(1)
Department of Surgery, Dar A lAlafia Medical Company, Qatif, Saudi Arabia
21.1 Introduction
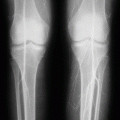
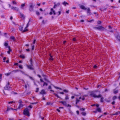
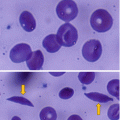
Sickle cell anemia is one of the common hemoglobinopathies worldwide.
Till now, there is no widely available cure.
The current management is directed toward relieving their symptoms and treating complications.
The goals of treating patients with sickle cell anemia include:
To relieve pain
To prevent infections
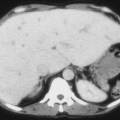
To prevent organ damage
To prevent stroke
To control and treat complications
The modern management of sickle cell anemia is based on these therapeutic approaches:
Symptomatic treatment of painful crisis
Blood transfusion
Immunization and penicillin therapy
Treatment of complications
Hydroxyurea
Bone marrow transplant
It was found that patients with sickle cell anemia and high levels of HbF have a relatively milder disease than those with low HbF. Fetal hemoglobin helps prevent red blood cells from sickling and improves anemia.
Increased fetal hemoglobin (HbF) production has long been recognized as one of the key factors which can ameliorate SCA, and in the 1970s, 5-azacytidine was investigated as an HbF promoting agent because of its potential ability to reactivate silenced γ-globin genes by inhibiting the methylation of deoxycytidine.
Although 5-azacytidine successfully increased HbF levels in patients with SCA and thalassemia, it was found to be relatively toxic.
Hydroxyurea was used to treat leukemia and showed particular activity in myeloproliferative disorders.
Hydroxyurea was also found to promote HbF synthesis.
Hydroxyurea was developed as a safer alternative to 5-azacytidine and showed significant increase in both HbF and total hemoglobin.
In 1995, a multicenter study evaluated the value of hydroxyurea for patients with sickle cell anemia. In this, the hydroxyurea group showed reductions in the rate of acute pain (median 2.5 vs. 4.5 episodes per year, P < 0.001), acute chest syndrome (25 vs. 51, P < 0.001), and blood transfusion (48 vs. 73, P < 0.001) when compared to the placebo group.
Hydroxyurea also reduced the number of hospitalizations (P = 0.0016) and total days in hospital (P = 0.0027) in patients with sickle cell anemia.
There was also an increased survival associated with long-term hydroxyurea use. A 40 % reduction in mortality was reported in those who continued using hydroxyurea after 9 years (P = 0.04).
Blood and bone marrow stem cell transplants:
This may offer a cure for a small number of patients with sickle cell anemia.
Blood and bone marrow stem cell transplants usually are used for young patients who have severe sickle cell anemia.
The transplant must come from a closely matched donor. This limits the number of people who may have a donor.
The transplant is expensive and not readily available.
The transplant is risky and can lead to serious side effects or even death.
Research on blood and bone marrow stem cell transplants, gene therapy, and new medications for sickle cell anemia is ongoing.
Gene therapy is being studied as a possible treatment for sickle cell anemia.
Researchers are also studying several medications for the treatment of sickle cell anemia. They include:
Decitabine
Adenosine A2a receptor agonists
5-HMF
Butyric acid
Clotrimazole
Nitric oxide
21.2 Hydroxyurea
Hydroxyurea was first synthesized in 1869 in Germany by Dressler and Stein.
A century later, phase I and II trials began testing the safety of this drug in humans with solid tumors.
It was first approved by the FDA in 1967 for the treatment of neoplastic diseases.
In subsequent years, clinical trials demonstrated the efficacy of this drug for the treatment of chronic myeloid leukemia, psoriasis, and polycythemia vera.
In February 1998, hydroxyurea was used for the treatment of sickle cell anemia.
It is approved for use in reducing the frequency of painful crises and the need for blood transfusions in adult patients with recurrent moderate-to-severe painful crises (generally at least three during the preceding 12 months).
Hydroxyurea is also approved for use in the treatment of melanoma, resistant CML, and recurrent, metastatic, or inoperable carcinoma of the ovary.
21.3 Mechanism of Action
The precise mechanism by which hydroxyurea produces its varied effects is unknown.
Assays conducted in cell-free bacterial systems have demonstrated that its target is the enzyme ribonucleotide reductase, with hydroxyurea acting as a free radical that is specific for the tyrosyl groups of this enzyme.
Ribonucleotide reductase is essential for deoxyribonucleic acid (DNA) synthesis, and its inhibition by hydroxyurea results in S-phase cell cycle arrest.
Hydroxyurea is an S-phase cytotoxic agent which does not demethylate DNA; it is thought to directly reduce DNA synthesis by inhibiting ribonucleotide reductase activity.
This nonspecific interruption of the cell cycle probably accounts for most of the HbF promoting activity.
Hydroxyurea acts on both early and late erythroid progenitors to increase the total intracellular hemoglobin, γ-globin mRNA, and HbF levels.
Other mechanisms may be responsible for the fact that this drug acts as a radiation sensitizer, inhibiting the repair of damaged DNA.
The efficacy of hydroxyurea in the treatment of sickle cell anemia is generally attributed to its ability to increase the levels of fetal hemoglobin (HbF, α2γ2).
This lowers the concentration of Hb S resulting in less polymerization of the abnormal hemoglobin.
However, the mechanisms by which it increases HbF are unclear.
Early studies suggested that hydroxyurea is cytotoxic to the more rapidly dividing late erythroid precursors, an effect that leads to the recruitment of early erythroid precursors with an increased capacity to produce HbF.
Others have suggested that it acts directly on late precursors to reprogram them to produce HbF.
Alternatively, it may interrupt the transcription factors that selectively bind to promoter or enhancer regions around the globin genes, thereby altering the ratio of Hb A to HbF.
There is also evidence that hydroxyurea can act as a nitric oxide donor and increase cGMP levels which accelerates translation of the γ-globin gene.
A recent study has provided evidence for a nitric oxide-derived mechanism for HbF induction by hydroxyurea.
Another study has suggested that it increases HbF production by inhibiting ribonucleotide.
Hydroxyurea may be of benefit in sickle cell anemia for reasons unrelated to HbF production, including:
Its ability to increase the water content of red blood cells
Decrease the neutrophil count
Alter the adhesion of red blood cells to the endothelium
The effects of hydroxyurea on the blood in SCA are dose dependent and include:
Increases HbF levels
Increases erythrocyte volume
Increases hemoglobin content
Decreases reticulocyte count
Decreases white cell count
Increases segmentation of the neutrophil nucleus
Reduces RBC dehydration by increasing their water content
Reduces phosphatidylserine exposure
Reduces expression of adhesion molecules and alters the adhesion of red blood cells to the endothelium
The clinical benefit of hydroxyurea is directly related to the increased HbF levels and reduced hemoglobin polymerization within the red cell, resulting in reduced red cell damage.
Hydroxyurea acts directly on vascular endothelium to decrease the expression of some adhesion molecules, which is clearly independent of any effects on the β-globin gene family.
Hydroxyurea may be more effective in preventing acute pain caused by microvascular complications than cerebrovascular disease in large blood vessels.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree