49.1
Aging and human immunodeficiency virus
With the availability of effective antiretroviral therapy (ART) in the mid-1990s, human immunodeficiency virus (HIV) transformed from a fatal disease into a manageable chronic illness in many parts of the world. Prior to this, HIV ranked among the most significant sources of morbidity and mortality among young people in the United States . With current ART, sometimes referred to as highly active ART (HAART), life expectancy among people living with HIV (PLWH) is approaching that of their HIV-uninfected peers . Nevertheless, even with the availability of increasingly less toxic and less cumbersome therapies, an important survival gap remains between people living with and without HIV infection .
In addition to the changes in survival, there has been a notable transition in the types of diseases affecting PLWH. Non-AIDS conditions, such as cardiovascular disease, liver disease, and cancer, have eclipsed AIDS conditions as the major source of morbidity and mortality among PLWH on ART . Another consequence of the success of ART has been a shift in the demographics of the HIV population. The majority of PLWH in the United States is >50 years and is expected to increase to >70% by 2035 . As a result, the management of aging-related conditions and comorbidities, such as osteoporosis, has become a critical part of HIV care. The risk of many of these conditions appears to be higher compared to HIV-uninfected peers, and their appearance is generally at an earlier age , leading some to speculate whether those with chronic HIV infection represent a phenotype of “accelerated aging,” although this notion is controversial . It is clear, however, that the pathogenesis of non-AIDS-related comorbidities, including osteoporosis, is complicated and results from a poorly understood interaction between HIV disease-related factors (e.g., inflammation/immune activation), ART factors, and host factors, both environmental and genetic.
49.2
Human immunodeficiency virus medicine 101
HIV-1 and HIV-2 belong to the retrovirus family and contains a single-stranded RNA genome. Infection of the host cell is initiated by the binding of the surface envelope protein of the virion to the T-lymphocyte CD4 molecule and a coreceptor, either the chemokine receptor CCR5 or CXCR4 on the surface of the target cell. Binding of the coreceptor allows the fusion of the virus and host cell membranes and entry of viral contents into the cytoplasm. Reverse transcription (copying of the viral genetic material from RNA to DNA) occurs, and then the copied DNA is inserted into the host chromosomal DNA by the viral enzyme integrase. The integrated DNA (provirus) can remain latent for years before becoming active through transcription. The transcribed viral RNA serves as genetic material for new virions, as well as critical viral enzymes such as the protease that serves to cleave viral proteins into functional components.
Transmission of HIV occurs through sexual exposure, injection drug use, mother-to-child transmission, and rarely through transfusion of contaminated blood products and occupational exposure. HIV infects immune cells, such as CD4+ (helper) T lymphocytes, macrophages, and dendritic cells and leads to progressive failure of the immune system through direct killing of the infected cells, increased rates of apoptosis in infected cells, and cytotoxic killing of infected cells. Early during the course of HIV infection, many of the immune cells in the gut-associated lymphoid tissue (GALT) are destroyed and do not regenerate despite ART. After acute HIV infection a relative equilibrium between viral replication and the host’s cell-mediated immune response is established such that a viral “set point” is reached. During this clinically “latent” period, however, HIV replication remains active, and the CD4+ T-cell counts drop by approximately 50–90 cells/μL per year, accelerating over time. When CD4+ T cells drop below 200 cells/μL, the individual is at imminent risk for developing opportunistic infections without ART. Mean survival after diagnosis of AIDS by either CD4+ T-cell count <200 cells/μL or an opportunistic infection or malignancy was less than 1 year prior to availability of ART .
Current HIV therapies inhibit the virus at the stage of binding and entry (CCR5 antagonists, postattachment inhibitors, and fusion inhibitors), reverse transcription [nucleoside reverse transcription inhibitors (NRTIs) and non-NRTIs (NNRTIs), respectively], integration (integrase strand transfer inhibitors, INSTIs), and protein cleavage [protease inhibitors (PIs)]. There are multiple FDA- and EMA-approved antiretrovirals in these classes, including fixed-dose combinations, but some antiretrovirals are seldom used. Current guidelines recommend initiation of therapy with combinations of two NRTIs [tenofovir disoproxil fumarate (TDF)/emtricitabine (FTC), tenofovir/alafenamide, or abacavir/lamivudine] with either an INSTI, NNRTI or PI, with alternative regimens for other specific concurrent conditions . The US DHHS guidelines recommend NRTIs plus INSTIs in fixed-dose combinations as initial regimens for most people with HIV . In addition, tenofovir alafenamide (TAF) is favored over TDF given fewer bone and kidney toxicities . Current guidelines also recommend initiation of ART irrespective of CD4+ T-cell thresholds, given the recognition of the role that chronic inflammation mediated by HIV infection has on non-AIDS mortality, improvements in safety and tolerability of newer ART regimens, and the potential for the public health benefits of decreasing HIV transmission with earlier viral suppression. Since eradication of HIV is not currently possible, treatment involves lifelong ART to maintain suppression of HIV viral replication, restored CD4+ T-cell count levels, and improved immune function.
49.3
Epidemiology of low bone mineral density in human immunodeficiency virus
Several years into the HAART era, reports began to emerge regarding the prevalence of low bone mineral density and osteoporosis among PLWH . A metaanalysis of cross-sectional studies performed between 1966 and 2005 showed that the overall prevalence of osteoporosis in the HIV-infected persons was 15%, which was more than three times more common in HIV-infected persons compared to age-matched HIV-uninfected controls (OR 3.7, 95% CI 2.3–5.9) . Subsequent studies have found a similar high prevalence of osteoporosis in HIV-infected populations . As described next, the higher than expected prevalence of low bone mineral density (BMD) in HIV-infected patients is multifactorial, with contributions from the effects of chronic infection with HIV, antiretroviral therapies, and patient-specific comorbidities (e.g., hypogonadism), behaviors (e.g., cigarette smoking and opiate use), and coinfections [e.g., hepatitis C virus (HCV)] ( Table 49.1 ).
| HIV disease factors | Antiretroviral therapy | Patient factors |
|---|---|---|
| Low CD4 cell count | Tenofovir (TDF) | Older age |
| HIV duration | Zidovudine (AZT) | Male gender |
| Inflammation/immune activation | Protease inhibitors | Low body weight/lean mass |
| Lopinavir/ritonavir | Hepatitis C coinfection | |
| Atazanavir/ritonavir | Opiate use | |
| Antiretroviral initiation (first 2 years) | Smoking | |
| Hypogonadism | ||
| Vitamin D deficiency | ||
| Concomitant medications | ||
| Glucocorticoids a | ||
| Thiazolidenedione | ||
| Proton-pump inhibitor | ||
a Glucocorticoid concentrations may increase with concomitant protease inhibitor use due to CYP3A4 interaction.
Longitudinal changes in BMD in PLWH compared to HIV-uninfected controls:
In the general population, aging men and women lose bone at an average rate of 0.5%/year and women experience a transiently accelerated 1%/year loss of BMD in the first few years after menopause . In middle-aged PLWH, prospective cohort studies investigating the longitudinal changes in BMD over time have generally shown stable or increasing BMD . In a metaanalysis of six longitudinal cohorts, Bolland et al. reported similar rates of bone loss at the total hip or femoral neck between HIV-infected persons, mostly receiving ART, and controls followed for 1.7–2.7 years . Most of these studies have examined relatively young populations in whom BMD changes are not pronounced. In a study of BMD change in postmenopausal women , HIV-infected women on stable treatment were found to have greater loss of BMD compared to matched HIV-uninfected women, with annualized rate of bone loss 2.3–3.7-fold higher in women with HIV infection depending on the anatomic site. In another study of a single HIV clinic in Italy, BMD declined at approximately twice the rate in middle-aged women compared to men over 4–5 years, even after adjustment for key covariates, including age and menopausal state . Further longitudinal studies are needed to understand bone loss as PLWH enter their seventh decade and beyond.
49.3.1
Fracture risk in human immunodeficiency virus
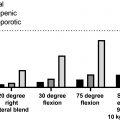
A critical question is whether lower BMD results in increased fracture risk among PLWH. Data from two large health-care database studies suggest that prevalence of ICD9-coded or self-reported fractures is greater among PLWH than in the general population of similar age, sex, and race , especially among older individuals ( Fig. 49.1 ). In addition, two other database studies found the incidence of fragility and nonfragility fractures was higher among PLWH than in the general population . Subsequent studies have utilized cohorts with individual-level data, to determine whether increased risk of fracture is attributable to increased prevalence of traditional fracture risk, HIV infection or ART exposure. A study of the United States male veterans found a higher rate of fracture among HIV, but the effect was attenuated after adjustment for weight . The Multicenter AIDS Cohort Study (MACS), a prospectively recruited cohort of men with or at risk of HIV, found a higher incidence of fracture (all fractures or fragility fractures) among men with HIV aged 50–59 compared to controls, but not in younger men . Similarly, the Women’s Interagency HIV Study, a prospectively recruited cohort of women with or at risk of HIV, found a higher fracture rate for all fractures in middle-aged women with HIV as compared to controls, even after adjustment for traditional risk factors . A recent metaanalysis of 10 studies found an odds ratio of 2.17 (95% CI 1.29–3.66) for all fractures (fragility and nonfragility) in PLWH . In addition, studies that have compared the prevalence of clinical or morphometric vertebral fractures suggest that risk of vertebral fractures is also higher among PLWH .
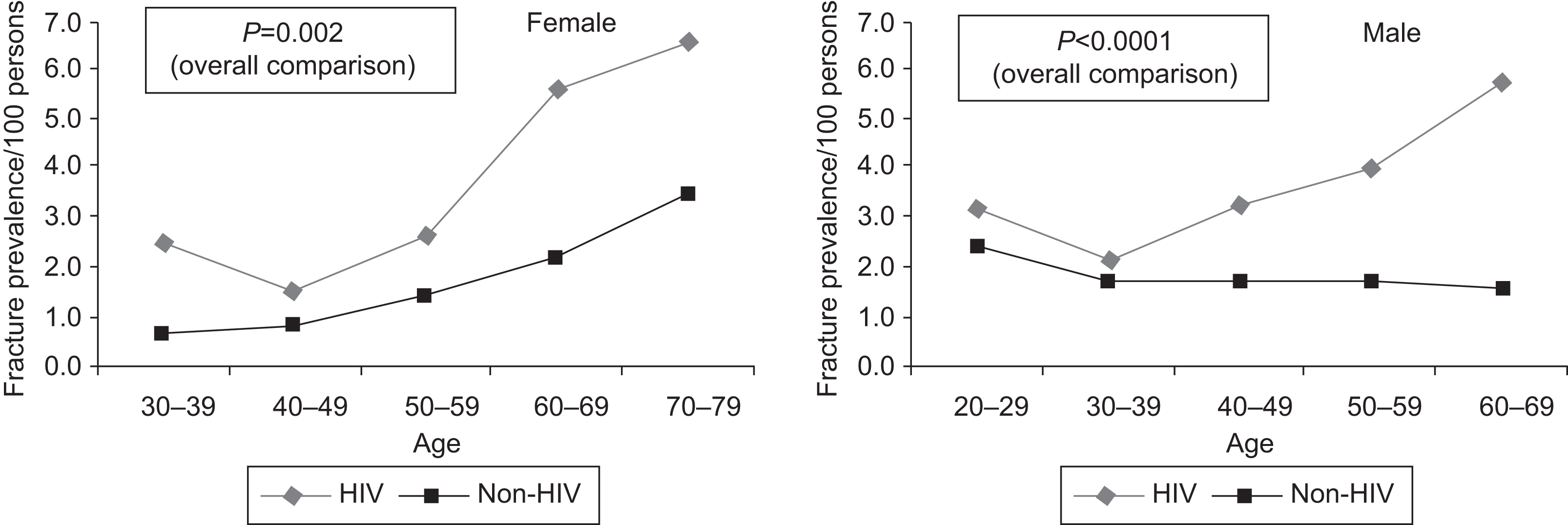
Several other large cohort studies examined the incidence and predictors of fracture among PLWH . Among PLWH the main predictors for fracture were traditional factors such as smoking , use of glucocorticoids or proton-pump inhibitors , alcohol or substance abuse , low weight/body mass index (BMI) , comorbidities such as diabetes and renal insufficiency , and HCV coinfection , as well as frailty and recurrent falls .
The overall burden of fracture among PLWH will likely increase as the population ages worldwide, despite improvements in ART management and a growing appreciation for the need for fracture prevention among PLWH.
49.4
Etiology of low bone mineral density in human immunodeficiency virus
49.4.1
Effects of chronic inflammation
The normal adult skeleton continually remodels via tightly coordinated activities of osteoblasts and osteoclasts, regulated by the Osteoprotegerin (OPG)/receptor activator of nuclear factor kappa-B (RANK)/RANK ligand (RANKL) system (see also Baron, Chapter 12 , WNTsignaling in skeletal homeostasis and diseases). The rate of bone resorption is determined by the relative amounts of RANKL and OPG produced by osteoblasts . Systemic hormones (estrogen, PTH, 1,25(OH) 2 D) and locally secreted cytokines (TNFα, IL-6) that increase bone resorption do so, in part, by increasing expression of RANKL by bone marrow mesenchymal stem cells (BMSCs) and osteoblasts. T cells are increasingly recognized as key regulators of bone remodeling in health and disease . Bone marrow T cells regulate bone remodeling via direct interactions with BMSCs and osteoblasts. Activated T cells express RANKL on their surface and produce soluble RANKL and other osteoclastogenic cytokines, such as TNFα, IL-6, and secreted osteoclastogenic factor of activated T cells . In particular, the Th17 subset of CD4+ T cells may be osteoclastogenic . It is well established that chronic infection and chronic inflammatory diseases, such as psoriatic and rheumatoid arthritis, lead to T-cell activation and production of RANKL and TNFα.
A hallmark of the chronic phase of HIV infection is generalized immune activation. There are several potential underlying mechanisms for activation of immune cells, including microbial product translocated from the GALT, persistent HIV replication and viral proteins exposure in lymphoid tissue, and host immune responses . Massive CD4+ T-cell depletion occurs in the GALT soon after HIV infection causing loss of mucosal integrity and function. Microbial products such as lipopolysaccharide, a potent activator of the immune system since it triggers the release of proinflammatory factors from macrophages and dendritic cells, are detected at higher levels in the circulation and associated with cellular markers of immune activation in patients with HIV infection . Soon after infection, HIV establishes latent viral reservoirs in lymphoid tissue. Low levels of ongoing viral replication occurring in these CD4+ T cells could also be a source of chronic immune activation. The consequences of generalized immune activation include skewing of lymphocytes toward more activated and differentiated subpopulations and increased cell turnover and induction of cellular exhaustion and senescence, polyclonal B-cell activation, and production of cytokines such as TNFα, IL-6, IL-1, IL-12, and IFN-α . Despite effective ART and viral suppression, the proportion of activated (CD38+) T cells remains higher than in uninfected controls .
Many investigators have hypothesized that inflammation, T-cell activation, and elaboration of proresorptive cytokines are the dominant mechanisms of HIV-associated bone loss. B cells are also an important source of OPG production . Disruption of T-cell and B-cell communication and B-cell function with HIV infection would also result in elevated RANKL and decreased OPG production, favoring osteoclastogenesis . Other evidence comes from in vitro studies demonstrating that exposure of T cells to gp120 or other HIV-1 viral particles results in T-cell activation and elaboration of RANKL, an effect that can be further potentiated by certain PIs . An HIV-1 transgenic rat model also provides some evidence for the association between inflammation and bone loss in HIV, without the influence of ART . In comparison to wild type, the HIV-1 transgenic rat has decreased bone size, trabecular volume, trabecular number, and increased trabecular spacing and bone marrow with increased osteoclast numbers on histology, as well as increased RANKL and decreased OPG mRNA expression in bone marrow and spleen .
Evidence for the causal role of inflammation in humans comes primarily from studies with serum/plasma measures of cytokines and either bone turnover or Dual-energy x-ray absorptiometry (DXA) data. In a study of ART-naïve PLWH, plasma RANKL and OPG levels were both increased in comparison to healthy controls, RANKL was positively correlated with plasma HIV-1 RNA levels, and subjects with low BMD had a decreased OPG/RANKL ratio . In PLWH not on ART, serum levels of soluble TNF receptor and C-telopeptide were elevated, while osteocalcin levels were decreased . After 24 months of ART, soluble TNF receptor levels decreased and osteocalcin levels increased, resulting in a high correlation of C-telopeptide and osteocalcin levels. These data suggest that bone remodeling is resynchronized after the suppression of viral replication and inflammation with ART . Other studies have described decreases in RANKL and OPG levels after treatment with ART . However, there is some evidence that proresorptive cytokines (TNF, RANKL) and OPG levels in PLWH on ART remain higher than levels in uninfected controls and remain associated with lower BMD . Similarly, some studies have demonstrated an association between higher levels of cellular markers of immune activation or senescence and low BMD in PLWH on ART in some , but not other studies .
Taken together, these studies indirectly suggest that inflammation associated with HIV infection plays a role in bone loss in acute infection, advanced untreated infection and after immune reconstitution with chronic suppressive ART; however, the magnitude of effect of inflammation and immune activation on bone resorption is variable, and less than that of antiretrovirals.
49.4.2
Effects of antiretroviral therapy
49.4.2.1
Protease inhibitors
After the widespread uptake of HAART in the late 1990s, there was increasing recognition of metabolic abnormalities in PLWH with well-controlled HIV, including body composition changes (collectively known as lipodystrophy), dyslipidemia, and insulin resistance. Although some of the NRTIs, namely, stavudine and zidovudine, were implicated in the pathogenesis of these metabolic comorbidities, most of the concern focused on the medications in the PI class.
Medications in the PI class were also the first implicated in the pathogenesis of bone loss in HIV-infected persons. A 2006 metaanalysis of cross-sectional studies also showed that PLWH receiving PIs had a higher prevalence of low BMD compared to non-PI-treated patients . From the available data at the time, it could not be determined if other differences existed between PI- and non-PI-treated patients (e.g., longer HIV duration, lower CD4 cell counts) that may have confounded the relationship between PI use and BMD. Subsequent cross-sectional studies have confirmed these findings .
The most robust data regarding the BMD effects of PIs come from randomized controlled trials that compare PIs to non-PIs. In ACTG 5224s, ART-naïve, PLWH randomized to an initial regimen containing the PIs, atazanavir/ritonavir (ATV/r), had a significantly greater decrease in lumbar BMD compared to those randomized to the NNRTI, efavirenz at 96 weeks (−3.1% vs −1.7%, P =.035) . However, the change in total hip BMD was similar in the two groups (−3.4% vs −3.1%, P =.61). Whether these differences in the effect of the PI by site are related to a specific effect on trabecular bone is not clear. In ACTG 5260s, ART-naïve, PLWH were randomized to TDF/FTC plus either ATV/r (a PI regimen), darunavir/ritonavir (DRV/r, a PI regimen), or raltegravir (an INSTI regimen) . Bone loss over 96 weeks did not differ in the PI arms but was greater in the combined PI arms compared to the raltegravir arms at the spine and the hip [spine: −3.8% vs −1.8% ( P <.001); hip: −3.7% vs −2.4% ( P =.005)]. In another study, examining the BMD effects of ART initiation with PI versus INSTI, PLWH were randomized to either TDF/FTC plus elvitegravir/cobicistat (a INSTI-based regimen) or TDF/FTC/ATV/r, and bone loss was similar over 96 weeks each arm (hip: −3.16 vs −4.19, P =.069; spine: −1.96 vs −3.54, P =.049). It should be noted that both arms in this study contained a potent cytochrome P450 (CYP) 3A4 inhibitor used as a pharmacologic booster for either the PI or the INSTI. These drugs also boost by approximately 30%, the concentrations of TDF, which has a known detrimental effect on BMD (see next). It is possible that some of the negative BMD effects attributed to PIs, which are most often combined with potent CYP3A4 inhibitor (e.g., ritonavir), are mediated through the elevated TDF concentrations .
Similar to the heterogeneous effects of PIs on BMD, mixed results have been observed in epidemiologic studies that have examined the association between PIs and fracture . The largest of these studies, however, using the Veteran’s Administration Clinical Registry showed that cumulative exposure to PIs was associated with an increased fracture risk, HR 1.11 (95% CI 1.05–1.18, P <.001), although this effect was attenuated after adjustment for BMI and other variables to 1.05 (0.97–1.13, P =.24) . Of the PIs examined, lopinavir/ritonavir, an older but previously highly prescribed PI, was associated with a 9% increased risk of incident wrist, hip, or vertebral fracture for each year of exposure . More recently, two large European epidemiologic studies showed no association between PI use and fracture .
The mechanisms underlying the effect of PIs on bone metabolism are not clear and in vitro studies have shown mixed effects. PIs have been shown to have variable effects on osteoblast and osteoclast function in in vitro models . In addition, in vitro studies have shown that certain PIs, including ritonavir, indinavir, and nelfinavir, impair 1-alpha hydroxylase activity and the production of 1,25 dihydroxyvitamin D . Clinical studies examining the effect of PIs on vitamin D metabolism, and its downstream effect on bone are limited.
49.4.2.2
Tenofovir
TDF has been one of the most widely used antiretroviral worldwide. TDF has also been specifically associated with bone loss. In studies of PLWH initiating ART, TDF has been independently associated with a 1%–2% decrease in BMD compared to other NRTIs . In a randomized controlled clinical trial, for example, those who were randomized to TDF had a 2% larger decrease in the spine and a 1.4% larger decrease in the hip over 96 weeks compared to those receiving another commonly used NRTI, abacavir . The effect of TDF on bone occurs quickly, peaks at 24–48 weeks, but does not return to baseline with continued therapy. A similar effect of TDF was observed in treatment-experienced virologically suppressed subjects randomized to FTC/TDF or ABC/3TC . TDF is also approved for use in HIV-uninfected persons for HIV preexposure prophylaxis, and recent randomized controlled trials of TDF-based regimens in this population have showed a statistically significant BMD decrease over 48 weeks of approximately 1% , confirming an HIV-independent effect of TDF, although this effect on bone is reversible with TDF discontinuation .
The clinical relevance of the TDF-induced bone loss has not yet been clearly established. None of the current randomized trials comparing TDF to other agents has been adequately powered to address whether TDF increases the risk of fracture . Cohort studies investigating the relationship between TDF exposure and fragility fracture have had mixed results. The Veterans Affairs Clinical Case Registry, which aimed to determine the effect of cumulative TDF compared to other ART on the risk of fragility fractures in HIV-infected persons, found an increased risk of incident fracture with cumulative exposure to tenofovir in the HAART era [HR 1.12 (95% CI 1.03–1.21; P =.01)], independent of traditional bone risk factors, HIV severity, or exposure to other ART , suggesting an important clinical impact. In a large cohort study in Europe, fracture risk was higher in those with current (HR 1.25; 1.05–1.49) or ever (HR 1.40; 1.15–1.70) TDF use . In contrast, a large case–control study from France showed no association between TDF use and fracture .
Although the effect of TDF on bone has been consistent in the randomized trials and may increase the risk of fracture, the underlying cause has not been elucidated. One of the leading hypotheses is that TDF induces subclinical phosphate wasting leading to impaired bone mineralization and lower BMD. TDF is associated with impaired bone mineralization in nonhuman primates and also has been associated with renal phosphate wasting, a manifestation of tubular dysfunction in humans . Although severe hypophosphatemia is uncommon in TDF-treated patients , an observational study showed that subclinical urinary phosphate wasting was seen in 41% of TDF-treated subjects and was correlated with increased alkaline phosphatase . In addition, other factors may influence the effect of TDF on phosphate handling. Vitamin D deficiency may also modify the effect of TDF on renal phosphate handling. In cross-sectional studies, bone turnover markers in TDF-treated persons were higher in those with vitamin D deficiency, and the use of TDF has been associated with increases in parathyroid hormone and higher bone turnover. As an alternate mechanism, in vitro studies have suggested that TDF may have direct effects on osteoblast function .
A new form of tenofovir, TAF, was approved by the FDA in 2016. TAF is a prodrug of tenofovir that is converted to intracellularly to active tenofovir in peripheral blood mononuclear cells, such as lymphocytes. As a result, much lower doses of TAF can be given to achieve the same virologic efficacy compared to TDF. These lower plasma concentrations of tenofovir result in less bone toxicity compared to TDF. In antiretroviral naïve PLWH, ART initiation with a TAF-containing regimen leads to less bone loss compared with TDF. In a metaanalysis of five randomized controlled trials, the mean percentage decrease in BMD over 48 weeks was less in TAF group compared to the TDF group at both the hip (−0.7% vs −3.25%, P =.005) and the spine (−1.29% vs −3.28%, P =.002), accompanied by a smaller increase in bone turnover markers . Similar results are seen in ART-experienced PLWH with virologic suppression on a TDF-containing regimen who switch to TAF, with BMD at the spine and the hip increases by approximately 2% over 96 weeks after the switch and remained stable in those who stayed on TDF . The largest differences in BMD between TAF and TDF are observed in PLWH who are also receiving the pharmacologic booster ritonavir or cobicistat . While the BMD differences between TAF and TDF are well established, it is unclear whether TAF is associated with a decrease risk of fragility fracture compared to TDF.
Antiretroviral therapy initiation
Initiation of effective ART has multiple benefits in reducing the incidence of AIDS events. In addition, control of the HIV virus may also have an impact on non-AIDS events, such as cardiovascular disease, prompting, in part, the recommendation for ART initiation at a higher CD4 cell count . ART initiation is also associated with physiological changes that would be predicted to have a salutary effect on bone metabolism, including reductions in systemic inflammation , increases in lean body mass , increases in testosterone among men , and decreases in RANKL concentrations . Early reports regarding the effects of ART on bone turnover documented an increase in both markers of bone formation and bone resorption and suggested that ART was associated with a resynchronization of osteoblast and osteoclast activity, resulting in net benefits to bone health .
Subsequent longitudinal studies have clearly demonstrated a consistent decrease in BMD of approximately 1%–6% in the 48–96 weeks after ART initiation, regardless of the type of ART initiated ( Fig. 49.2 ). This effect is greater than what would be expected with aging alone. In an analysis of PLWH initiating ART compared to age, sex, and gender-matched HIV-uninfected controls, annualized BMD loss in PLWH was greater at the spine (−0.76%/year vs 0.09%/year) and the hip (−1.56%/year vs −0.31%/year) with the greatest differences occurring in the first 96 weeks after initiating ART . The magnitude of bone loss with ART initiation is comparable to the bone loss seen in women ages 50–59 over 2 years . Further evidence of the negative effect of ART on BMD comes from the SMART study, which compared outcomes in HIV-infected persons who were randomized to stopping ART at a high CD4 cell count (350 cells/mm 3 ) and restarting when the CD4 cell count dropped below 250 cells/mm 3 or remaining on continuous ART. In the bone substudy of SMART, stopping ART was associated with either an attenuation of BMD loss or a BMD increase compared to continuous ART .
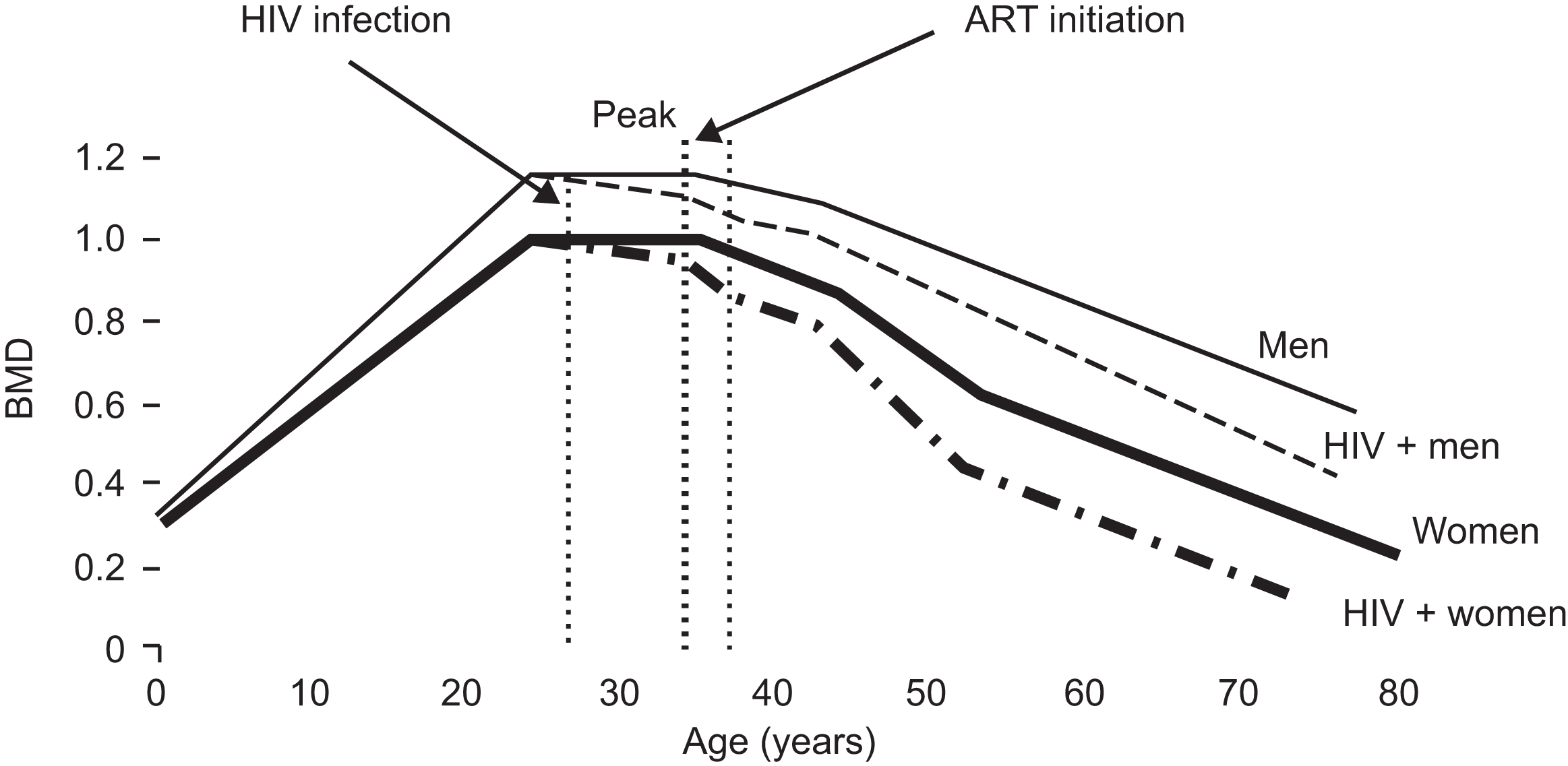
Early bone loss after ART initiation may be clinically relevant. In a nested, case–control study of HIV-infected persons initiating ART, the incidence of self-reported fractures was higher within the first 2 years after initiation of ART than in subsequent years , suggesting that BMD loss associated with ART initiation results in an immediate increase in fracture risk, similar, but likely lower in magnitude, to initiation of glucocorticoids.
Insights into the mechanisms underlying the bone effects of ART initiation can be obtained from an examination of longitudinal changes in bone turnover markers during this period. Prior to ART initiation, markers of bone resorption are generally in the upper range of normal, whereas markers of bone formation are relatively depressed, consistent with uncoupling of bone formation and bone resorption . With ART initiation, there is a rapid acceleration of bone turnover with marked increases in both markers of bone formation and bone resorption which is associated with bone loss . Increases in bone resorption markers are greater than increases in bone formation markers and increase at an earlier time point after ART initiation, creating a “catabolic widow” . Markers of bone turnover tend to level off after 48–96 weeks but do not return to baseline, which mirrors the effect on BMD.
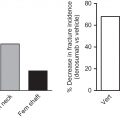
The mechanisms underlying the effect of ART initiation on bone are unclear. One consistent risk factor for both bone loss after ART initiation and increases in bone turnover is a lower pre-ART CD4 cell count . These findings suggest that CD4 cell depletion prior to ART initiation and subsequent immune reconstitution after ART leads to bone loss. The importance of T cells in the bone turnover is demonstrated by preclinical models that have shown preservation of BMD in ovariectomized, T-cell nude mice, which is lost after T-cell reconstitution . Similarly, in an animal model of HIV, T-cell repletion led to rapid acceleration of bone turnover and BMD loss . These preclinical results suggest that T cells may be critical in the regulation of bone turnover in the setting of HIV infection. The observation that low pre-ART CD4 is associated with greater bone loss upon ART initiation suggests that starting ART at a higher CD4 cell count would attenuate the bone loss related to ART initiation. Bisphosphonates at the time of ART initiation have been shown to be effective at preventing bone loss related to ART initiation. In a randomized clinical trial a single dose of zoledronic acid (5 mg) at the time of ART initiation was associated with a 1% increase in the lumbar spine over 144 weeks compared to a 4.3% decrease in the placebo group . Zoledronic acid may therefore be an important strategy to prevent bone loss with ART initiation in persons at higher risk of fracture because of older age, lower BMD, or other risk factors.
49.4.3
Other contributing factors
49.4.3.1
Low body weight and lean mass
In the general population, low body weight is a major risk factor for bone loss and fracture . Similarly, low body weight is an important risk factor for osteoporosis among HIV infection and explains some, but not all, of the differences in the prevalence of low BMD. In a metaanalysis of cross-sectional studies, low BMI accounted for a significant proportion of the difference in BMD between HIV-infected and -uninfected persons .
Chronic HIV infection may influence body weight, and this effect may be in the causal pathway between HIV status and bone loss. In cohorts that include HIV-infected and -uninfected populations, BMI is lower among HIV-infected participants, and longitudinal analysis suggests that age-related increases in BMI are attenuated in HIV-infected persons . It is unclear whether these differences in BMI are related to changes in fat mass or changes in lean mass. Lean body mass is tightly correlated to BMD in both the general population and the HIV-infected population . In general, HIV-infected populations have lower BMI and lean mass than HIV-uninfected control populations . In the FRAM study, for example , HIV-infected men, but not women, had lower muscle mass compared to their respective HIV-uninfected controls. One possible explanation for differences in lean mass by HIV serostatus is the effect of chronic HIV infection on muscle metabolism. Inflammatory markers, such as TNFα and IL-6, have direct effects on muscle breakdown, and these markers have been associated with sarcopenia in the general elderly population . In this way, increased inflammation associated with untreated HIV infection or residual inflammation associated with treated HIV infection may lead to increased muscle proteolysis ultimately resulting in decreased BMD .
49.4.3.2
Hepatitis C coinfection
In the United States, 1%–2% of the population has chronic HCV infection and up to 75% of infected persons are unaware of their infection . Infection occurs after exposure to infected blood through injection drug use or sexual transmission. After acute HCV infection, 50%–80% of HIV-uninfected individuals and more than 80% of HIV-infected individuals develop chronic infection , which may progress to cirrhosis or hepatocellular carcinoma. Chronic HCV coinfection may also contribute to the risk of osteoporosis and fracture in HIV-infected individuals. Hepatic osteodystrophy has many etiologies. The prevalence of osteoporosis in patients with chronic hepatic disease is highest among patients with chronic cholestasis and higher in patients with cirrhosis of any etiology. The pathogenic mechanisms are multifactorial and include lifestyle risks such as smoking, alcohol or drug use, low body weight, vitamin D insufficiency/deficiency, and hypogonadism, which may vary with increasing liver dysfunction.
Prevalence of osteoporosis is 14%–28% in HCV mono-infected and 19%–55% in HCV/HBV-coinfected individuals . The severity of osteoporosis may be related to severity of liver disease by histology even without cirrhosis . HCV mono-infection is also associated with increased fracture risk independently of cirrhosis . Furthermore, fracture risk decreases in postmenopausal women who were successfully treated for HCV mono-infection , suggesting that dysregulation of bone metabolism with chronic HCV mono-infection may be related to HCV viral replication or the inflammatory consequence of chronic infection.
Among HIV-infected individuals, Lo Re et al. reported lower BMD in HIV/HCV-coinfected than HIV-mono-infected women, but not in men . Data also indicate that HCV coinfection is an independent risk factor for incident fractures . The increased risk of fracture is approximately 1.5–2 times greater in HIV/HCV-coinfected than HIV-mono-infected individuals .
While the clinical impact of HIV/HCV coinfection on fracture risk is quite clear, the complex mechanisms of how chronic viral coinfections affect bone metabolism require further study. Studies have demonstrated higher levels of T-cell activation in HIV/HCV-coinfected individuals than HIV-mono-infected individuals , possibly augmented by increased microbial translocation in HIV/HCV-coinfected individuals . Chronic immune activation may also be associated with increased HIV and HCV viral replication resulting in the observed rapid progression to liver failure in HIV/HCV-coinfected individuals. Similarly, increased immune activation may result in enhanced bone resorption and greater bone loss and fracture among HIV/HCV-coinfected individuals. However, in a study of HIV/HCV-coinfected individuals, stage of fibrosis or necroinflammatory activity on liver biopsy was not associated with lower Z -score of the spine and hip . Since 2015, well-tolerated regimens to cure hepatitis C after an 8- to 12-week course have been available and widely used. It is unclear, however, whether cure of HCV also improves bone density and reduces fracture risk.
