INTRODUCTION
Hospital-acquired pneumonia (HAP) and ventilator-associated pneumonia (VAP) remain important causes of morbidity and mortality despite recent advances in antimicrobial therapy and the use of a wide range of prevention measures (
1,
2,
3). HAP is an infectious disease of lung parenchyma occurring ≥48 hours after admission that was not incubating at the time of admission. VAP refers to pneumonia that occurs >48 hours after endotracheal intubation.
Bacterial pathogens causing HAP and VAP originate from the host’s endogenous flora, other patients, hospital staff, and environmental sources. Bacterial entry for VAP may result from aspiration during intubation, leakage around the endotracheal tube cuff, or endotracheal tube colonization (
1,
4). Over the past decade, there has been an increase in HAP and VAP caused by multidrug-resistant (MDR) pathogens, such as
Pseudomonas aeruginosa,
Acinetobacter baumannii, other resistant Enterobacteriaceae, and methicillin-resistant
Staphylococcus aureus (MRSA) (
1,
5,
6,
7,
8,
9).
This chapter highlights the epidemiology, diagnosis, treatment, and prevention of HAP and VAP. Our primary focus is on the wide spectrum of bacterial pathogens causing HAP and VAP. The readers are referred to other chapters for information on pulmonary infections caused by mycobacteria, viruses, and fungal pathogens.
EPIDEMIOLOGY
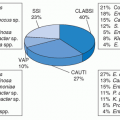
Each year, there are between 5 and 10 episodes of HAP per 1,000 hospital admissions, but the incidence is decreasing (
1,
5,
10). HAP and VAP account for 15% of all healthcare-associated infections (HAIs) and ˜25% of all intensive care unit (ICU) infections (
11). HAP and VAP rates and etiology are influenced by underlying host disease, length of hospital stay, and transfers to acute care facilities from other healthcare venues. The overall rates of HAP and VAP tend to be higher in academic vs. nonteaching hospitals.
The Centers for Disease Control and Prevention’s (CDC) National Healthcare Safety Network (NHSN) data summary from January through December 2010 reported >3,525 VAPs in the United States with an incidence between 0.0 and 5.8 per 1,000 ventilator-days (
12). Crude rates of HAP and VAP vary by patient population and method of diagnosis (
1,
5,
10). The rates of VAP increase with the duration of mechanical ventilation, and have been estimated to be ˜3% per day during the first 5 days, 2% per day from days 6 to10, and 1% per day thereafter (
13,
14). Improved prevention strategies have led to lower rates of HAP and VAP in the past decade (
2,
15).
OUTCOMES: MORTALITY, MORBIDITY, LENGTH OF STAY, AND COSTS
The crude mortality rates for VAP range from 20% to 50%, reflecting, in large part, the severity of the patient’s underlying disease, presence of organ failure, and infections caused by MDR pathogen(s) (
1,
5,
10,
13,
15). In two studies of VAP, the mortality rate varied between 4% in patients without prior antibiotic exposure and 73% in those with VAP due to MDR pathogens (e.g.,
P. aeruginosa or
A. baumannii), and attributable mortality ranged from 6% to 14% (
16,
17).
VAP increases the duration of hospitalization by 12 days, mechanical ventilation by 10 days, and ICU stay by 6 days, at a cost of $40,000 (
13). In a more recent study, when compared with patients without VAP, VAP was associated with a 13-day-longer hospital stay and a similar increase in cost (
18). Other analyses have suggested that the morbidity associated with VAP results in estimated cost per case of nearly $15,000. The discordance in these cost estimates reflects the differences in accounting for patient charges vs. costs (
13,
19,
20). VAP survivors who have experienced long-term mechanical ventilation and then discharged to chronic care facilities have a significant risk of readmission and increased morbidity and healthcare costs. In a recent study, the average cost per ICU survivor 1 year following discharge was ˜$3.4 million (
21). These data underscore the importance of preventing HAP and VAP. In addition, there is a growing trend toward public reporting of institution-specific HAI rates is increasing, which in the future may be linked to reduced hospital reimbursement for care.
PATHOGENESIS
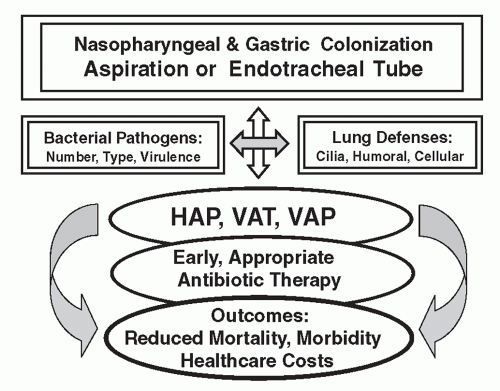
The pathogenesis of HAP and VAP involves the direct interaction between the invading pathogen(s), risk factors, and host and host defenses (
Figure 32.1). Microaspiration in non-ventilated patients is the primary route of bacterial entry into the lower respiratory tract for HAP (
5,
10). Patients who are sedated, postoperative, or have abnormal swallowing are at high risk for aspiration (
5,
10). Direct inoculation, bacteremic spread, and translocation of bacteria from the gastrointestinal tract (GI) are less well-documented routes of infection.
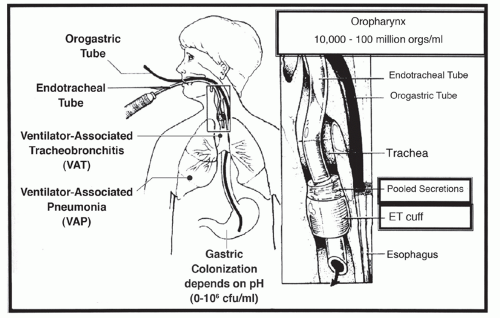
In the intubated patient, lower airway colonization may occur during intubation, by leakage around the endotracheal tube cuff or through the endotracheal tube (
Figure 32.2). In mechanically ventilated patients, inhalation of aerosols, contaminated tubing condensate, and leakage of bacteria and oral secretions around the endotracheal cuff are routes of bacterial entry into the lower respiratory tract (
Figure 32.2) (
22). In addition, local trauma and inflammation from the endotracheal tube increase tracheal colonization and prevent clearance of organisms and secretions from the lower respiratory tract. The development of biofilm-encased bacteria increases over time in the endotracheal tube lumen and may result in bacterial embolization to the bronchi and alveoli during suctioning or bronchoscopy (
23).
In contrast to healthy people, critically ill patients and those with VAP have high rates of oropharyngeal colonization with bacterial pathogens (
5,
10). Colonization with gram-negative bacilli was present in 16% of moderately ill patients vs. 57% of critically ill patients, and the rates of pneumonia were increased 6-fold in ICU patients with bacterial colonization (
24). Host factors, types of bacteria colonizing the pharynx, and the use of antimicrobials may alter colonization and adherence of gram-negative bacilli. Oral epithelial cells rich in fibronectin bind gram-positive organisms, such as streptococci and
S. aureus. Conversely, those poor in fibronectin preferentially bind gram-negative bacilli such as
P. aeruginosa (
25).
The stomach contents are usually sterile when the pH is <2 due to the potent bactericidal activity of hydrochloric acid, but increases significantly with increases in gastric pH. The use of antacids or histamine-2 (H2) antagonists or proton pump inhibitors, commonly prescribed for stress bleeding prophylaxis in mechanically ventilated patients, increases gastric pH, resulting in bacterial colonization that may exceed 1 million organisms per milliliter (
Figure 32.2) (
10,
26,
27,
28). Reflux of bacteria from the stomach to the oropharynx and lung may occur when patients are supine or not kept upright during transport from the ICU for X-rays, surgery, or other tests, thus increasing the risk of bacterial entry into the lung.
The response of pulmonary host defenses to invading microorganisms plays an integral part in the pathogenesis and
outcome of infection, which may result in HAP, ventilator-associated tracheobronchitis (VAT), or VAP, as shown in
Figures 32.1 and
32.2 (
1,
5,
29). Mucociliary and mechanical clearance in the upper airway are important factors in the defense against infection. The role of bacterial antigens and cytokines that alter the activity and efficacy of ciliary cells in clearing bacteria from the lower airway should be studied further. The ability of macrophages and polymorphonuclear leukocytes to eliminate bacterial pathogens and the interaction of these cells with inflammatory cytokines probably play important roles in the pathogenesis of pneumonia. Cell-mediated immune response is controlled by a complex array of lipids, peptides, and cytokines, including interleukin-1 and -2, interferons, growth factors, and chemotactic factors. Leukotrienes, complement components, and platelet-activating factor also assist in the inflammatory response and contribute to the pathogenesis of pneumonia. On the basis of an improved understanding of the molecular interaction between bacterial cell walls, toxins, and host defenses, strategies should be considered to enhance host defenses and supplement current antimicrobial regimens.
SPECTRUM OF BACTERIAL PATHOGENS
The wide spectrum of etiologic agents causing HAP and VAP, shown in
Table 32.1, varies with time, facility size, geographic location, type of ICU, patient population, and method of diagnosis (
5,
9,
10,
13,
30,
31). Bacteria causing HAP and VAP may originate from various sources, including the patient’s endogenous oropharynx or gastric flora, other patients, medical staff, contaminated devices, or the environment (
4,
32,
33). Gram-negative bacilli have been implicated in most episodes of HAP and VAP, and
S. aureus (often MRSA) accounts for ˜20% to 40% of infections (
9,
10,
34). The overall rates of MDR pathogen infections are increasing in the United States (
34,
35,
36).
HAP and VAP occurring during the first 5 days of hospital stay are more likely to be caused by more “antibiotic-sensitive” bacteria, such as
Streptococcus pneumoniae,
Moraxella catarrhalis,
Haemophilus influenzae, methicillin-sensitive
S. aureus, or anaerobic bacteria (
Table 32.1) (
1,
5,
28).
STREPTOCOCCUS PNEUMONIAE AND HAEMOPHILUS INFLUENZAE
S. pneumoniae and
H. influenzae usually cause early-onset HAP and VAP. Penicillin or ceftriaxone are the drugs of choice for pneumonia due to
S. pneumoniae, but resistance may occur (
37). Generally, patients infected with strains with low or moderate levels of resistance to penicillin have clinically improved when treated with the antibiotic (
38). All of the penicillin- and ceftriaxone-resistant strains in the United States are currently sensitive to vancomycin and linezolid, and many isolates remain sensitive to fluoroquinolones. Resistance of
H. influenzae to antibiotics other than penicillin and ampicillin is rare.
METHICILLIN-SENSITIVE STAPHYLOCOCCUS AUREUS (MSSA)
MSSA usually causes early-onset HAP and VAP. Data from the SENTRY program revealed that the incidence of MSSA in HAP has remained remarkably consistent for the past 14 years worldwide (
39). In a recent multinational study of HAP and VAP in patients admitted to ICUs,
S. aureus was documented as a causative pathogen in 5.5% of cases in Europe and 11.1% of cases in four countries in Latin America, with MSSA being identified in 63% and 55% of the
Staphylococcus isolates, respectively. As in previous studies, mortality was higher in the MRSA group (
40). In the United States, as in other parts of the world, the incidence of
S. aureus is lower among patients with VAP than among patients with HAP (19.5% vs. 26.6%) (
41).
LEGIONELLA PNEUMOPHILA
The rates of HAP due to
L. pneumophila vary among hospitals, but are increased in immunocompromised patients (e.g., organ transplant recipients or patients with HIV disease) and in those with diabetes mellitus, underlying lung disease, or end-stage renal disease (
4,
10,
42). Serotype 1 is most common and can be cultured on special media, but may be more rapidly diagnosed by urinary antigen testing. HAP due to
Legionella spp. is more common in hospitals where the organism is present in the water supply or may surge during construction.
Owing to the widespread use of Legionella urinary antigen rather than culture for the diagnosis of
Legionella, infection due to serotypes other than serotype 1 may be underdiagnosed. Detailed strategies for the prevention of
Legionella spp. infection and eradication procedures for
Legionella spp. in cooling towers and the hospital water supply are outlined in
Chapter 44 (
10).
MDR PATHOGENS
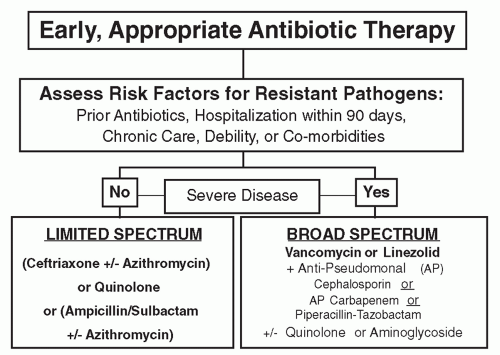
By comparison, late-onset HAP and VAP are more commonly caused by MDR pathogens, including MRSA,
Klebsiella pneumoniae,
A. baumannii, or
P. aeruginosa (
Table 32.1) (
29,
43). As summarized in the American Thoracic Society (ATS)/Infectious Diseases Society of America (IDSA) guidelines and shown in
Figure 32.3, patients who have received prior antibiotics, been hospitalized in the past 90 days, resided in a chronic care facility, or have debility or serious underlying diseases or comorbidities are at greater risk of infection due to MDR pathogens (
29). Finally, individuals with HAP or VAP, who have severe disease, such as septic shock or multiple organ failure, should be given initial broad-spectrum antibiotic therapy as shown in
Figure 32.3. Specific MDR pathogens causing HAP and VAP are shown in
Table 32.1 and discussed below.
Pseudomonas Aeruginosa
P. aeruginosa, the most common MDR gram-negative bacterial pathogen causing late-onset HAP and VAP, has intrinsic resistance to many antimicrobial agents (
5,
44). This resistance is mediated by multiple efflux pumps, which may be expressed continuously or may be upregulated by mutation. Resistance to piperacillin, ceftazidime, cefepime, other oxyimino-
β-lactams, imipenem and meropenem, aminoglycosides, and fluoroquinolones is increasing in the United States. Decreased expression of an outer membrane porin channel (OprD) can cause resistance to both imipenem and meropenem or specific resistance to imipenem but not other
β-lactams (
45). A systematic literature review reported significant increases in imipenem resistance, from 15% at initiation of imipenem treatment for VAP to 54% during treatment (
46). Although currently uncommon in the United States, there is concern about the acquisition of plasmid-mediated metallo-
β-lactamases active against carbapenems and antipseudomonal penicillins and cephalosporins. Currently, some MDR isolates of
P. aeruginosa are susceptible only to polymyxin B.
Klebsiella, Enterobacter, and Serratia Species
Klebsiella spp. are intrinsically resistant to ampicillin and other aminopenicillins and can acquire resistance to other penicillins and aztreonam by the production of extended-spectrum
β-lactamases (ESBLs) (
5). Carbapenem antibiotics have historically been considered the drugs of choice for ESBL-producing organisms. Plasmids encoding ESBLs often carry resistance to fluoroquinolones, aminoglycosides, and other antibiotics.
Enterobacter,
Citrobacter, and
Serratia spp. as well as
Escherichia coli can have chromosomal AmpC
β-lactamases with consequent resistance to oxyimino-
β-lactams and a-methoxy-
β-lactams, but have susceptibility to carbapenems. Recent reports of increasing rates of carbapenemase-producing Enterobacteriaceae (CPE) or Klebsiella-producing carbapenemases (KPCs) have emerged. CPEs and KPCs confer resistance to carbapenems and have been increasingly observed in isolates of
K. pneumonia,
E. coli, and
Enterobacter sp. Treatment options for CPE infections should be made on the basis of antibiotic-susceptibility reports.
Acinetobacter Baumannii, Stenotrophomonas Maltophilia, and Burkholderia Cepacia
Although generally less virulent than
P. aeruginosa,
Acinetobacter spp. have nonetheless become problematic pathogens because of their increasing resistance to commonly used antimicrobial agents (
47). Owing to mechanisms including
β-lactamases, porins, and efflux pumps,
Acinetobacter spp. can manifest resistance to commonly used antibiotics for treating pneumonia (
17,
48). Resistance to carbapenems through either IMP-type metalloenzymes or carbapenemases of the OXA type has increased to at least 40% of isolates in the United States (
49). Alternative therapies include sulbactam, which is usually employed as an enzyme inhibitor but has direct antibacterial activity against
Acinetobacter spp., as well as polymyxins and intravenous and inhaled aminoglycosides as adjuvant agents.
Stenotrophomonas maltophilia, which shares with
Burkholderia cepacia a tendency to colonize the respiratory tract rather than cause invasive disease, is uniformly resistant to carbapenems because of a ubiquitous, metallo-
β-lactamase.
S. maltophilia and
B. cepacia are most likely to be susceptible to trimethoprim/sulfamethoxazole, ticarcillin/clavulanate, or a fluoroquinolone.
B. cepacia usually is susceptible to ceftazidime and carbapenems.
METHICILLIN-RESISTANT STAPHYLOCOCCUS AUREUS
HAP and VAP caused by MRSA are associated with significant morbidity, mortality, and healthcare costs, making it a challenge for infection control (
49,
50). MRSA produces a penicillin-binding protein with reduced affinity for
β-lactam antibiotics that is encoded by the
mecA gene, which is carried by one of a family of four mobile genetic elements (
5). Strains of
S. aureus with the
mecA gene are resistant to all commercially available
β-lactams and many other antistaphylococcal drugs with considerable geographic variability worldwide. Vancomycin and linezolid are the preferred agents for MRSA pneumonia. Treatment with these two agents was studied in a recently published multicenter, prospective, randomized, controlled trial that demonstrated an ˜10% improvement in clinical response with linezolid; however, the lower bound of the 95% confidence interval approached zero. There was no difference observed in mortality (
51). Decisions to use vancomycin or linezolid for MRSA pneumonia should be made on the basis of patient-specific criteria, microbiologic data, and relevant considerations about potential renal toxicity for vancomycin or serotonin syndrome for linezolid. Linezolid therapy should be considered in patients who are nonresponders to vancomycin or when MRSA isolates have a vancomycin MIC >1
µg/mL (
52).
Although vancomycin-intermediate
S. aureus (VISA) with an MIC of 4 to 8
µg/mL and high-level vancomycin-resistant
S. aureus (VRSA) with MIC ≥16
µg/mL have been isolated from clinical specimens, all of these isolates have been sensitive to linezolid (
53,
54). Additionally, some
S. aureus isolates demonstrating heteroresistance have been associated with poor response to vancomycin. Although linezolid resistance has emerged in
S. aureus, it is currently rare. Daptomycin resistance also has been reported, but this drug is not indicated for the treatment of HAP and VAP because of its inactivation by lung surfactant. Ceftaroline, a broad-spectrum cephalosporin with activity against MRSA, was recently introduced in the United States, but does not have a Food and Drug Administration (FDA) indication for HAP or VAP.
CHANGES IN THE CDC SURVEILLANCE DEFINITIONS FOR VAP
The CDC, in conjunction with several professional organizations has been working to improve surveillance definitions for VAP in adult patients. The new definitions are planned for implementation by NHSN in 2013. VAP rates in many hospitals have decreased significantly over the past decade, and ranged from 0 to 5.8 per 1,000 ventilator-days. However, the current definition involves a wide spectrum of clinical criteria that lack specificity, and unfortunately, there is no current “gold standard” for VAP diagnosis.
Since the VAP surveillance definition is critical for assessing and implementing better VAP prevention strategies and comparing rates between hospitals, the CDC convened a multidisciplinary working group to develop new surveillance definitions that will be implemented in 2013. All of these events are measured by incidence per 1,000 ventilator-days. The new terms and definitions for VAP include the following with specific details included in
Table 32.3.
“Ventilator-Associated Condition” (VAC) is associated with changes in oxygenation, which may be due to either an infection or a spectrum of noninfectious causes, such as congestive heart failure, adult respiratory distress syndrome, pulmonary emboli, or atelectasis.
“Infection-Related Ventilator-Associated Complication” (IVAC), which includes temperature criteria, changes in white blood cell count, and use of antibiotic therapy.
“Possible or Probable” VAP includes purulent secretions and bacterial cultures of sputum, BAL, protected brush samples, or lung tissue.
These new CDC surveillance definitions are designed to be suitable for use in internal quality improvement and are scheduled to begin in 2013. Details are available on the CDC Web site: www.cdc.gov/nhsm/psc_da-vae.html. Note that these new surveillance definitions rely on changes in oxygenation, clinical signs, and microbiologic criteria to define VAP, and that chest X-ray criteria for the diagnosis of probable or possible VAP were not included, because of lack of sensitivity and specificity of new pulmonary infiltrates. A recent multicenter evaluation of the above-proposed new surveillance paradigm by Klompas et al. evaluated 597 patients, and found that 9.3% had VAP (8.8 per 1,000 ventilator-days) (
69). Compared with matched controls, VAP patients had prolonged mechanical ventilation (5.8 vs. 6.0 days) and longer days in the ICU (5.7 vs. 5.0 days). VAC was associated with increased mortality, but VAP was not. The authors concluded that VAC assessment was fast, and that objective surveillance definitions that include both clinical and quantitative evidence of respiratory deterioration are strongly predictive of increased length of ventilation, ICU stay, and increased healthcare costs.