a common radiofrequency source, allowing the radiofrequency fields from all antennas to add together to form a focus in the center of the array. The focus can usually be shifted off center and into the tumor by variation of the relative phase of the signals driving each amplifier. For an array with multiple antennas in phase, one can achieve better and deeper power deposition into tissue than is possible with operation of the antennas independently (i.e., driving the antennas without phase addition). By varying the phase and amplitude of multiple antennas, the phased array technique has more flexibility for controlling power deposition than the magnetic induction and capacitive techniques, which depend heavily on tissue properties and have few power control variables. Figure 21-7 shows one example of a 12-antenna annular phased array applicator mounted on the patient table of an MRI system. This MR-compatible 100-MHz radiofrequency phased array applicator fits inside the bore of a 1.5-T magnet, allowing MR imaging of a temperature rise inside the body during HT treatment.
Ultrasound Heating
Energy is coupled into tissue using temperature-controlled degassed water. Single- and multiple-transducer unfocused devices have been designed for heating superficial tumors (2 to 5 cm); these devices typically operate in the 1- to 5-MHz range.110,124–126 Deep heating with ultrasound is accomplished by using scanned focused transducers, phased arrays, or multiple scanned focused transducers, normally in the 0.5- to 2-MHz range.127,128
Measurement of Temperatures during Hyperthermia
Invasive Thermometry
Invasive thermometry, the current standard, involves physically placing thermometry probes into the tumor within implanted needles or catheters to read subsurface temperatures.129,130,131 Although the accuracy of invasive thermometers is sufficiently precise (typically, ±0.2° C) to resolve important differences in thermal dose (as described above), this type of thermometry has many disadvantages. Disadvantages include discomfort to the patient and risk of hemorrhage and/or infection, the expense of physician time required for catheter placement, and the necessity for imaging to verify placement of thermometers. In addition, the sparse nature of the data obtained makes it difficult to spatially control power deposition or to alter treatment to improve temperature distributions.132,133 The most common method for doing invasive thermometry is to insert blind-ended catheters into a tumor, using either ultrasound or computed tomography (CT) guidance.130 Alternatively, for deep-seated tumors, thermometers may be placed inside orifices that are surrounded by tumor, such as the rectum, urethra, or cervix.134,135 Thermometers are then placed inside these catheters during treatment. The probes can be multipoint sensors that remain fixed in place, or the sensors can be moved cyclically during treatment to record temperatures at many points along the catheter. There are guidelines published for how these measurements should be taken for all HT devices.130,136–138 In spite of its sparse nature, invasive thermometry can provide valuable information about the quality of a treatment. A number of clinical reports have correlated temperature-related parameters to clinical response.66,139,140,141–144