49.4.4
Gonadal dysfunction
Among HIV-infected men, low testosterone may contribute to low bone mass and fracture. Prior to the availability of effective ART, hypogonadism was observed in up to 50% of patients and was linked to HIV disease severity and the presence of HIV wasting . In the HAART era the prevalence of hypogonadism remains approximately 20% . It is known that testosterone concentrations increase after ART initiation , and that free testosterone concentrations are lower in HIV-infected men compared to HIV-uninfected controls .
In addition to the effects of chronic infection with HIV and the resulting inflammation on the gonadal axis , low testosterone in HIV may also be related to poor nutritional status, prescription drug use (opiates, megestrol acetate, steroids), HCV infection, or illicit drug use. Both primary and secondary hypogonadism have been reported in HIV-infected men . Low testosterone may affect BMD through multiple mechanisms. Lower testosterone in HIV-infected patients has been associated with lower lean mass and other comorbidities, such as diabetes mellitus . It is unclear in HIV-infected men the extent to which low testosterone relates to lower BMD and fracture risk. Long-term testosterone treatment is very common among men with HIV and has been associated with higher BMD, suggesting a long-term salutary effect on bone. In a substudy of the MACS, for example, 22% of the men with HIV ages 50–69 years reported testosterone use, which was associated with higher T -scores at the spine and hip (0.95 and 0.45, respectively) . However, as in the general population, it is unclear if testosterone use can prevent fractures among men with HIV.
Among women with HIV, data regarding gonadal dysfunction and early menopause are mixed. In the Women’s Interagency HIV Cohort, women with HIV experienced menopause at an earlier median age than HIV-uninfected women with similar risk factors (46 vs 47 years), and a greater proportion had menopause before age 40 , while another study found no difference . Comparisons of the age of menopause between women with and without HIV are complicated by differences in weight, opiate use, and ART that may alter normal cyclic production of LH and FSH by the hypothalamus and lead to irregular menses .
It is not clear whether menopause has a larger effect on BMD in women with HIV compared to HIV-uninfected controls. In contrast to longitudinal studies showing a similar rate of decline in BMD in premenopausal women with and without HIV, postmenopausal women with HIV were found to have a faster rate of BMD decline compared to matched HIV-uninfected controls . As women with HIV age with HIV, it will be important to determine whether these differences translate into an increased risk of fragility fracture.
49.4.5
Alcohol, drugs, smoking
Many studies have found that the frequency of cigarette smoking, alcohol, and drug use is higher among PLWH than the general population. While the prevalence of cigarette smoking in the United States has declined to approximately 20%, recent surveys of PLWH in New England and New York found that 70% and 59%, respectively, of respondents were current smokers . Illicit drug use is also higher among PLWH. In a cross-sectional study, 86% of PLWH reported lifetime use of illicit drugs as compared to 57% of controls . In addition, there is a significant association between smoking status, excessive alcohol use, and cannabis consumption .
Cigarette smoking and alcohol use have been clearly associated with both increased risk of osteoporosis and fracture. Opiates have been associated with increased fracture risk in the general population , but whether this is mediated by hormonal dysregulation or by increased risk of falls has not been clearly defined.
Among PLWH, cigarette smoking has been found to be associated with either lower bone density or fracture in multivariate models. Similarly, increased alcohol use was also associated with lower bone density or fracture in multivariate models. In most studies, HIV status remains a risk factor for either low BMD or fracture even after adjustment for cigarette use, alcohol, and illicit drug use, suggesting that despite the increased prevalence of these factors amongst PLWH they do not wholly explain the observed group differences in the prevalence of low BMD or rates of fracture.
49.4.6
Fat distribution and low bone mineral density
Abnormalities in body fat distribution are common in HIV-infected persons and are often grouped under the term lipodystrophy that includes both subcutaneous lipoatrophy and central lipohypertrophy. Lipoatrophy is the diffuse loss of subcutaneous adipose tissue that most apparent in the face, buttocks, legs, and arms and has been attributed due to exposure to stavudine and zidovudine , which are no longer in widespread use. These medications likely cause lipoatrophy by inducing mitochondrial dysfunction in adipocytes, which in turn leads to adipocyte apoptosis . However, since discontinuation of stavudine or zidovudine only minimally improves lipoatrophy, there are many prevalent cases among highly treatment-experienced patients , and adverse metabolic effects of these drugs may persist many years after discontinuation . Lipoatrophy has been associated with lower BMD , and it is possible that the limited subcutaneous tissue overlying the femoral head in lipoatrophic patients may fail to attenuate forces applied to the femur after a fall and contribute to hip fracture risk .
Lipohypertrophy is generally seen as the relative excess of abdominal fat in the visceral adipose tissue compartment and occasionally dorsocervical fat pad enlargement and the emergence of subcutaneous lipomata. Excess visceral adipose has also been associated with ectopic adipose tissue deposition in the liver, epicardium, and muscle . Unlike lipoatrophy, which does not occur in the absence of stavudine or zidovudine, the pathogenesis of lipohypertrophy is not clear. Although lipohypertrophy was first recognized after the introduction of PIs, there is very limited evidence for a causal role. Current hypotheses for the development of lipohypertrophy in HIV-associated patients include (1) the dysregulation of fatty acid metabolism due to HIV or antiretroviral agents, which leads to selective deposition in the visceral adipose tissue depot in the setting of blockage of the subcutaneous depot ; (2) increased local cortisol concentrations owing to aberrant conversion from cortisone ; (3) relative growth hormone deficiency ; and (4) fat accumulation in the setting of successful immune reconstitution and control of HIV replication (return to health) .
Similar to observations in the general population , relative visceral fat accumulation has been associated with lower bone mineral density in HIV-infected populations . While generalized adiposity is associated with higher BMD and decreased fracture risk , it has been hypothesized that adipocytokines secreted by visceral fat may have a negative effect on bone metabolism . In addition, abnormalities in growth hormone metabolism may contribute to low BMD. It is not clear whether visceral fat accumulation in HIV-infected patients is associated with fracture or whether interventions to decrease visceral fat will result in improved BMD and decrease the risk of fracture.
49.5
Vitamin D deficiency
Vitamin D deficiency (serum 25OHD <20 ng/mL) and insufficiency (<30–32 ng/mL) are common conditions in the general population . The classic actions of the active form of vitamin D, 1,25(OH) 2 D, involve regulation of calcium and phosphate flux across bone, gut, and kidney primarily through its interactions with PTH and FGF23. In vitro studies also suggest that 1,25(OH) 2 D affects bone cells directly, by increasing osteoblast differentiation and activity and downregulating secretion of OPG by osteoblasts, which indirectly results in increased osteoclastogenesis . Vitamin D also has a role in extraskeletal organ systems, including cardiovascular, pancreatic, and immune function in addition to cancer.
Vitamin D deficiency has been reported in many cohort studies of PLWH from the United States, Europe, and Africa, with prevalence in larger studies ranging from 24% to 74% . However, in studies from cohorts, including HIV-uninfected controls with similar demographics and risk factors, mean 25OHD levels and proportion with vitamin D insufficiency/deficiency are not higher among individuals with HIV . In multivariate analyses from these and other studies, vitamin D deficiency is associated with traditional risk factors such as older age , African-American race or dark skin , decreased dietary intake , decreased sun exposure , evaluation during winter , and increased BMI . Among PLWH a few studies also found an association with vitamin D deficiency and low CD4 count or a positive correlation between 25OHD levels and CD4 count , or an association between higher vitamin D levels and a greater rise in CD4 counts after ART .
