Major Concepts
Diseases
Human herpesvirus 8 (HHV-8) is a gamma-2 herpesvirus that is present in people presenting with all five forms of Kaposi’s sarcoma (KS). These types are classic KS, affecting elderly men of Mediterranean or European Jewish ancestry; endemic KS, affecting younger men, very young children, and women in East Africa; iatrogenic KS, affecting individuals receiving immunosuppressive therapy; epidemic KS, affecting HIV-positive persons, especially gay men; and nonepidemic gay-related KS, found among HIV-negative gay men. KS is a cancer of endothelial cells lining blood vessels and begins as a skin cancer. It may remain confined to the skin, causing mild disease, or in more aggressive forms, such as epidemic KS, may travel to mucocutaneous areas or internal organs, resulting in life-threatening illness. HHV-8 infection appears to also play an important role in causing primary effusion lymphoma (PEL), a non-Hodgkin’s lymphoma with poor prognosis, and multicentric Castleman’s disease, a nonmalignant proliferative disorder of B lymphocytes. Individuals with sarcoidosis, multiple myeloma, and pemphigus may also be infected with HHV-8.
Infection
HHV-8 is closely related to the oncogenic Herpesvirus saimiri of monkeys and Epstein-Barr virus, which causes mononucleosis and Burkitt’s lymphoma in humans. Other herpesviruses cause herpes simplex, chickenpox and shingles, sixth disease (roseola), and a severe infection of the liver and central nervous systems of immunosuppressed persons. HHV-8 is a large, enveloped DNA virus whose genome contains an extensive unique region encompassing approximately 25 genes, many of which appear to have been pirated from human chromosomes. These viral homologues to cellular genes include viral interleukin-6, viral Bcl-2, a receptor for vascular endothelial growth factor, and several viral chemokines. These genes help bring potential viral host cells into the area and trigger their excessive growth and survival. Other HHV-8 genes aid the virus in escaping death by CD8+ T killer cells and inhibit the activity of the antiviral cytokine IFN-γ.
Immune System
HHV-8 has a complex relationship with the host immune response. Virally infected cells are killed by CD8+ T killer cells and natural killer cells, but B lymphocyte–derived antibodies are less protective. HHV-8 infects monocytes and B lymphocytes, leading to the B cell proliferative disorders, PEL, and multicentric Castleman’s disease. Host inflammatory cytokines stimulate the growth of KS and PEL cells.
Protection
Several antiherpes drugs effectively kill lytic viruses but do not kill latent viruses. A number of anticancer medications, particularly those used to treat leukemia and lymphoma, decrease the size of KS lesions and slow disease progression. These medications are not without their drawbacks—relapses often occur, many of the drugs are expensive, and some have potentially dangerous side effects, including damage to the heart and bone marrow, decreased neutrophil numbers, fever, muscle inflammation, anemia, diarrhea, nausea, and depression.
Kaposi’s sarcoma (KS) is one of several diseases definitively linked to infection by the gamma-2 herpesvirus HHV-8. KS is a cancer of endothelial cells lining the blood vessels and may present as a mild skin cancer or may be aggressive, spreading to internal organs and causing death. Several other cancers or proliferative disorders, including those of B lymphocytes, have been linked to HHV-8 infection. Prior to the early 1980s, the majority of cases of KS were found among discrete populations, including elderly men of Mediterranean or Ashkenazi (eastern European) Jewish ancestry, younger men and very young children in restricted areas of Africa, and immunosuppressed persons. Between 1981 and 1982, an unusual number of cases of life-threatening KS began to turn up among young homosexual men in California. This population with aggressive KS was soon found to be HIV-positive, and the discovery of KS in these men was one of the first indications that a new virus (HIV) was spreading through the United States and later the world. Persons with all forms of KS were subsequently found to be infected with HHV-8, discovered in 1994. HHV-8 appears to play a causal role in primary effusion lymphoma and multicentric Castleman’s disease and perhaps several other diseases. KS in HIV-positive persons and primary effusion lymphoma are very dangerous diseases, and the latter usually results in death within 60 days of initial diagnosis. Other gamma herpesviruses, such as Epstein-Barr virus, are linked to cancers in humans and monkeys, and the beta herpesvirus, cytomegalovirus, may cause severe pathology in HIV-positive persons. It is possible that HHV-8 or other presently unknown herpesviruses may contribute to other malignancies. More widespread screening for herpesvirus infection may aid in determining the extent of infection among different populations and may help define cofactors, such as coinfection with HIV-1, that may trigger HHV-8 to cause serious disease.
In 1872, a Viennese dermatologist, Moritz Kaposi, recognized an unusual pigmented skin cancer in elderly men of the area. This disease was later named classic Kaposi’s sarcoma. Two other forms of KS were subsequently identified: one is endemic in some regions of Africa and affects younger men and children, and the other occurs among persons receiving immunosuppressive therapy, often following organ or bone marrow transplantation. In the early 1980s, an unusually aggressive form of KS was increasingly reported in young, otherwise healthy homosexual men from California. Between June 1982 and April 1983, eight cases of this disease turned up in Los Angeles and Orange Counties. They were linked to infection of at least 11 other men from eight additional cities. This new form of the disease spread beyond the skin, affecting internal organs and often resulting in death. This group of young men was later found to have other unusual conditions, including life-threatening infections with normally harmless commensal microbes. These men were subsequently found to be infected with a new human retrovirus, later named HIV-1. HIV-positive men with this aggressive form of KS were later found to be coinfected with a novel gamma herpesvirus, HHV-8, that is related to the oncogenic Epstein-Barr virus. HHV-8 was first identified in 1994 in KS tissues from an AIDS patient but was later detected in lesions and other tissues from all types of KS, including those occurring in HIV-negative persons. HHV-8 infection is also associated with many of the cases of primary effusion lymphoma and one subtype of multicentric Castleman’s disease.
Infection with the human herpesvirus HHV-8 leads to several cancers of cells lining blood vessels or malignancies of B lymphocytes. Some of these diseases are mild, treatable skin malignancies, while others cause progressive illnesses that terminate in death. The most common of these cancers is Kaposi’s sarcoma, but several other diseases are also linked to HHV-8 infection. Other members of the herpesvirus group may also cause malignancies, as seen by the link between infection with Epstein-Barr virus (EBV) and B cell lymphomas and nasopharyngeal carcinoma.
Table 17.1 Types of Kaposi’s sarcoma
| Type | Individuals Affected | Symptoms |
| Classic | Men over 50 years old of Mediterranean or Ashkenazi Jewish ancestry | Red, purple, or brownish lesions on legs and feet |
| Endemic | Men and young children in East Africa | Mild to aggressive cancer, with a tendency to metastasize to bone or internal organs |
| Iatrogenic | Persons receiving medical immunosuppressive treatment | Fever, rash, flulike symptoms, marrow aplasia, diarrhea; graft rejection in transplant recipients |
| Epidemic | HIV-positive persons, especially gay men | Very aggressive; begins as skin lesions leading to mucocutaneous and visceral lesions of lungs and digestive tract; difficulty in eating and swallowing, restriction of food passage, shortness of breath |
| Nonepidemic gay-related | HIV-negative gay men | Lesions primarily on arms, legs, and genitals |
Kaposi’s Sarcoma
Five forms of Kaposi’s sarcoma have been described. These are commonly found in the skin, where they appear as flat, painless purple-red spots that do not blanch when pressure is applied to them. They also occur in the lining of the mouth, nose, and eyes. More serious or life-threatening disease results when these cells spread to the lungs, liver, stomach, intestines, or lymph nodes.
Classic KS
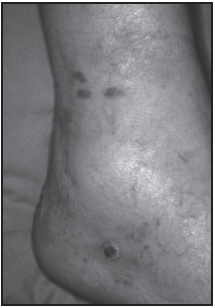
Classic KS typically occurs primarily in men over the age of 50 years who are either of Mediterranean (particularly Italian) or Ashkenazi Jewish ancestry. Corticosteroid usage and infrequent bathing increase the risk of developing this form of the disease. Classic KS is limited to the skin as one or more red, purple, or brownish lesions usually on the legs and feet, especially the ankles and soles. Lesions grow slowly in both size and number but rarely result in death, although affected persons have an increased risk of developing lymphomas, and lesions may form internally in areas such as the stomach and intestines, leading to gastrointestinal bleeding. Approximately 300 to 400 cases of classic KS are diagnosed each year in the United States. Prior to 1986, this was the predominant form of KS in the United States.
Endemic KS
Endemic KS is the prevalent form found in East Africa, particularly in northeastern Democratic Republic of Congo, Tanzania, and western Uganda and was known prior to the advent of HIV infection in the continent. It was responsible for 8% of all cancers in Ugandan men during the mid-1960s. Endemic KS is more common in men and very young children than women; however, it occurs more frequently in women than other forms of KS. The average age of affected men is 35 years.
Endemic KS presents itself in four clinical forms, one of which has a mild course similar to that seen in classic KS but affecting younger adults. The other three forms are more aggressive, particularly in infected Africans as opposed to Americans or Europeans, with an increased tendency to spread to bone or other tissues. A variant found in some young African children spreads via the lymphatic system to internal organs, with rapidly fatal results. In recent years, the incidence of endemic KS has increased among the HIV-negative population in Africa.
Iatrogenic KS
Iatrogenic KS is found in persons receiving medically related immunosuppressive regimens, especially organ or bone marrow transplant recipients. Renal transplant patients and those receiving cyclosporine (frequently used following this type of transplantation) are particularly vulnerable. Iatrogenic KS occurs equally in females and males. The immunosuppressive regimens required to prevent rejection may reactivate latent virus, often leading to serious visceral involvement, which may result in graft rejection and the death of the recipient. In patients receiving bone marrow transplants, fever, rash, flulike symptoms, marrow aplasia, diarrhea, and increased viral load have been reported, followed by death.
Epidemic (HIV-Associated) KS
This form of KS emerged rapidly in California at the beginning of the AIDS epidemic, and its appearance was one of the first indications of a serious condition sweeping the gay community. It is the most common cancer in HIV-positive men, affecting approximately 20% of HIV-positive people. The decline in CD4+ T helper cell numbers during the progression of HIV infection increases the chances of developing KS, and the Th1 subset of CD4+ lymphocytes appears to protect the host against HHV-8-associated disease (described later in this chapter). Although HHV-8 infection is similar among those coinfected with HIV-1 and HIV-2, epidemic KS is almost exclusively restricted to those who test positive for HIV-1.
Epidemic KS is particularly common in the gay population of HIV-positive men, especially those who have multiple sex partners; it is less common in individuals who acquired HIV via heterosexual contact, IV drug use, or blood transfusion; and it is much less common in HIV-positive women or HIV-positive hemophiliacs. Women who contracted HIV through heterosexual contact with bisexual men are at higher risk for developing KS. In 1990, the incidence of KS in the United States was 300 times more common in AIDS patients than in other immunosuppressed persons and 20,000 times that of the general population. In areas of Africa that are severely affected by the AIDS pandemic, such as Uganda, it is the most common cancer of men and one of the most common in women. The number of KS cases in that region rose tenfold between the 1950s and 1991. HIV-positive children are also afflicted with this cancer.
HIV-associated KS is a very aggressive form of cancer that most commonly begins as small lesions on the face and trunk. The lesions initially appear to be bruises with discolored edges and later become scaly, brown, and elevated. These often then develop into mucocutaneous and visceral lesions involving the lungs and digestive tract. If the disease is restricted to the skin, it is not life-threatening. The prognosis is far worse if the lesions spread to internal organs. These may lead to difficulty in eating or swallowing if lesions are found in the esophagus, restriction of food passage if found in the intestines, shortness of breath if found in the lungs, and lymphadenopathy if found in the lymph nodes. HIV-associated KS appears to consist of a large number of primary lesions rather than metastases from a single initial lesion.
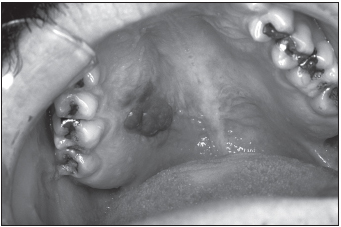
FIGURE 17.2 Kaposi’s sarcoma of the hard palate of an HIV-positive person
Source: CDC/Sol Silverman Jr.
Nonepidemic Gay-Related KS
Nonepidemic KS occurs in homosexual men who are HIV-negative. Its progression is slow, and new lesions appear every few years, most often on the arms, legs, and genitals, but can form anywhere on the skin.
Primary Effusion Lymphoma
Primary effusion lymphoma (PEL), an AIDS-associated non-Hodgkin’s lymphoma, contains primary malignant lymphomatous effusions of the pleural, pericardial, or peritoneal cavities with large-cell immunoblasts or anaplastic large cells. The morphology of these cells differs from Burkitt’s lymphoma, although they may contain the Epstein-Barr virus. They do not have oncogenic rearrangements in c-myc, bcl-2, or p53 oncogenes but do have clonal immunoglobulin rearrangements, similar to B lymphocytes. The cells express the CD45 antigen of dividing B cells. No tumor is present, and the lymphoma cells do not leave the body cavity in which they originated. These cells do not have other B lymphocyte or T lymphocyte antigens, nor do they rearrange DNA encoding the T cell antigen receptor. PEL frequently occurs in men, particularly men who have sex with men (MSM). The prognosis is poor, with a length of survival after diagnosis of approximately 60 days.
Multicentric Castleman’s Disease
Multicentric Castleman’s disease (MCD) is a rare, polyclonal nonmalignant atypical lymphoproliferative disorder of B lymphocytes in germinal centers. It is more common in men and is often accompanied by malignancies, such as KS or non-Hodgkin’s lymphoma. MCD takes two forms, the more common of which presents as a tumor mass in the mediastinum of the thoracic cavity or in the retroperitoneum. It is not associated with HHV-8 infection. The less common form involves infiltrates of plasma cells (B lymphocytes producing antibody) and is associated with HHV-8 infection. This form is characterized by immune system dysfunction, generalized lymphadenopathy, autoimmune disorders, rashes, and generalized symptoms. HHV-8-associated MCD is most common among HIV-positive persons, especially MSM.
Other Diseases Potentially Associated with HHV-8 Infection
Sarcoidosis
Sarcoidosis is a multisystem disease of unknown origin that may affect a number of organs, including the lungs, lymph nodes, and skin. It is characterized by the presence of noncaseous granulomas in more than one tissue. Conflicting reports have either found an increased association of HHV-8 infection in persons with sarcoidosis or failed to find such an association.
Multiple Myeloma
Multiple myeloma
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree