Hodgkin’s disease (HD; also Hodgkin’s lymphoma) has a bimodal age distribution. The first peak is from age 25 to 30 years, and the second peak is from age 75 to 80 years (63).
HD nearly always begins in the lymph nodes.
More than 80% of patients with HD present with cervical lymph node involvement, and more than 50% have mediastinal disease.
Patients with HD generally present with painless lymphadenopathy.
Some patients may note systemic symptoms such as unexplained fevers, drenching night sweats, weight loss, generalized pruritus, and alcohol-induced pain in tissues involved by HD.
The theory of contiguity of spread and the development of treatment programs, including presumptive treatment of uninvolved sites, were important conceptual advances in the treatment of HD (56).
Nearly all patients with hepatic or bone marrow involvement by HD have extensive involvement of the spleen (44).
HD rarely involves Waldeyer’s ring, Peyer’s patches, the upper aerodigestive tract, central nervous system, or skin.
One third of patients present with B symptoms: fever, night sweats, or 10% weight loss in the past 6 months. Fevers may present in a classic waxing and waning Pel-Ebstein pattern. Night sweats may be drenching, requiring a change of bedclothes.
HD may be diagnosed during pregnancy, and many women become pregnant after successful treatment.
Historically, children had a better prognosis than adults; however, advances in treatment have lead to equivalent outcomes in the adult population.
Diagnostic and staging procedures commonly used for HD are listed in Table 40-1.
Important factors such as patient age and presence of intercurrent disease influence the selection of staging studies.
The erythrocyte sedimentation rate (ESR) is a prognostic factor for patients with stage I—II disease; anemia, lymphopenia, and hypoalbuminemia are adverse prognostic factors for patients with stage III—IV disease (36, 37).
Computed tomography (CT) scan is less sensitive, specific, and accurate (65%, 92%, and 87%, respectively) for detection of disease in the retroperitoneal nodes than classic bipedal lymphangiography, although the latter technique has largely fallen out of favor (13).
The criterion for enlarged lymph nodes on CT scan is typically a short-axis diameter that exceeds 1 cm (14).
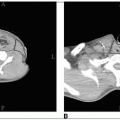
The criterion for bulky mediastinal lymphadenopathy is when the maximum width of the mediastinal mass divided by the maximum transthoracic diameter (near the level of the diaphragm) on a standing posteroanterior chest radiograph exceeds 1/3. Other definitions include a mass greater than 10 cm and a ratio of mediastinal mass to the chest diameter at T5-6 exceeding 0.35 (used by the European Organization for the Treatment of Cancer [EORTC]).
Gallium imaging has been replaced by 2-fluoro-2-deoxy-D-glucose positron emission tomography (FDG-PET). FDG-PET is routinely used as part of the initial staging in HD, and is more sensitive than CT or gallium imaging (52, 73).
Magnetic resonance imaging (22) is an alternative to CT, and is useful in the staging of women who are pregnant (12).
The incidence of bone marrow involvement in HD is only 5%. Therefore, a bone marrow biopsy is performed only in those patients who present with stage III—IV disease, bulky adenopathy, or B symptoms.
Low-risk populations for subdiaphragmatic extension are (a) patients with clinical disease limited to intrathoracic sites (yield approximately 0%), (b) women with stage I disease (yield approximately 6%), (c) men with stage I disease and lymphocyte predominance or interfollicular histology (yield approximately 4%), and (d) women with stage II disease and with three or fewer sites of clinical involvement and who are younger than 27 years of age (yield approximately 9%) (40).
TABLE 40-1 Diagnostic and Staging Procedures for HD | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
TABLE 40-2 Ann Arbor Staging Classification | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||
The Ann Arbor staging system for HD, used since 1971, is outlined in Table 40-2 (11). The lymphoid regions defined in this system are shown in Figure 40-1.
Since staging laparotomy has been discontinued, the generic term stage is now employed instead of the clinical and pathologic stage.
Inadequacies of the Ann Arbor system include failure to consider bulk of disease and lack of a more precise definition of the E lesion (15).
However, the descriptor “X” is now employed to designate bulky mediastinal adenopathy in Cotswolds modification of the Ann Arbor system (61). See also Table 40-4 for the AJCC stage groups.
The neoplastic cell of HD is the Reed-Sternberg cell. It is typically binucleate, with a prominent, centrally located nucleolus in each nucleus. It also has a well-demarcated nuclear membrane and eosinophilic cytoplasm with a perinuclear halo.
Reed-Sternberg cells are derived from monoclonal B cells and are CD15 and CD30 positive.
They usually account for less than 1% of the cells in a HD-infiltrated lymph node. The majority of the cells in involved nodes are nonmalignant lymphoid cells, plasma cells, eosinophils, and other benign cells.
The World Health Organization (WHO) modification of the Lukes and Butler system has five histologic subtypes (see WHO online index, 2007). These include the four subtypes of classical HD: nodular sclerosis, lymphocyte rich, mixed cellularity, and lymphocyte depleted; as well as nodular lymphocyte predominance HD (nLPHD).
nLPHD is predominantly composed of normal-appearing lymphocytes and has few abnormal cells. Unlike the subtypes of classical HD, the abnormal cells in nLPHD are strongly positive for CD20 and CD45 and negative for CD15 and CD30. The abnormal cells are called “L and H cells” or “popcorn cells.” Patients frequently present with a solitary peripheral node and early-stage disease. Systemic symptoms are uncommon (less than 10%). Some patients may have a late relapse, but it has the best overall survival (OS) of any HD histologic subtype.
Lymphocyte predominant HD (LPHD) is often diagnosed in young people. Patients frequently present with early-stage disease, and systemic symptoms are uncommon. The natural history is the most favorable of the classical HD histologic subtypes.
Nodular sclerosing HD (NSHD) is the most common histologic subtype. The mediastinum is often clinically involved. One third of these patients have B symptoms. The natural history of NSHD is less favorable than that of LPHD.
Mixed cellularity HD patients more commonly present with advanced disease (e.g., subdiaphragmatic involvement). Its natural history is less favorable than that of NSHD.
Lymphocyte depleted HD tends to occur in older patients and is more likely to be associated with advanced disease and B symptoms. It has the worst prognosis of all histologic subtypes of HD.
Men have a slightly worse outcome than women (75).
After the extent of disease has been determined, histologic subtype seems to have little additional impact on prognosis.
Ann Arbor stage is the most important prognostic factor influencing therapy.
Bulky disease, especially in the mediastinum, is reported to be associated with greater risk of relapse after single-modality therapy.
The “International Prognostic Score” was created from a large study, which evaluated prognostic factors for 5,141 patients with advanced HD. There were seven factors identified, which have a significant impact on prognosis: age, gender, albumin, white cell count, lymphocyte count, hemoglobin, and Ann Arbor stage. If patients have three or more adverse risk factors, they are considered to be in an unfavorable prognostic group for advanced HD (36).
Irradiation is the most effective single agent for the treatment of patients with HD. Actuarial relapse-free survival at 5 years for all stages ranges from 94% for LPHD, 74% for NSHD, and 75% for mixed-cellularity HD to 45% for lymphocyte-depleted HD (41).
However, irradiation as a monotherapy has largely been abandoned in favor of multidrug chemotherapeutic approaches, often in a combined-modality setting incorporating involved-field radiotherapy (IFRT).
The first successful drug combination for treating HD was mechlorethamine hydrochloride (nitrogen mustard), vincristine sulfate (Oncovin), procarbazine hydrochloride, and prednisone (MOPP) (17).
After the development of doxorubicin (Adriamycin), many trials utilized this drug in novel combinations. Adriamycin, bleomycin, vinblastine, and dacarbazine (ABVD) is now the “gold standard” based on a randomized trial comparing MOPP, ABVD, and MOPP/ABVD (6, 10).
Typically, treatment is initiated with chemotherapy, which has the advantage of treating all disease sites at the outset (particularly important in stages III and IV). Another advantage of initial chemotherapy treatment is a reduction in the size of bulky disease to facilitate subsequent radiotherapy with minimization of normal tissue irradiation (especially in the mediastinum).
Combined-modality therapy programs generally use a reduced number of cycles of chemotherapy and/or “safer” drugs, as well as reduced irradiation fields and/or doses.
Is chemotherapy alone a reasonable option in early-stage HD? Two trials with MOPP gave contradictory results (4, 62). The decision not to treat with radiotherapy in these trials was not linked to tumor response. Subsequent numerous studies using more modern chemotherapy regimens indicate that combined-modality therapy offers improved relapse-free survival/eventfree survival compared with chemotherapy alone. The EORTC-GELA H9-F trial randomized early-stage favorable HD patients to no IFRT, 20 Gy IFRT, or 36 Gy IFRT after achieving a complete response to six cycles of epirubicin, bleomycin, vinblastine, and prednisone (EBVP) chemotherapy; the 4-year event-free survival was inferior in the no IFRT arm (70%) versus the 20 Gy (84%) and 36 Gy (87%) arms (Noordijk EM et al. [91]).
The principal objective of radiation therapy in HD is to treat involved and contiguous lymphatic chains to a dose associated with a high likelihood of tumor eradication using IFRT.
In the rare circumstance when radiation alone is used in the modern era, the Patterns of Care Study recommendation for a tumoricidal dose of radiation is 35 to 44 Gy fractionated at a rate of 7.5 to 10.0 Gy per week (45). In this setting, generally extended fields of irradiation are used (e.g., subtotal nodal irradiation) rather than IFRT. Indeed, a key component in curative irradiation monotherapy programs is the use of “prophylactic” treatment to clinically uninvolved areas. A relatively recent study by the German Hodgkin’s Disease Study Group (GHDSG) indicates that 30 Gy is an adequate dose for such prophylactic treatment (23).
Most commonly, combined-modality therapy is used in the modern management of HD, for which the range of irradiation doses is 20 to 30 Gy for nonbulky and 20 to 36 Gy for bulky disease (42).
Evenly weighted opposed-field treatments are generally used; all fields are treated daily with fractions of 1.5 to 1.8 Gy.
The beam energy used is typically in the 6 to 15 MV range. If an energy greater than 6 MV is used for the supraclavicular and inguinofemoral lymph nodes, bolus should generally be added.
As combined-modality treatment has become more prevalent, “classic” radiation fields (e.g., total lymphoid irradiation, subtotal lymphoid irradiation, full mantle, inverted-Y) are being used less often in favor of more tailored IFRT.
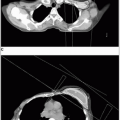
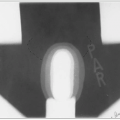
The full mantle is rarely treated in contemporary management; however, involved fields are portions of the classic treatment field. A description of one setup for a classic mantle field is given in Tables 40-3. An example of typical mantle field blocking is shown in Figure 40-2.
In addition to lung blocks, blocks can be placed over the occipital region and spinal cord posteriorly, the larynx anteriorly, and the humeral heads both anteriorly and posteriorly.
Spinal cord shielding may not be necessary with compensated fields if the prescribed tumor dose is only 36 Gy but should be used when the prescribed dose is more than 40 Gy.
For significant mediastinal disease with subcarinal extension or pericardial involvement, the entire cardiac silhouette is irradiated to 15 Gy, with a block placed over the apex of the heart thereafter.
After a dose of 30 Gy has been delivered, a block is placed in the subcarinal region (approximately 5 cm below the carina), shielding the pericardium and myocardium.
Bolus can be used if disease extends to the anterior chest wall.
If the pulmonary hilar lymph nodes are involved and a patient is being treated with irradiation alone, a 37% transmission lung block can be used to deliver 15.0 to 16.5 Gy to the lung (72).
Given issues of potentially excessive pulmonary irradiation with bulky mediastinal disease, the postchemotherapy width of disease (plus about 1.5- to 2.0-cm margin) is often used as the target volume. However, superior and inferior field margins should encompass the initial (prechemotherapy) extent of disease.
TABLE 40-3 Classic Mantle Setup | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||
TABLE 40-4 AJCC Clinical Anatomic Stages for Lymphomas | |||||
|---|---|---|---|---|---|
|