Tamoxifen
Tamoxifen continues to be an important hormonal therapy for the prevention and treatment of breast cancer worldwide. The continued importance of tamoxifen is reflected in the fact that it is the only hormonal agent approved by the U.S. Food and Drug Administration (FDA) for the prevention of premenopausal breast cancer,
1 the treatment of ductal carcinoma in situ (DCIS),
2 and the treatment of surgically resected premenopausal estrogen receptor (ER)-positive breast cancer.
3The standard daily dose of tamoxifen is 20 mg, and the optimal duration depends on the underlying clinical setting. Although the recommended duration in the prevention and DCIS settings is 5 years, recently published prospective studies have demonstrated that for the adjuvant treatment of invasive breast cancer, a duration of 10 years (compared to 5 years) further reduced the risk of breast cancer mortality and improved overall survival.
4The most common toxicity from tamoxifen is hot flashes, affecting approximately 50% of treated women. These hot flashes are of varying intensity and duration. Tamoxifen-induced hot flashes appear to increase over the first 3 months of therapy and then plateau. They appear to be more prominent in women with a history of hot flashes or estrogen replacement use. Tamoxifen-induced hot flashes can be ameliorated by a number of different pharmacotherapies, including low doses of megestrol
5; antidepressants such as venlafaxine,
6 desvenlafaxine,
7 citalopram,
8 escitalopram,
9 and paroxetine
10; and the anticonvulsant drugs gabapentin
11 and pregabalin.
12 There is evidence that drugs that inhibit CYP2D6 (e.g., paroxetine) alter the metabolic activation of tamoxifen to endoxifen, a critical metabolite associated with in vivo tamoxifen efficacy.
13The estrogenic properties of tamoxifen are responsible for both beneficial and deleterious side effects. Tamoxifen increases the incidence of endometrial cancer in postmenopausal (but not premenopausal) women, with the increase in the annual incidence of endometrial cancer being approximately 2.58 (ratio of incidence rates).
14 The absolute risk depends on the duration of tamoxifen administration. For women who receive 10 years of adjuvant tamoxifen, the cumulative risk is 3.1% (mortality, 0.4%) versus 1.6% (mortality, 0.2%) for 5 years of tamoxifen.
4 The incidence of a rarer form of uterine cancer, uterine sarcoma, is also increased after tamoxifen use.
15 This form of endometrial cancer comprises approximately 15% of all uterine malignancies that develop after tamoxifen use.
15 Beneficial estrogenic effects from tamoxifen include a decrease in total cholesterol
16 and the preservation of bone density in postmenopausal women.
17 In premenopausal women, however, tamoxifen has a negative effect on bone density.
18 Although most patients do not complain of vaginal symptoms, a few complain of vaginal dryness, whereas others have increased vaginal secretions and discharge, the latter of which is an indication of the estrogenic activity of tamoxifen on the vagina. In the Arimidex, Tamoxifen, Alone or in Combination (ATAC) trial, a commonly observed tamoxifen side effect was vaginal bleeding, leading to a higher hysterectomy rate for patients randomized to tamoxifen (5%) compared to anastrozole (1%).
19 An uncommon effect from tamoxifen is retinal toxicity. This drug can also increase the risk of cataracts. However, no difference in the rate of vision-threatening ocular toxicity has been seen among prospectively treated tamoxifen patients.
20 Tamoxifen predisposes patients to thromboembolic phenomena, especially if used with concomitant chemotherapy. Depression has also been described, but the association with tamoxifen is not clear. Although liver cancers have been noted in laboratory animals, there is no established association between tamoxifen and liver cancers in humans.
Pharmacology
Tamoxifen acts by blocking estrogen stimulation of breast cancer cells, inhibiting both translocation and nuclear binding of the ER. This alters transcriptional and posttranscriptional events mediated by this receptor.
21 Tamoxifen has agonistic, partial agonistic, or antagonistic effects depending on the species, tissue, or endpoints that have been assessed. Additionally, there are marked differences between the antiproliferative properties of tamoxifen and its metabolites.
22Resistance to tamoxifen can be intrinsic or acquired, and the potential mechanisms for this resistance are reviewed in the following paragraphs. At each step of the signal transduction pathway with which tamoxifen or its metabolites interferes, there is the potential for an alteration in response. The most important factor appears to be the level of ER, which is highly predictive for a response to tamoxifen. Tamoxifen is ineffective in ER-negative breast cancer. Although decreased or absent expression of the progesterone receptor (PR) is associated with a worse prognosis, the relative risk reduction in tamoxifen-treated patients is the same regardless of the presence or absence of the PR.
Following binding to the ER, subsequent translocation of the tamoxifen/ER complex to the nucleus and binding to an estrogenresponse element may occur. This binding prevents transcriptional activation of estrogen-responsive genes. Laboratory and clinical data have demonstrated that ER-positive breast cancers that overexpress HER2 may be less responsive to tamoxifen and
to hormonal therapy in general.
23,24,25,26 In these tumors, ligand-independent activation of the ER by mitogen-activated protein kinase (MAPK) pathways may contribute to resistance.
27,28,29 In addition, the expression of AIB1, an estrogen-receptor coactivator, has been associated with tamoxifen resistance in patients whose breast cancers overexpress HER2.
30 In some cases, resistance may result from a decrease or loss of ER expression.
31,32 Although mutations in the ER ligand binding domain (LBD) are rare in newly diagnosed breast cancer, ER mutations are present in up to 20% of recurrent breast cancers.
33,34,35,36 These mutations lead to a conformational change in the LBD, which mimics the conformation of activated ligand-bound receptor and constitutive, ligand-independent transcriptional activity, resulting in resistance to hormonal therapy. Preclinical studies suggest that some of these mutations, although insensitive to aromatase inhibitors, retain sensitivity to higher dose selective estrogen-receptor modulators (SERM), such as endoxifen, as well as fulvestrant.
35The carcinogenic potential of tamoxifen has been recognized in rat studies
37,38,39 and in humans (endometrial cancer).
40 It has been proposed that the generation of reactive intermediates that bind covalently to macromolecules underlies the process. Such reactive intermediates have been demonstrated in vitro.
40,41,42,43 In addition, the induction of covalent DNA adducts in rat livers treated with tamoxifen has been reported.
44 Both constitutive and inducible cytochrome P-450 (CYP) enzymes have been implicated in the formation of metabolites with tamoxifen,
45,46 and the flavonecontaining monooxygenase has been implicated in the formation of the
N-oxide of tamoxifen. Reactive intermediates from such metabolic steps are being evaluated for their carcinogenic potential in vitro and in vivo.
Multiple studies to evaluate tumor gene expression profiling have identified gene expression patterns or specific genes associated with resistance to tamoxifen therapy. A commonly utilized gene expression assay, Oncotype DX 21 gene assay (Genomic Health, Redwood City, California), measures the expression of genes known to be involved in estrogen signaling (e.g., ER, PR), HER2, proliferation (e.g., Ki-67), and others. In multiple different data sets, the recurrence score has been associated with a higher risk of breast cancer recurrence in patients treated with hormonal therapy (e.g., tamoxifen or aromatase inhibitors) without concomitant chemotherapy.
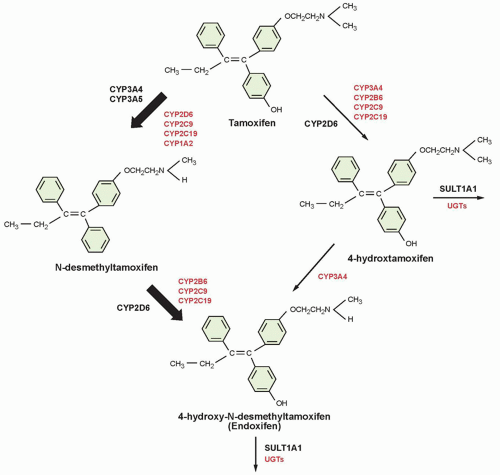
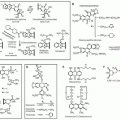
47,48,49The pharmacokinetics of tamoxifen is complex. The chemical structure and metabolic pathway of tamoxifen are shown in
Figure 27.1. Metabolic activation of tamoxifen is associated with greater pharmacologic activity. The two most active tamoxifen metabolites are 4-hydroxytamoxifen (4-OH tamoxifen) and 4-OH-N-desmethyltamoxifen (endoxifen). A series of studies carried out to characterize endoxifen pharmacology have demonstrated that it has equivalent potency in vitro to 4-hydroxytamoxifen in ER-α and -beta (ER-β) binding,
50 for the suppression of ER-dependent human breast cancer cell line proliferation,
22,50 and in global ER-responsive gene expression.
51 A recent study suggests that endoxifen’s effect on the ER may differ from 4-hydroxytamoxifen based on the observation of ER-α degradation.
52In women who receive tamoxifen at a dose of 20 mg per day, plasma endoxifen steady-state concentrations are generally 6 to
10 times higher than 4-hydroxytamoxifen.
53 Although the metabolism of tamoxifen to 4-OH-tamoxifen is catalyzed by multiple enzymes, endoxifen is formed predominantly by the CYP2D6-mediated oxidation of
N-desmethyltamoxifen, the most abundant tamoxifen metabolite (see
Fig. 27.1).
54 Multiple clinical studies have demonstrated that common
CYP2D6 genetic variation (leading to low or absent CYP2D6 activity) or the drug-induced inhibition of CYP2D6 significantly lowers endoxifen concentrations.
53,55 The
CYP2D6 gene is highly polymorphic, with more than 70 major alleles with four well-defined phenotypes: poor metabolizers (PM), intermediate metabolizers (IM), extensive metabolizers (EM), and ultrarapid metabolizers (UM).
The clinical studies to evaluate the association between
CYP2D6 polymorphisms and tamoxifen outcomes have yielded conflicting results. Initial
56 and follow-up data
57,58 demonstrated that CYP2D6 PM had an approximately two- to threefold higher risk of breast cancer recurrence (compared to CYP2D6 EM) and these data led an FDA special emphasis panel to recommend a tamoxifen label change to incorporate data that the
CYP2D6 genotype was an important biomarker associated with tamoxifen efficacy.
5 However, this label change has been delayed, in part because of conflicting data from secondary analyses of 5-year tamoxifen prospective trials (ATAC,
59 BIG 1-98,
60 and ABCSG8
61) as well as meta-analyses,
62 which demonstrate that the
CYP2D6 genotype is associated with tamoxifen efficacy when tamoxifen is administered as monotherapy for the adjuvant treatment of postmenopausal, ER-positive breast cancer. Additional support for the importance of endoxifen concentrations came from a secondary analysis of a prospective study, which demonstrated a higher risk of recurrence for women with low endoxifen concentrations.
13Many drugs are known to inhibit CYP2D6 activity. In tamoxifen-treated women, the coadministration of potent CYP2D6 inhibitors, such as paroxetine, converts a patient with normal CYP2D6 metabolism to a phenotypic PM.
63 Many other clinically important drugs have been reported to inhibit the CYP2D6 enzyme system, but their effects on tamoxifen metabolism have not been prospectively studied. As with the data regarding
CYP2D6 genotype, the data regarding CYP2D6 inhibitors has additionally been controversial, including two studies that reported opposite findings with regard to CYP2D6 inhibitor use and breast cancer
recurrence or death.
64,65 Although the
CYP2D6 data remain controversial, we conclude that until results from prospective adjuvant studies are available, women should be counseled regarding the potential impact of the
CYP2D6 genotype on the effectiveness of adjuvant tamoxifen, and potent CYP2D6 inhibitors should be avoided. Additional caution should be used with drugs that induce CYP3A, such as rifampicin, as a these drugs have been demonstrated to substantially reduce (up to 86%) the concentrations of tamoxifen and its metabolites.
66Strategies to overcome low endoxifen concentrations include dose escalation of tamoxifen to 40 mg per day, which has been demonstrated to significantly increase endoxifen concentrations,
67,68 as well as the direct administration of endoxifen itself. The latter strategy is ongoing in multiple different clinical trials, and early reports suggest clinical activity in aromatase inhibitors (AI)-resistant breast cancer.
69Following the metabolic activation of tamoxifen, the hydroxylated metabolites undergo both glucuronidation and sulfation. Peak plasma levels of tamoxifen (maximum concentration [Cmax]) are seen 3 to 7 hours after oral administration. Assuming an oral bioavailability of 30%, the volume of distribution has been calculated to be 20 L/kg, and plasma clearance ranges from 1.2 to 5.1 L per hour.
70 The terminal half-life of tamoxifen has been reported to range between 4 and 11 days.
71,72 The elimination half-life of tamoxifen increases with successive doses, which is consistent with saturable kinetics.
71,73 The drug’s distribution in tissues is extensive. Levels of the parent drug and metabolites have been reported to be higher in tissue than in plasma in animal studies.
74,75 Reports of tamoxifen concentrations 10- to 60-fold higher than plasma concentrations in the liver, lungs, brain, pancreas, skin, and bones are reported.
76,77 Elevated levels of tamoxifen with biliary obstruction have been reported.
78Tamoxifen has been reported to interact with warfarin,
73,79,80,81 digitoxin, phenytoin,
82 and medroxyprogesterone.
73 Tamoxifen-induced activation of human transcription factor pregnane X receptor (hPXR), resulting in the induction of CYP3A4, may increase the elimination of concomitantly administered CYP3A substrates,
83 such as anastrozole.
84
Pharmacology
Raloxifene is partially estrogenic in bone
99 and lowers cholesterol.
100 It is antiestrogenic in mammary tissue
101,102 and uterine tissue.
103The pharmacokinetics of raloxifene have been studied principally in postmenopausal women.
104,105,106 Pharmacokinetic parameters of raloxifene show considerable interindividual variation. Limited information is available on the pharmacokinetics of raloxifene in individuals with hepatic impairment, renal impairment, or both.
Raloxifene is rapidly absorbed from the gastrointestinal tract. Because raloxifene undergoes extensive first-pass glucuronidation, oral bioavailability of unchanged drug is low. Although approximately 60% of an oral dose is absorbed, the absolute bioavailability as unchanged raloxifene is only 2%. However, systemic availability of raloxifene may be greater than that indicated in bioavailability studies, because circulating glucuronide conjugates are converted back to the parent drug in various tissues.
After the oral administration of a single 120- or 150-mg dose of raloxifene hydrochloride, peak plasma concentrations of raloxifene and its glucuronide conjugates are achieved at 6 hours and 1 hour, respectively. After the oral administration of radiolabeled raloxifene, less than 1% of total circulating radiolabeled material in plasma represents the parent drug.
Results of a single-dose study in patients with liver dysfunction indicate that plasma raloxifene concentrations correlate with serum bilirubin concentrations and are 2.5 times higher than individuals with normal hepatic function. In postmenopausal women who received raloxifene in clinical trials, plasma concentrations of raloxifene and the glucuronide conjugates in those with renal impairment (i.e., estimated creatinine clearance values as low as 23 mL per minute) were similar to values in women with normal renal function.
Raloxifene and its monoglucuronide conjugates are more than 95% bound to plasma proteins. Raloxifene binds to albumin and α
1-acid glycoprotein. Raloxifene undergoes extensive first-pass metabolism to the glucuronide conjugates raloxifene 4′-glucuronide, 6-glucuronide, and 6,4′-diglucuronide. UGT1A1 and -1A8 have been found to catalyze the formation of both the 6-β-and 4′-β-glucuronides, whereas UGT1A10 formed only the 4′-β-glucuronide.
107 The metabolism of raloxifene does not appear
to be mediated by CYP enzymes (such as CYP2D6), because metabolites other than glucuronide conjugates have not been identified.
The plasma elimination half-life of raloxifene at steady state averages 32.5 hours (range, 15.8 to 86.6 hours). Raloxifene is excreted principally in feces as an unabsorbed drug and via biliary elimination as glucuronide conjugates, which, subsequently, are metabolized by bacteria in the gastrointestinal tract to the parent drug. After oral administration, less than 0.2% of a raloxifene dose is excreted as the parent compound and less than 6% as glucuronide conjugates in urine.
Fulvestrant
Fulvestrant is an ER antagonist that has no known agonist activity and results in ER downregulation.
108,109,110,111 Like tamoxifen, fulvestrant competitively binds to the ER but with a higher affinity—approximately 100 times greater than that of tamoxifen,
108,112,113,114—thus preventing endogenous estrogen from exerting its effect in target cells.
Results from two phase III clinical trials using the 250 mg per month dose demonstrated fulvestrant to be as effective as anastrozole in the treatment of postmenopausal women with advanced hormone receptor-positive breast cancer previously treated with antiestrogen therapy (mainly tamoxifen).
112,113,114,115,116 In the setting of first-line hormone-responsive metastatic breast cancer, a randomized phase III clinical trial to compare tamoxifen to fulvestrant (250 mg per month) demonstrated no differences in response or time to progression.
117 Because of pharmacology data (discussed in the following paragraphs), the 500 mg per day dose was developed. A randomized trial comparing the 250 mg per month with 500 mg per month dose demonstrated a 4-month improvement in median overall survival advantage for the higher dose.
118 For this reason, the higher dose is now the standard recommended dose.
Fulvestrant is well tolerated. The most common drug-related events (greater than 10% incidence) from the randomized phase III studies were injection-site reactions and hot flashes. Common events (1% to 10% incidence) included asthenia, headache, and gastrointestinal disturbances such as nausea, vomiting, and diarrhea, with minor gastrointestinal disturbances being the most commonly described adverse event.