49.5.1
Antiretrovirals and vitamin D metabolism
Several studies have found associations between vitamin D deficiency and the use of NNRTIs , non-PI-based regimens , or specifically efavirenz . Case reports of osteomalacia in patients receiving efavirenz have been published . Data from longitudinal studies demonstrate that initiation of an efavirenz-containing regimen results in a mean decrease in 25OHD of 5 ng/mL after 6–12 months , and switching from an efavirenz-containing to nonefavirenz containing regimens results in increases in 25OHD levels . The effect of efavirenz on vitamin D metabolism is hypothesized to occur through the induction the 24-hydroxylase, a cytochrome P450 enzyme, that inactivates 25OHD and 1,25(OH) 2 D, similar to the effects of antiepileptics . However, the exact mechanism and whether 24-hydroxylase activity is attenuated over time have not been established. Other NNRTIs inhibitors such as nevirapine, etravirine, and rilpivirine do not appear decrease 25OHD levels to the same degree as efavirenz , and efavirenz use has been declining since 2015 as newer, safer ART has become available. Despite the high prevalence of vitamin D deficiency in PLWH, and the putative negative effects of antiretrovirals on vitamin D metabolism, low 25OHD has been associated with low BMD only in a few studies .
49.5.2
Vitamin D supplementation/treatment studies
Despite the strength of association from observational studies, vitamin D treatment studies in PLWH have had mixed results. Results from the placebo arms of bisphosphonate studies in PLWH suggest BMD remains stable or increases 1%–2% over 1 year in those who take calcium and low doses (400–800 IU) vitamin D . Several randomized controlled trials evaluating the effect of vitamin D3 supplementation on BMD , PTH, and bone turnover markers in PLWH have been published, focusing on the most at-risk patient groups, children/adolescents, and older adults. Recent studies have evaluated vitamin D3 supplementation in children and adolescents with HIV, given in different doses and intervals ranging. Arpadi et al. found that 100,000 IU of vitamin D3 given every other month along with calcium supplementation increased 25OHD levels but did not increase total body or spine BMC or BMD after 2 years over placebo, either before or after adjustment for stage of sexual maturation . Similarly, Rovner et al. found no difference in BMD change between children and adolescents given 7000 IU vitamin D3 versus placebo after 12 months, despite improvements in 25OHD levels . In contrast, Havens et al. observed a greater increase in lumbar spine BMD among adolescents on TDF-containing regimens who received 50,000 IU of vitamin D3 versus placebo . Studies that have compared higher dose to low-dose vitamin D3, without a placebo control, have generally not found statistically significant differences between treatment groups, whether in children/adolescents or postmenopausal women with HIV .
49.5.3
Screening and treatment of vitamin D deficiency
There are no clear data to suggest that PLWH should receive a more aggressive screening approach for vitamin D deficiency or treatment; therefore guidelines for the general population should be followed for PLWH. The European AIDS Society guidelines does not recommend routine screening of vitamin D levels in PLWH but do recommend checking levels in persons with history of low BMD or fracture or at high risk of fracture, similar to the general population . Similarly, none of the guidelines suggest that target levels for 25OHD should be different for PLWH than the general population.
49.6
Children and adolescents
Children and adolescents, who acquire HIV infection either perinatally or early in life through sexual transmission, have the greatest cumulative exposure to the negative effects of HIV infection and ART on bone metabolism. Peak bone mass is a key determinant of osteoporosis and fracture risk with aging . Fracture risk may be increased during adolescence, at a time when fracture rates are typically increased in boys, and further increased in adulthood if impairments in bone acquisition during childhood prevented the achievement of genetically determined peak bone size and mass. While there have been dramatic reductions in perinatal acquisition of HIV in the United States and Europe due to successful implementation of mother-to-child transmission prevention strategies, early-life acquisition of HIV remains a substantial problem worldwide.
Several cross-sectional studies have found both lower BMC and BMD by DXA in children with perinatal HIV than healthy children of similar age and sex . A major limitation of DXA is that it does not measure the anteroposterior diameter of bone; therefore areal BMD (aBMD) by DXA may underestimate volumetric BMD (vBMD) in children with impaired growth and pubertal development. Growth delays have been described in some but not all studies of children with HIV in comparison to uninfected controls. There are few published studies of bone structure in children with HIV, but available studies suggest that vBMD by lumbar spine CT and peripheral quantitative CT (pQCT) does not differ between children with and without HIV . In contrast, in a study of peak bone mass, comparing bone mass and structure by DXA and high-resolution quantitative CT in HIV+ and HIV− young men at age 20–25, when peak bone mass should be attained, deficits in bone by high-resolution pQCT in cortical and trabecular compartments were detected, as well as decreased bone stiffness, a measure of bone strength . In addition, among the HIV+ young men, bone stiffness was lower in those with perinatally acquired versus adolescence-acquired HIV .
From cross-sectional studies, several traditional factors associated with low BMD among children and adolescents with HIV have been identified, including delayed growth and puberty, low lean body mass, vitamin D deficiency, malabsorption, and physical inactivity. In addition to other hormonal influences, disturbances in somatotropic axis could also be important to bone accrual during childhood and adolescence, either directly or indirectly through effects on overall growth. IGF-1 modulates osteoblast–osteoclast interactions and is linked to longitudinal bone growth and bone mass acquisition during childhood . Perturbations of IGF-1 and IGFBP3, possibly in response to IL-6 overproduction, have been described and are associated with failure to thrive, growth failure, reduced bone mass in children with HIV .
Some studies have also identified an association with serum cytokine levels , stage of HIV , class of antiretrovirals, or specific antiretrovirals. It is especially difficult to isolate the effects of antiretrovirals on bone metabolism from changes due to growth in children, since the treatment paradigm involves lifelong therapy with sequential regimens of multiple ART from multiple drug classes . Suppression of viral replication and modulation of inflammatory cytokines with ART and resulting improvements in overall health leads to improvements in growth and increases in BMC and BMD, which may mask the negative impact of specific antiretrovirals on bone metabolism. Growth impairment and bone toxicity due to TDF fetal exposure have been described in animal models. There has been concern that children born to mothers with HIV on TDF would have growth impairments. However, current evidence indicates that in utero TDF exposure does not negatively impact on growth, bone turnover marker levels, or mortality .
Alterations in regional fat have also been reported in association with poor bone accrual in children receiving ART. HIV+ children with lipodystrophy had lower BMC and BMD than HIV+ children without lipodystrophy . A shared underlying mechanism related to alterations in differentiation of the common MSC progenitor of osteoblasts and adipocytes has been suggested, although relationships between bone and fat homeostasis in children and adolescents with HIV infection are not well understood.
Thus far there has only been one study of fracture risk in children with HIV. PACTG 219/219c compared rates of fracture in a cohort of 1326 children with perinatal HIV and 649 uninfected children with a mean age of 5.8–7.1 with a median of 2.26–4.97 years . Rate of fracture was similar in the HIV+ and HIV− groups (1.2 vs 1.1 per 1000 person years); however, there are no data on fracture rates among individuals with perinatal HIV during early and late adulthood.
49.7
Nonskeletal risk factors for fracture
aBMD accounts for only 50% of fracture risk . In addition to skeletal factors not measured by BMD (e.g., bone quality), nonskeletal risk factors for fracture, such as the risk of falls, also likely to contribute to the incidence of fracture among PLWH. Traditional risk factors for falls, such as polypharmacy, cognitive impairment, lower extremity neuropathy, and muscle weakness, are common among PLWH. In a cross-sectional study of patients with HIV from a single center, 30% reported had experienced one or more falls in the previous 12 months. While HIV-related variables such as current or CD4 cell count or HIV-RNA concentrations were not associated with falls, the presence of diabetes and other comorbidities, treatment with antidepressants or sedatives, opiate use all increased the risk of falls .
As in the general population , the presence of the frailty phenotype, a clinical syndrome characterized by loss of muscle mass, weight, and energy; slowed motor performance; and low physical activity, also increases the risk of falls and fractures in PLWH . There is emerging evidence suggesting that frailty is observed at an earlier age and a higher prevalence in PLWH, compared to HIV-uninfected individuals . As the HIV-infected population ages and the prevalence of frailty increases, there is concern that the decreased functional performance among PLWH may further increase the risk of falls and fractures .
49.8
Screening considerations in persons living with human immunodeficiency virus
Several societies have published guidelines on DXA screening in HIV+ populations. Given the increased risk of fracture among PLWH compared to their HIV-uninfected counterparts, the most recent “Primary Care Guidelines for Management of Persons with HIV” published by the HIV Medical Association of the Infectious Diseases Society of America in 2014 recommends DXA screening in all postmenopausal women with HIV and men with HIV ≥50 years . This guidance is consistent with recommendations from the National Osteoporosis Foundation Guidelines (2014) that recommended DXA testing in women ≥65 and in men ≥70 and older, and in postmenopausal women and men age 50–69 when clinical risk factors for fractures are present which includes HIV . This approach is also advocated by the European AIDS Clinical Society (version 10.0, 2019) and other expert groups .
Based upon existing data on fractures, earlier DXA screening for HIV-infected individuals is reasonable. The extent to which these recommendations have been adopted by clinicians in United States and Europe is unknown, as are the cost-effectiveness of the proposed screening measures. With increasing proportion of HIV-infected persons over 50 years , the costs both in terms of increased DXA utilization and additional treatment for fracture prevention are substantial.
49.9
Treatment considerations
Use of FRAX: FRAX is a population-specific algorithm that estimates the absolute risk of fracture to aid in screening and treatment decisions in the general population. Studies suggest that fracture rates calculated using clinical risk factors underestimate observed fracture rates older men and women with HIV . Adjustment with the utilization of “secondary osteoporosis” as a surrogate for HIV status in the FRAX calculator helps to improve the accuracy of FRAX for HIV, as does the addition of BMD data for hip fracture estimates .
Screening and treatment for secondary causes of low BMD: As in the general population, screening for secondary causes is important to identify reversible causes of low BMD among PLWH. Although the optimal evaluation has not been delineated in the general population, a measurement of thyroid-stimulating hormone, serum calcium, parathyroid hormone, 25 hydroxyvitamin D, and serum testosterone (for males) is generally recommended . Among PLWH, there are some additional considerations. First, because sex hormone-binding globulin is generally higher in HIV-infected men compared to HIV-uninfected controls, particularly among men with hepatitis B or C coinfection, total testosterone levels may be normal, even in the setting of a low free testosterone concentration. In a study from the MACS, the use of total testosterone alone failed to identify 30% of men with biochemical hypogonadism . For this reason, screening for hypogonadism with a free testosterone (from a sample drawn in the morning) is recommended . Another important secondary cause of low BMD in PLWH is phosphate wasting, particularly among patients treated with TDF. Since TDF use can be associated with proximal tubule dysfunction, a high degree of suspicion for phosphate wasting is prudent for TDF-treated patients. For TDF-treated patients with low BMD, renal phosphate wasting, using simultaneous measurements of serum and urine phosphate and creatinine to calculate the fractional excretion of phosphate, should be assessed. If hyperphosphaturia is present (i.e., if the fractional excretion of phosphate is >20%–30%), transition to a nontenofovir-containing ART regimen should be considered. Patients with severe phosphate wasting may also require phosphate supplementation. Serum phosphate alone may not be adequately sensitive to identify more mild, yet potentially clinically significant, phosphate wasting. The newer formulation of tenofovir, TAF, does not appear to have the same effects on renal proximal tubule function, so renal phosphate wasting will likely diminish in importance as TDF use decreases.
Switching ART: Because certain antiretroviral medications are associated with decreased BMD and increased fracture risk, it is reasonable to switch ART in patients who have an increased risk of fragility fracture who are also receiving TDF or PIs, assuming antiretroviral resistance or intolerance have not limited treatment options. Studies that have investigated the effect of switching from tenofovir to abacavir, another nucleoside reverse transcriptase inhibitor, have found increases in BMD . It should be noted that there is concern that abacavir may not be as effective in patients with a high viral load and may increase the risk of cardiovascular disease . In addition, abacavir hypersensitivity is a potential major source of morbidity in patients with a genetic predisposition but can be prevented with appropriate genetic testing. Studies that have examined switches from TDF to TAF have also shown improvements in BMD, and switching to a regimen that does not contain an NRTI may also be a potential strategy to improve bone health .
Among the PIs, lopinavir/ritonavir has been associated with an increased risk of fracture in one large study , and ATV/r has been associated with a larger decrease on lumbar spine BMD compared to efavirenz . In addition, ATV/r or DRV/r was both associated with greater bone loss than the integrase inhibitor, raltegravir in a randomized trial of PLWH initiating ART . If feasible, PI-sparing ART should be considered in PLWH with clinical risk factors for fracture.
Bone-specific treatments: Similar to the general population, bisphosphonates are considered first line for the treatment of osteoporosis in HIV-infected patients. Of the bisphosphonates, alendronate and zoledronic acid have been specifically evaluated in HIV-infected populations . These short-term studies have consistently shown increases in lumbar spine and total hip BMD with treatment, similar in magnitude to the BMD change with bisphosphonates in the postmenopausal women with osteoporosis in the general population . There are no available data regarding the effects of bisphosphonates on the risk of fracture in HIV-infected patients. Similarly, the long-term risks of prolonged suppression of bone turnover, including atypical femoral fractures and osteonecrosis on the jaw, have not been determined in HIV-infected populations. The incidence of avascular necrosis of the hip in HIV-infected patients is approximately 100-fold greater than the general population and is not associated with any specific ART medication or class . Whether the underlying pathogenesis involved in avascular necrosis of the hip among HIV-infected patients also contributes to the risk of osteonecrosis of the jaw in HIV-infected patients on long-term bisphosphonates has not been examined.
For second-line treatment, raloxifene can be considered in postmenopausal HIV-infected women with osteoporosis but has not been specifically studied in HIV-infected populations. Alternatively, estrogen-replacement therapy may also be considered in postmenopausal women who also have significant vasomotor symptoms. The anabolic agents, teriparatide, abaloparatide, and romosozumab, and the potent antiresorptive, denosumab, also have not been specifically evaluated in HIV-infected populations, although no specific drug–drug interactions would be expected. In the case of denosumab, given the importance of the RANKL/RANK interaction in immune regulation and the risk of skin infections observed in the general population , further studies regarding the safety of denosumab with respect to immune function are needed before its use can be recommended in HIV-infected persons.
49.10
Conclusion
As the population with ages, osteoporosis and fragility fractures behave become increasingly common and are likely to be a major source of morbidity in these patients. The etiology of osteoporosis in PLWH is multifactorial, including the effects of HIV disease and antiretroviral treatment, as well as traditional risk factors, such as comorbidities, hypogonadism, smoking, and low body weight. The optimal screening and treatment recommendations have not yet been determined. Given the higher risk of low BMD, compared to HIV-uninfected populations and emerging evidence suggesting a higher risk of fragility fracture, DXA screening in postmenopausal women with HIV and men with HIV age 50 years or older, particularly those with additional risk factors, is reasonable. Until more data become available, treatment guidelines should follow those established for the general population.
References
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree