HUMAN CHORIONIC GONADOTROPIN
hCG is the signature hormone of the placenta. It is produced in large amounts, has pervasive actions, and has a profile of an early rise followed by a decrease to low steady-state levels. Although trophoblast is the major source, a wide variety of normal tissues, including anterior pituitary, can make hCG. Nontrophoblastic hCG is not glycosylated and its levels are very low in the circulation due to rapid clearance. Some nontrophoblastic tissues may not even release hCG, so that it will serve as a local ligand for hCG/luteinizing hormone (LH) receptors.24,25 This chapter focuses on trophoblastic hCG and some of its newly discovered actions in the fetoplacental unit and elsewhere in the body that are necessary for completion of a successful fullterm pregnancy.
STRUCTURE AND REGULATION OF HUMAN CHORIONIC GONADOTROPIN SYNTHESIS
hCG is a heterodimeric glycoprotein hormone consisting of noncovalently bound α and β subunits.26 The α subunit is identical to that of others in the glycoprotein hormone family, which consists of LH, follicle-stimulating hormone (FSH), and thyroid-stimulating hormone (TSH) (see Chap. 15 and Chap. 16). The β subunit, on the other hand, is hormone specific and, while similar, is not identical to the β subunit of LH.26 The primary difference is that the hCG-β subunit contains 30 additional amino acids at the carboxyterminus with four O-linked oligosaccharide chains, enabling intact hCG to survive in the circulation longer than does LH and also establishing a basis for developing highly specific immunoassays. The β -hCG assay measures not only the β subunit but also intact hCG. The circulatory half-life of intact hCG, 24 to 36 hours, is much longer than that for LH (2–3 hours), for free hCG-α (10–15 minutes), and for free hCG-β (35–45 minutes). Thus, not only the amount of carbohydrate on the protein backbone but also the conformation of the native hormone plays an important role in the clearance from the circulation.11,26,27
The α subunit of hCG contains 92 amino acids with 10 half-cystine residues, forming five intrachain disulfide linkages. The β subunit of hCG contains 145 amino acids with 12 half-cystines that form six conserved disulfide bridges.27 The folding pattern of the α and β subunits, with three disulfide bonds, forms a distinct seatbelt-like motif that is found in a family of cystineknot growth factors, which include nerve growth factor, TGF-β, and platelet-derived growth factor-β.28
A single gene on chromosome 6q21.1–23 encodes the α subunit of hCG.29 It contains five introns, four exons, and a consensus TATA box located 30 base pairs (bp) upstream from a single transcription initiation site. The β subunit of hCG is encoded by a cluster of six genes spanning more than 52 kbp on chromosome
19q13.3.30,31 The genes are a closely spaced array of tandem inverted copies. The hCG-β subunit genes 5, 3, and 8 contain a natural promoter and have high transcriptional activity. The 5′-flanking region of other hCG-β subunit genes contains several gaps and deletions that make them transcriptionally less active; sensitive methods are required to detect their expression.32
19q13.3.30,31 The genes are a closely spaced array of tandem inverted copies. The hCG-β subunit genes 5, 3, and 8 contain a natural promoter and have high transcriptional activity. The 5′-flanking region of other hCG-β subunit genes contains several gaps and deletions that make them transcriptionally less active; sensitive methods are required to detect their expression.32
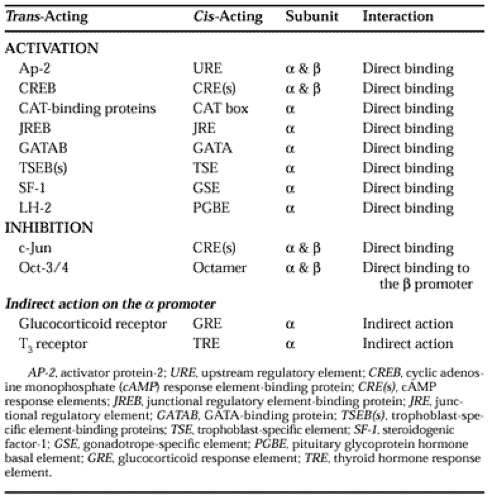
Analysis of sequence divergence in the coding and noncoding regions of hCG-β subunit genes suggests that multiple copies are probably derived from duplication of the ancestral LH-β subunit gene.33 During this duplication, deletion of a single base resulted in read-through of the translation stop codon. Thus, a part of the 3′-untranslated region became the coding sequence for the additional 30 amino acids in the carboxyterminus of the hCG-β subunit.34 Unlike the gonadotropin-α subunit gene, which contains a single promoter site, the evolution of the hCG-β subunit gene introduced a new promoter and a number of cis-acting elements that confer cell-specific transcription.35 The α subunit gene is transcriptionally more active than are the hCG-β subunit genes. However, the hCG-β subunit is rate limiting in the synthesis of intact hCG.27,36 The trans-acting factors and cisacting elements that are involved in transcriptional activation or inhibition of the α and β subunit genes of hCG are summarized in Table 112-2.35,36,37 and 38 These confer the specificity of regulation by agents that modulate the hCG synthesis. The regulation of hCG synthesis also involves posttranscriptional mechanisms such as the stability of the hCG-subunit mRNA and protein and posttranslational modifications.30,31,32,33,34,35,36,37,38,39,40 and 41
The placenta primarily secretes intact hCG and, to a small extent, the free α subunit, nicked hCG, and the β -core fragment.26,27 The control mechanisms for their release are probably different and are not well understood. Intact hCG is bioactive by virtue of its ability to bind to hCG/LH receptors. The free subunits, on the other hand, are bioinactive because they cannot bind to hCG/LH receptors. It is difficult to reconcile the few reports42,43 and 44 claiming that free subunits are also bioactive because no separate receptors for subunits have been identified. The functional relevance of nicked hCG and β -core fragment is unknown.
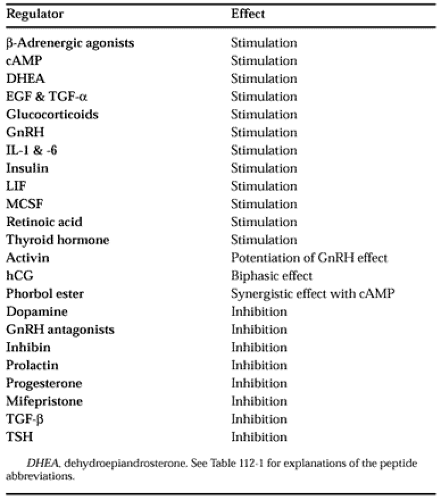
A number of agents produced by the placenta can regulate the synthesis of hCG. Some of them inhibit, whereas others stimulate; depending on the concentration, hCG can do both. Many of the regulatory agents that control hCG synthesis are listed in Table 112-3.2,8,15,36,41
hCG synthesis can be controlled at two different levels: (a) by increasing the number of syncytiotrophoblasts that produce large amounts of hCG45 (these are formed from cytotropho-blasts, which produce very little hCG) and (b) by regulating the expression of hCG-subunit genes in syncytiotrophoblasts. The self-regulation of hCG synthesis is a new concept, which has developed from the discovery that cyto- and syncytiotropho-blasts contain hCG/LH receptors and that exogenous, as well as endogenous, hCG can promote the differentiation of cytotrophoblasts and the expression of hCG-subunit genes by both transcriptional and posttranscriptional mechanisms. The effects of hCG are concentration dependent. Lower concentrations stimulate and higher concentrations inhibit the differentiation of cytotrophoblasts as well as the expression of hCG-subunit genes.17,40 Although hCG/LH receptors are present in the first-trimester placenta, they are not functional in self-regulation of hCG biosynthesis.
Self-regulation can potentially explain the pregnancy profile of hCG. Its rapid increase to peak levels is probably due to lack of feedback inhibition by hCG during the first trimester. Once peak levels are reached, feedback inhibition results in a drop in hCG levels. When they decrease, they can never fall to zero because low levels stimulate synthesis. When they begin to rise, they can never return to previous high levels because high concentrations inhibit synthesis. Thus, low steady-state levels are maintained throughout the second and third trimester of pregnancy.2 This profile is quite different from that of other placental hormones (i.e., human placental lactogen and the steroid hormones; see Chap. 108 and Chap. 109), which progressively increase throughout pregnancy.
The self-regulation of hCG biosynthesis is probably not an all-or-none phenomenon. Its onset may be gradual, potentially explaining the decrease in doubling times as hCG levels rapidly increase during the first trimester. The presence or absence and the strength of self-regulation may explain individual variations in hCG levels, variations in the same woman during different pregnancies, and the high hCG levels in preeclampsia and Down syndrome as compared to those found in normal pregnancy.7,9,46
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree