Hodgkin Lymphoma
The management of Hodgkin lymphoma (HD) continues to evolve. Since the last edition of this text, molecular imaging has taken on an expanded role as a means for “interim evaluation,” that is, individualization of subsequent therapy based upon the imaging response after just a portion of treatment has been completed. Programs of combined-modality therapy have been established as the standard for early-stage disease, although challenges to the use of radiation in this setting have been raised. New, more precise techniques for radiation therapy delivery have been adopted. For the first time in more than three decades, a new systemic agent has been approved by the U.S. Food and Drug Administration (FDA) for treatment of Hodgkin lymphoma. Large prospective, randomized clinical trials have enabled us to refine treatments, and data regarding late effects continue to influence the development of management approaches.
 ANATOMY
ANATOMY
Hodgkin lymphoma almost always begins in lymph nodes. More than 80% of patients with Hodgkin lymphoma present with cervical lymph node involvement, and >50% have mediastinal disease. Isolated extralymphatic involvement in the absence of nodal disease is rare.
 EPIDEMIOLOGY AND RISK FACTORS
EPIDEMIOLOGY AND RISK FACTORS
The reported incidence of Hodgkin lymphoma is slightly less than 3 per 100,000. It accounts for 0.56% of all cancers diagnosed but only 0.23% of all cancer deaths in the United States each year, a death rate that decreased by more than one-third between 1990 and 2006 (0.85 per 100,000 to 0.56 per 100,000).1 There is a slight male predominance (1.2:1). Hodgkin lymphoma is rare in children <10 years of age. The median age of patients at the time of diagnosis is 26 years, and the incidence has a bimodal peak as a function of age.2 The early peak, from ages 25 to 30 years, shows an incidence of approximately 5.5 per 100,000 per year. A second peak, from age 75 to 80 years, shows a similar incidence. However, this peak in older adults may be “contaminated” by cases that were actually anaplastic large-cell or diffuse large-cell lymphoma.
Geographic clusters of patients with HD have been reported, but these are probably only coincidental.3 A relation between HD and previous infection with Epstein-Barr virus (EBV) has been proposed.4 Weiss et al.5 identified components of the EBV genome in the cellular DNA of Reed–Sternberg cells in lymph nodes involved by Hodgkin lymphoma. In addition, Mueller et al.6 identified elevated levels of immunoglobulin G and immunoglobulin A against the EBV capsid antigen and elevated levels of antibody against the EBV nuclear antigen and early antigen D in the serum of patients with Hodgkin lymphoma 3 to 156 months before the diagnosis of Hodgkin lymphoma.
The risk for development of Hodgkin lymphoma is 2.55 times higher among individuals who have a history of infectious mononucleosis than among noninfected control subjects.7 Several series demonstrated an association between EBV infection and mixed-cellularity Hodgkin lymphoma, especially in children in developing countries. An international analysis based on 1,546 patients with Hodgkin lymphoma showed an increased risk for EBV-associated Hodgkin lymphoma in Hispanics (vs. Whites), those with mixed-cellularity histologic subtype (vs. nodular sclerosis), children from economically less developed (vs. more developed) regions, and young adult men (vs. women).8 However, the ultimate relationship between EBV infection and development of Hodgkin lymphoma remains undefined. Studies attempting to link occupational exposures or other etiologic factors with the development of Hodgkin lymphoma have been inconclusive or contradictory.3
 NATURAL HISTORY AND CLINICAL PRESENTATION
NATURAL HISTORY AND CLINICAL PRESENTATION
Patients with Hodgkin lymphoma usually present with painless lymphadenopathy. Some may note systemic symptoms such as unexplained fevers, drenching night sweats, weight loss, generalized pruritus, fatigue, and alcohol-induced pain in tissues involved by Hodgkin lymphoma. Still other patients are diagnosed after detection of a mediastinal mass on a routine chest radiograph.
If contiguity is assumed among the supraclavicular lymph nodes and upper para-aortic nodes/celiac axis/spleen, 90% of patients present with contiguous sites of involvement.9,10 In addition, disease spread after treatment with limited irradiation also occurs in a contiguous fashion in most instances.9 The theory of contiguity of spread and the development of treatment programs including presumptive treatment of uninvolved sites were important conceptual advances in the treatment of Hodgkin lymphoma in the latter half of the twentieth century.
Organ involvement by Hodgkin lymphoma may be secondary to extension from adjacent lymph nodes, such as spread from enlarged mediastinal or bronchopulmonary (pulmonary hilar) nodes directly into the pulmonary parenchyma, or it may be hematogenous, such as nodular disease in the liver or multiple bony sites. Involvement of the bones may cause blastic changes, especially in the vertebrae (creating the classic “ivory vertebra” on plain radiographs), pelvis, sternum, or ribs.
The mechanism of spread of disease to the spleen is unclear. However, the likelihood of disseminated disease, including bone marrow and liver involvement, increases as the extent of disease in the spleen increases.11 Nearly all patients with hepatic or bone marrow involvement by Hodgkin lymphoma have extensive involvement of the spleen.12 Hodgkin lymphoma only rarely involves the gut-associated lymphoid tissues such as Waldeyer ring and Peyer patches. It also only rarely involves the upper aerodigestive tract, central nervous system, and skin.9
The rapidity of Hodgkin lymphoma growth and spread is not predictable. Disease may evolve over a period of several years, demonstrated on serial radiographs or suspected by clinical history, and it is unusual to document progression during evaluation and staging.
There are three “B symptoms” included as part of the staging system for Hodgkin lymphoma (see later discussion). They are fever, drenching night sweats, and significant weight loss. One-third of patients present with one of these symptoms. Fevers may present in the classic waxing-and-waning Pel-Ebstein pattern. Night sweats may be drenching and require a change of bedclothes. The B symptoms may occur even in patients with relatively limited disease (stage II) but are uncommon in stage I disease.
Historically, children have had a particularly good prognosis compared with adults, and therefore different treatment strategies have been developed for children (see Chapter 89).13,14–15 More recently, the results of treatment programs in adults have improved to the same level enjoyed by children. Older adults (>60 years) have a worse prognosis, which often may be secondary to intercurrent illness or the ability to tolerate standard therapies, especially bleomycin, anthracyclines, and extended-field irradiation.16,17
Hodgkin lymphoma may be diagnosed during pregnancy, and many women become pregnant after successful treatment. Special treatment considerations are warranted for the pregnant patient; however, no evidence exists that pregnancy per se has any effect on the natural history of the disease.18,19,20 Although patients infected with human immunodeficiency virus type 1 do not appear to be at increased risk for development of Hodgkin lymphoma, the disease tends to behave differently in infected persons.21,22,23
TABLE 77.1 DIAGNOSTIC AND STAGING PROCEDURES FOR HODGKIN LYMPHOMA
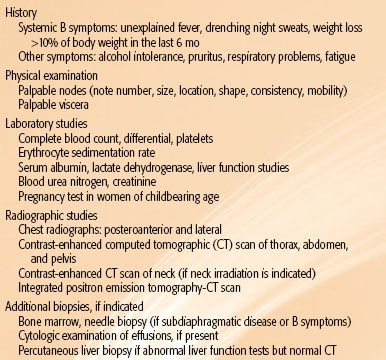
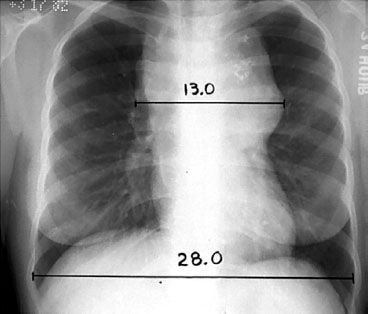
FIGURE 77.1. The mediastinal mass ratio (MMR). This ratio is defined as the maximum single horizontal mediastinal mass measurement divided by the maximum intrathoracic diameter, which is usually near the diaphragm. In this example, MMR = 13.0/28.0 = 0.46.
 DIAGNOSTIC WORKUP
DIAGNOSTIC WORKUP
Diagnostic and staging procedures commonly used for Hodgkin lymphoma are listed in Table 77.1. Patient age and the presence of intercurrent disease influence the selection of staging studies.24–26
Hematologic evaluation may reveal anemia, leukopenia, lymphopenia, or thrombocytosis. This is often a paraneoplastic effect, but it may be indicative of bone marrow involvement. Anemia, lymphopenia, and hypoalbuminemia are adverse prognostic factors, especially for patients with advanced disease (stage III–IV).27 The serum alkaline phosphatase level may serve as a nonspecific marker of tumor activity or hepatic, bone marrow, or bone disease. The erythrocyte sedimentation rate (ESR) may correlate with response to treatment and subsequent disease activity and is a prognostic factor for patients with limited disease (stage I–II).28 Other useful markers may include the lactate dehydrogenase and β2-microglobulin levels.
Radiographic evaluation should include posteroanterior (PA) and lateral chest radiographs. Mediastinal adenopathy may be quantitated by a measurement of the maximum width of the mediastinal mass divided by the maximum intrathoracic diameter (near the level of the diaphragm) on a standing PA chest radiograph, as shown in Figure 77.1. When this ratio exceeds 1:3, the disease is defined as bulky, and this affects assignment to many clinical trials. Other definitions of bulky mediastinal adenopathy include a mass >10 cm and a ratio of mediastinal mass to the chest diameter at T5-6 exceeding 0.35 (employed in European Organization for the Treatment of Cancer [EORTC] clinical trials). Contrast-enhanced (diagnostic) computed tomographic (CT) scans of the chest, abdomen, and pelvis may reveal adenopathy or organ involvement. If irradiation to the cervical nodes is contemplated, a CT scan of the neck may be indicated in order to identify their precise location for treatment planning. Lymph nodes are usually considered to be enlarged on CT if their short axis measurement exceeds 1 cm.29 Splenomegaly or hepatomegaly alone cannot be interpreted to represent involvement by Hodgkin lymphoma because enlarged spleens often are not involved at the time of splenectomy; however, the presence of focal nodules is usually indicative of involvement.
Positron emission tomography (PET) using 2-fluoro-2-deoxy-D-glucose (FDG), especially with fused CT images (PET-CT scan), has become an important component of initial staging in Hodgkin lymphoma. FDG-PET is more sensitive than CT for detecting disease.30 It may even reveal unsuspected bone or bone marrow disease.31 PET-CT is an essential study for response assessment, is particularly useful for the evaluation of residual masses detected by CT scanning, and may even be a useful prognostic indicator when repeated after just a portion of chemotherapy has been administered.29,32,33
Magnetic resonance imaging may be an alternative to chest or abdominal-pelvic CT scanning for initial staging but has not been used widely.34 Its main value may be in the staging evaluation of women during pregnancy.19
A needle biopsy of the posterior iliac crest bone marrow is appropriate in selected patients. Because the yield is exceedingly low in asymptomatic patients with limited clinical disease, it should be restricted to patients with B symptoms or clinical evidence of subdiaphragmatic disease. The overall incidence of bone marrow involvement in Hodgkin lymphoma is only ~5%.
 STAGING
STAGING
The Ann Arbor staging system for Hodgkin lymphoma, used since 1971, is outlined in Table 77.2.35 The lymphoid regions defined in this system are shown in Figure 77.2. The Ann Arbor system includes designation of a clinical stage, based on the results of the initial biopsy and clinical staging studies, and a pathologic stage, based on the results of any subsequent biopsies, including bone marrow biopsy and those obtained at staging laparotomy. With the exclusion of laparotomy, the generic term stage is now usually employed and reflects the final stage designation after completion of all appropriate staging studies. Deficiencies of the Ann Arbor system include its failure to consider bulk of disease and its lack of a more precise definition of the E-lesion (extralymphatic involvement).36 However, the Cotswolds modification of the Ann Arbor system employs the subscript “x” to designate large mediastinal adenopathy.37
FIGURE 77.2. The lymph node regions as defined in the Ann Arbor staging system. Note that the ipsilateral supraclavicular, cervical, preauricular, and occipital nodes are defined as a single region. The mediastinum and pulmonary hila are defined as separate regions. (From Hoppe RT. The non-Hodgkin lymphomas: pathology, staging, treatment. Curr Probl Cancer 1987;11:363–447; with permission.)
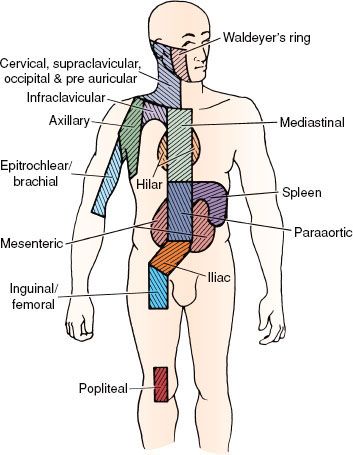
TABLE 77.2 THE ANN ARBOR STAGING CLASSIFICATION FOR HODGKIN’S DISEASE

 PATHOLOGIC CLASSIFICATION
PATHOLOGIC CLASSIFICATION
The neoplastic cell of classic Hodgkin lymphoma is the Reed–Sternberg cell. It is typically binucleate, with a prominent, centrally located nucleolus in each nucleus, a well-demarcated nuclear membrane, and eosinophilic cytoplasm with a perinuclear halo. However, these cells usually account for <1% of the cells in a lymph node involved by Hodgkin lymphoma. The majority are lymphoid cells, eosinophils, plasma cells, and other normal cells.38
Reed–Sternberg cells probably originate from B-lineage cells at various stages of development, including pre–B-cell and germinal center B-cell origin.39,40 In most instances, the Reed–Sternberg cells stain positively with the lymphocyte activation marker CD30, PAX5, and with variable expression of the antigranulocyte monoclonal antibody CD15. CD20, a marker of mature B cells, may be expressed on a minority of tumor cells with variable intensity in as many as 40% of cases. They stain negatively with CD45, ALK, and J chain.41
There are five histologic subtypes of Hodgkin lymphoma as defined by the World Health Organization modification of the Lukes and Butler system. These include nodular lymphocyte-predominant Hodgkin lymphoma and four subtypes of classic Hodgkin lymphoma: nodular sclerosis, mixed cellularity, lymphocyte-rich, and lymphocyte-depleted.42
Nodular lymphocyte predominant Hodgkin lymphoma (nLPHD) is characterized by an abundance of normal-appearing lymphocytes and a scarcity of abnormal cells. Unlike the other subtypes of Hodgkin lymphoma, the abnormal cells (“L and H cells” or “popcorn cells”) in nLPHD are strongly reactive for CD20, CD45, CD79a, and PAX5 and negative for CD15 and CD30.38,41 nLPHD is often diagnosed in young people. Patients frequently present with early-stage disease, usually in a solitary peripheral nodal site, and systemic symptoms are uncommon (<10%). The natural history is the most favorable of the histologic subtypes. Occasional patients demonstrate a pattern of late relapse but good survival, similar to that observed in the follicular (B-cell) lymphomas. Some investigators suggest that nLPHD would be more appropriately considered a form of non-Hodgkin B-cell lymphoma.43,44–45 As part of its natural history, as many as 14% of patients with nLPHD may transform to an aggressive B-cell lymphoma.46 A reactive process termed progressive transformation of germinal centers may be observed in conjunction with nLPHD.47,48
The other four histologic subtypes of Hodgkin lymphoma are variants of classic Hodgkin lymphoma (cHD). The Reed–Sternberg cells in these cases are CD15+, CD30+, PAX5+, and occasionally CD20+. Nodular sclerosis classical Hodgkin lymphoma (NSHD) is the most common histologic subtype diagnosed in developed countries. Involved nodes often have a thickened capsule and are traversed by broad bands of birefringent collagen that surround nodules of cells consisting of lymphocytes, eosinophils, plasma cells, and tissue histiocytes intermixed with a variable proportion of atypical mononuclear cells and Reed–Sternberg cells. These cells may be in empty (lacunar) spaces, which are artifacts of formalin fixation. The syncytial variant refers to cases in which there are prominent cellular aggregates of atypical cells and histiocytes. The clinical presentation includes common mediastinal involvement, and one-third of patients have B symptoms. The natural history of NSHD is less favorable than that of nLPHD.
Mixed-cellularity classic Hodgkin lymphoma (MCHD) is characterized by a diffuse effacement of lymph nodes by lymphocytes, eosinophils, plasma cells, and relatively abundant atypical mononuclear and Reed–Sternberg cells. Patients with MCHD present more commonly with advanced disease and tend to be slightly older than those with NSHD or nLPHD. The natural history of MCHD is less favorable than that of NSHD.
Lymphocyte-rich classic Hodgkin lymphoma (LRHD) is a relatively recently described entity.49,50 It usually has a nodular growth pattern, but occasionally it has a diffuse one. Previously, many cases of LRHD may have been confused with nLPHD; however, the staining characteristics of the malignant cells in LRHD clearly are consistent with those of classic Hodgkin lymphoma. The clinical characteristics of patients affected by LRHD are similar to those of nLPHD, that is, early stage, absence of B symptoms, and excellent prognosis.
Lymphocyte-depleted classic Hodgkin lymphoma (LDHD) is variable in its microscopic appearance but is generally characterized by a paucity of normal-appearing cells and an abundance of abnormal mononuclear cells, Reed–Sternberg cells, and Reed–Sternberg variants. This subtype may be difficult to differentiate from anaplastic large-cell lymphoma.51 It is an exceedingly uncommon subtype of Hodgkin lymphoma. It tends to occur in older patients and is more likely to be associated with advanced disease and B symptoms. It has the worst prognosis of all histologic subtypes of Hodgkin lymphoma.52
In addition to these major subtypes, interfollicular Hodgkin lymphoma is an uncommon pattern of focal involvement of a lymph node in which there is reactive hyperplasia with a small focus of Hodgkin lymphoma in the interfollicular zone. It is easy to confuse these cases with reactive lymphoid hyperplasia.53
Table 77.3 summarizes the characteristics of patients treated according to the major histologic subtypes of Hodgkin lymphoma at Stanford University from 1989 to 2010. These characteristics are similar to those reported from many other large centers in the United States and western Europe. However, the distribution of histologic subtypes and clinical behavior reported from South America, Asia, Africa, Eastern Europe, and even some parts of the United States indicates a greater proportion of unfavorable histologic subtypes and more aggressive clinical behavior in these developing areas.54,55
TABLE 77.3 MAJOR HISTOLOGIC SUBTYPES CORRELATED WITH CLINICAL CHARACTERISTICS OF 615 ADULT PATIENTS TREATED FOR HODGKIN LYMPHOMA AT STANFORD UNIVERSITY (1989–2011)
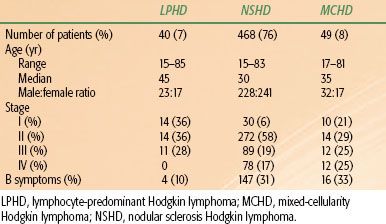
TABLE 77.4 INTERNATIONAL PROGNOSTIC SCORE FOR ADVANCED HODGKIN LYMPHOMA

 PROGNOSTIC FACTORS AND THERAPEUTIC IMPLICATIONS
PROGNOSTIC FACTORS AND THERAPEUTIC IMPLICATIONS
Because most patients with Hodgkin lymphoma are cured, prognostic factors are more important for defining therapy than for predicting outcome. Historically, when treatment programs were less effectie, prognostic factors did have great importance in predicting survival.56
The Ann Arbor stage is likely the most important factor influencing therapy. Data generated at a time when treatment programs were more limited show a marked impact of stage on prognosis. With current management programs, this distinction has been blurred.
The bulk of disease is important, especially in the mediastinum. Bulk may be defined by absolute measurements, ratio of mass to anatomic measurements, surface area on radiographs, or volumetric determinations. In general, the risk of relapse after treatment with single-modality therapy is greater in the presence of bulky mediastinal disease than in nonbulky disease.57 For this reason, combined-modality therapy is the established standard for patients with bulky disease.58,59,60–63
Other measurements of disease severity that may influence treatment selection for Hodgkin lymphoma are the presence of B symptoms, the number of sites of involvement, and the elevation of serum markers such as the ESR. Sophisticated assessments of total tumor burden correlate very well with prognosis.64
The histologic subtype of Hodgkin lymphoma has little impact on therapy, with the exception of identification of cases of nLPHD, for which different treatment algorithms will apply.62 Interobserver agreement among pathologists regarding subclassification is not perfect,65 and after the extent of disease has been determined, histologic subtype of classical Hodgkin lymphoma has little additional impact on prognosis.
Large series report a slightly worse outcome for men than for women.1 However, gender is more important because of its influence on the choice of treatment secondary to potential reproductive complications (see Sequelae of Treatment).
An important international study evaluated a series of prognostic factors among 5,141 patients with advanced Hodgkin lymphoma.27 Seven factors were identified, each of which had an independent and similar impact on prognosis. These included gender, age, Ann Arbor stage, hemoglobin, white cell count, lymphocyte count, and albumin (Table 77.4). This International Prognostic Score is now used for assignment of patients to clinical trials. Patients who have three or more adverse risk factors are often considered to be in an unfavorable prognostic group for advanced Hodgkin lymphoma.
Patients with stage I or II disease often have only one or two adverse factors based on this index. Large clinical trials groups have found other factors, such as number of sites of disease, age, ESR, and presence of B symptoms, to be helpful in stratifying patients according to prognosis. Unfortunately, the criteria for defining “unfavorable” presentations of stage I–II disease vary among clinical trial groups (Table 77.5).
TABLE 77.5 DEFINITION OF “UNFAVORABLE” STAGE I TO II HODGKIN LYMPHOMA IN RECENT CLINICAL TRIALS
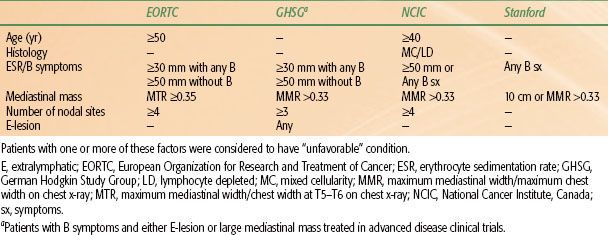
TABLE 77.6 COMMON DRUG COMBINATIONS USED IN THE TREATMENT OF HODGKIN LYMPHOMA
 GENERAL MANAGEMENT
GENERAL MANAGEMENT
Radiation Therapy
Radiation therapy is the most effective therapeutic agent for treating Hodgkin lymphoma and has been used in its management for more than a century.66 Optimal irradiation technique includes careful pretreatment evaluation of disease sites, precise simulation and the use of megavoltage photon beams, fields individually contoured to the patient’s anatomy and tumor configuration, an adequate dose, multifield fractionated treatment, and portal film verification during therapy. Careful attention must be paid to every detail of therapy in order to maximize outcome and minimize risks.67,68
Chemotherapy
The initial successful drug combination for treating Hodgkin lymphoma was nitrogen mustard, vincristine, procarbazine, and prednisone (MOPP), reported by DeVita et al.69 from the National Cancer Institute in 1970. The acute toxicities of treatment at that time were significant and included nausea, vomiting, peripheral neuropathy, constipation, leukopenia, and thrombocytopenia. Late effects of concern included sterility (especially in men) and the risk for secondary myelodysplastic syndrome or leukemia. A number of MOPP-like programs, such as chlorambucil, vinblastine, procarbazine, and prednisone (ChlVPP), achieve comparable results with similar drugs and less toxicity.70
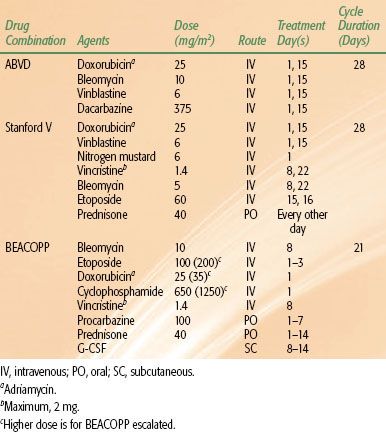
With the introduction of doxorubicin (Adriamycin), completely novel drug combinations were developed. The most successful of these is ABVD, which includes doxorubicin, bleomycin, vinblastine, and dacarbazine.71 ABVD has replaced MOPP as the gold standard of chemotherapy for Hodgkin lymphoma. This is based largely on the results of an intergroup trial that compared MOPP, ABVD, and MOPP/ABVD.72
More recently, in an effort to reduce toxicity, the Stanford V regimen, which almost always includes a component of radiation, was developed as an alternative to ABVD. As another approach, in an effort to enhance efficacy, the German Hodgkin Study Group (GHSG) developed the BEACOPP regimen, which may be administered in a baseline, escalated, or 14-day schedule.73 Table 77.6 summarizes the dosages and scheduling of these drug combinations.70
Most recently, a completely new systemic agent has been approved by the FDA for the treatment of Hodgkin lymphoma. Brentuximab vedotin (BV) is an anti-CD30 monoclonal antibody linked to an antitubulin agent. BV has demonstrated efficacy in CD30+ lymphomas, including Hodgkin lymphoma and anaplastic large-cell lymphoma. It is approved for patients who have had disease recurrence after stem cell transplantation and is being introduced in clinical trials to define its possible use in other settings.74
Combined-Modality Therapy
Combined-modality therapy has become the most common form of general management for patients with Hodgkin lymphoma. Important considerations include the sequence of therapy, the selection of irradiation fields, the decision to irradiate all involved sites, only initially “bulky” sites, or only sites that have not responded completely to chemotherapy, the prescription of dose, and potential overlapping toxicities. Treatment is almost always initiated with chemotherapy. This has the advantages of treating all sites of disease at the outset (especially important in stage III or IV) and reducing bulky disease to facilitate subsequent irradiation (especially in the mediastinum). The irradiation dose used in combined-modality studies in adults ranges from 20 to 36 Gy.
 RADIATION THERAPY TECHNIQUES
RADIATION THERAPY TECHNIQUES
The principal objective of radiation therapy in Hodgkin lymphoma is to treat involved nodes and regions at high risk for containing disease to a dose associated with a high likelihood of tumor eradication. This requires thoughtful evaluation of all imaging studies, especially diagnostic CT and integrated PET-CT scans, a precise simulation with appropriate immobilization and consideration of organ motion, detailed treatment planning, and effective treatment delivery, including portal imaging and appropriate quality assurance measures.67,68,75,76
The most common techniques for field blocking include multileaf collimation, divergent Cerrobend (Cerro Metal Products, Bellefonte, PA) blocks attached to a Lucite (Lucite International, Southampton, United Kingdom) plate and mounted to the head of the machine, or a combination of both for complex-shaped fields. The use of body molds reduces body movement and rotation and may increase patient comfort.
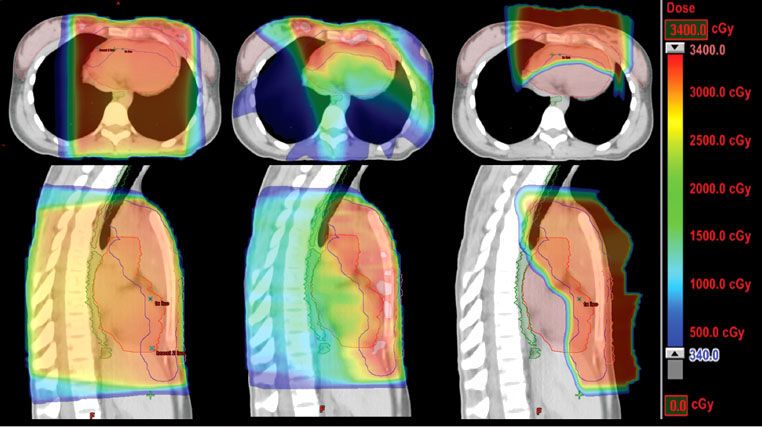
For the majority of clinical scenarios, three-dimensional conformal treatment planning and opposed-field treatment is appropriate, but occasionally intensity-modulated radiation therapy (IMRT) may be indicated.77 IMRT has the inherent advantage of better dose conformality, improved dose–volume histogram (DVH) criteria for the heart, coronary arteries, esophagus, and lungs, and less acute toxicity, but this is at the expense of the low-dose “bath,” which may put larger volumes of normal tissue at risk for the development of secondary cancer, for example, the lungs, breasts (in women), and thyroid gland (Figs. 77.3 and 77.4). This remains a key consideration whenever the use of IMRT is contemplated. IMRT may be most useful in situations of reirradiation, when disease has relapsed in a previously treated site.
Routine three-dimensional (3D) CT simulation is required to optimize treatment field design and analyze DVHs in order to ensure adequate tumor coverage and sparing of organs at risk (OARs). When treatment includes the mediastinum, axillary, and supraclavicular areas, an arms-up position pulls the axillary nodes away from the chest wall and thereby permits more generous lung shielding but may result in an increased dose to the breasts and heart and an enhanced skin reaction in the supraclavicular area. An arms-down or akimbo position permits shielding of the humeral heads and minimizes skin reaction in the tissue folds of the supraclavicular/low neck regions and to the breasts.78 When patients require separate treatment to adjacent regions, the calculation of field separation (gap) is exceedingly important.79 Special additional cord blocking should be used, if possible, when adjacent fields overlie the spinal cord.
FIGURE 77.3. Color-wash dose distributions for three different plans for treating mediastinal Hodgkin lymphoma: axial sections (top) and sagittal sections (bottom) for conventional photon three-dimensional conformal anteroposterior/posteroanterior fields (left), intensity-modulated radiation therapy photon (middle), and anterior proton field (right). Green outline, esophagus; red outline, heart; pink outline, breasts; blue outline, clinical target volume. (Courtesy of Bradford S. Hoppe, MD, MPH.)
In the uncommon situations when radiation therapy alone is used for the treatment of Hodgkin lymphoma (primarily for nLPHD), the National Cancer Center Network (NCCN) guidelines recommend a dose of 30 to 36 Gy to involved and 25 to 30 Gy to uninvolved regions, fractionated at a rate of 7.5 to 10 Gy per week to involved sites.62 Evenly weighted opposed-field treatments, all fields treated daily with fractions of 1.5 to 1.8 Gy, depending on field size and patient tolerance, are the general treatment recommendations. More commonly, radiation therapy is used in the combined-modality setting. Doses vary considerably in different trials, depending on the prognostic category of patients, bulk of disease, and type and duration of chemotherapy. The range of doses considered acceptable according to the NCCN guidelines is 20 to 30 Gy for nonbulky and 30 to 36 Gy for bulky sites of disease, 1.5 to 1.8 Gy per fraction.62
The classic field configurations for the treatment of Hodgkin lymphoma by radiotherapy alone included the mantle and inverted-Y. However, clinical trials have demonstrated an equivalence of “involved-field” treatment (IFRT) with “extended-field” or “subtotal-lymphoid” irradiation in the context of combined-modality therapy programs, and involved-field irradiation has been adopted as the standard for combined-modality therapy.80–82 More recent trials are testing the application of even more restricted fields, referred to as “involved-node radiotherapy” (INRT; discussed later)83 of “involved-region” or “involved-site” irradiation. Because these more limited fields are really portions of the classic treatment fields for Hodgkin lymphoma, an understanding of these classic fields makes the design of limited fields more logical.
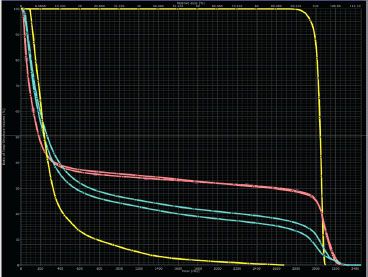
FIGURE 77.4. Representative dose–volume histograms for the three plans displayed in Figure 77.3. Anteroposterior/posteroanterior photons (top), intensity-modulated radiation therapy photon (middle), protons (bottom). Red, heart; green, esophagus; blue, lungs; pink, breasts. (Courtesy of Bradford S. Hoppe, MD, MPH.)
PET-CT “Simulation”
In the current treatment paradigm for Hodgkin lymphoma, radiation therapy is nearly always administered in the combined-modality therapy setting. Integrated PET-CT imaging is completed as part of the initial staging for all patients and provides accurate information regarding the initial extent of disease. However, this initial scan is not usually obtained in the treatment position or on a flat couch. After the completion of chemotherapy, the PET-CT scan is usually repeated, and generally one can expect normalization or near normalization of the PET component despite residual disease on CT.84 After the radiation therapy simulation study has been completed (postchemotherapy), the data from the initial staging PET-CT may then be merged with it in order to localize the initial sites of disease. The accuracy of the registration will vary, depending on patient positioning for the two studies, and this needs to be accounted for in ultimate design of the treatment fields.
FIGURE 77.5. A 28-year-old man with massive mediastinal nodular sclerosis Hodgkin lymphoma and right supraclavicular disease, following completion of chemotherapy, with a negative positron emission tomography (PET) scan. White, pretreatment PET+ disease; black, postchemotherapy residual abnormality on computed tomography (CT). A: Design of a modified “involved field” to include the mediastinum, bilateral hila, and supraclavicular areas with an anterior larynx block. Note that inferiorly the field includes the entire length of the original extent of disease plus 2-cm margin. However, laterally the field encompasses only the residual disease on CT plus 2-cm margin. B: Design of an “involved-site” field.
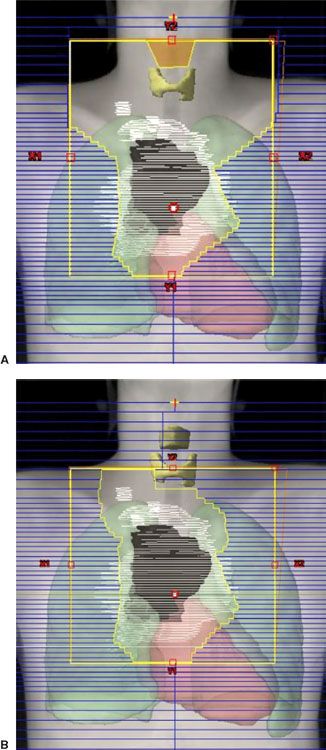
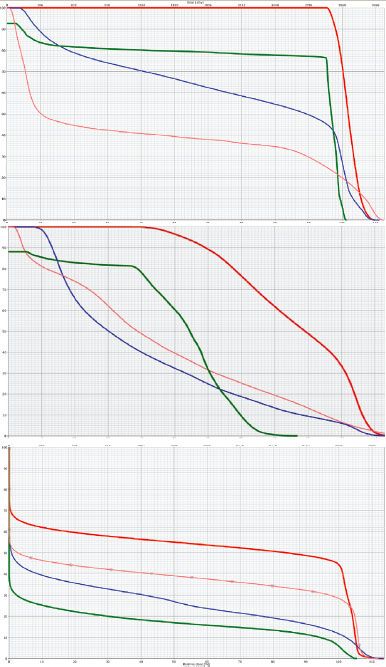
FIGURE 77.6. Representative dose–volume histograms for the fields displayed in Figure 77.5. Small triangles, involved field; small squares, involved site. Yellow, thyroid; pink, heart; blue, lungs. With this configuration of disease the most notable sparing of organs at risk is for the thyroid, with some sparing of the lungs. There is little difference in heart dose. If the disease was more superior in location, sparing of the heart using an involved region would have been more notable.
Supradiaphragmatic Fields
The classic mantle included all of the major lymph node regions above the diaphragm.85 The field extended from the inferior portion of the mandible almost to the level of the insertion of the diaphragm. Individually contoured lung blocks conformed to the patient’s anatomy and tumor localization. In addition to the lung blocks, blocks could be placed over the occipital region and spinal cord posteriorly, the larynx anteriorly, and the humeral heads both anteriorly and posteriorly. The use of these blocks depended on total dose planned and proximity of the adenopathy.
In the classic two-dimensional planned mantle field, the patient was set up supine, with the head fully extended. The superior margin of the field bisected the mandible and passed through the mastoid process. The lateral margins were set to flash the axillae (with humeral head blocks if the arms were at sides or akimbo). The inferior axillary margins were at the level of the inferior tips of the scapulae. The inferior mediastinal border was set at the level of the T10-11 interspace. The lung blocks were designed to provide ~1-cm margin around the mediastinal contours and also encompass the pulmonary hilar lymph nodes. The superiormost point of the lung blocks was no higher than the inferior tip of the head of the clavicle, with the tops of the lung blocks tapered laterally, often parallel to the projection of a posterior rib, in order to expose the high axillary/infraclavicular lymph nodes.
As noted previously, in contemporary management programs of combined-modality therapy, more limited fields are treated. Involved-field treatment includes just portions of the classic radiation treatment fields, and in the original definition implied treatment to the entirety of an involved lymphoid region when any portion of that region was involved. However, the definition of these regions, based on anatomic boundaries, is somewhat arbitrary. Logical considerations mandate modification of these regions in the context of individual patient management. For example, in the common scenario of supraclavicular disease (level IV) without disease any higher in the neck, the submaxillary and submandibular nodes (levels I and II) may be spared. On the other hand, the supraclavicular region is often included when the superior mediastinum is involved because a portion of the superior mediastinum actually superimposes on the lower supraclavicular area in the treatment position. The axillae are not treated unless they are involved. The two-dimensional design of these fields includes a superior border at the top or bottom of the larynx (depending on the extent of supraclavicular disease), lateral borders set at the coracoid processes of the scapulae (to include approximately two-thirds the length of the clavicle), and 0.5- to 1-cm margins beneath the clavicles. With 3D treatment planning, the initially involved lymph nodes (gross tumor volume [GTV]) and adjacent “at-risk” lymph nodes (clinical target volume [CTV]) are outlined on cross-sectional images, and field design is completed to ensure a dose range of 95% to 105% of the prescribed dose to the planning target volume (PTV). The CTV will generally extend 2 to 5 cm proximal and distal to initial PET- or CT-positive disease. The PTV expansion is then ~1 cm (Figs. 77.5A and 77.6).
In the setting of an initial large mediastinal mass, the postchemotherapy treatment fields can usually conform to the width of the residual disease only (unless there was pulmonary parenchymal extension), although the superior and inferior field margins should encompass the initial extent of disease, with margin as noted previously.67 Although special techniques such as deep inspiration breath hold, active breathing control, and respiratory gating are infrequently used in treatment of Hodgkin lymphoma, they do demonstrate improvements in DVH criteria for the lungs and heart that would be especially useful in the setting of treatment for patients with large mediastinal masses.86,87
Organs at Risk
The dose range used for Hodgkin lymphoma (20 to 36 Gy) is below the threshold tolerance for many organs, including the spinal cord. However, intrathoracic structures may be affected by these doses, and careful review of dose–volume histograms is warranted to minimize both acute and late effects. The risk for pneumonitis is related to volume of lung irradiated, total dose, and fraction size. The relationship between mean lung dose or Vx and pneumonitis is complex and may vary for different diseases and depend on patient age, smoking history, presence of intercurrent disease, prior chemotherapy, prior surgery, and so on.88 One study of patients with Hodgkin lymphoma identified a higher risk for Radiation Therapy Oncology Group grade 2 pneumonitis when the mean lung dose exceeded 14 Gy or the V20 exceeded 35%.89 The likelihood of radiation-related pulmonary complications may be increased by the use of bleomycin,90 and the effect that drug combinations with different doses of bleomycin may have on acceptable mean lung dose or V20 values is not known. A guideline followed at Stanford is not to exceed a mean lung dose of 15 Gy after treatment with ABVD or 17 Gy after treatment with Stanford V, treating with 1.5-Gy fractions. When these parameters have been followed, the risk for radiation pneumonitis has been negligible. With respect to late carcinogenesis, because data indicate that lung cancer risk may be increased after doses as low as 5 Gy, it is reasonable to define the lung V5 and try to minimize it if multiple plans are reviewed.91
The criteria for cardiac dose tolerance are not well defined. Again, tolerances will be affected by comorbidities, family history, and prior treatment with cardiotoxic drugs such as doxorubicin. With respect to acute effects (pericarditis), limited data suggest keeping the mean pericardial dose to <26 to 27 Gy and the V30 to <46%.92,93 Data for late cardiac events are less reliable; however, in one study of patients with Hodgkin lymphoma a threshold effect at 30 Gy was suggested for cardiac mortality.94 A more conservative estimate, based on patients irradiated for breast cancer, is that a cardiac V25 of <10% is associated with a very low risk of cardiac mortality.95 This level may be difficult to achieve in patients who present with large mediastinal adenopathy. Treatment techniques such as IMRT may succeed in reducing the cardiac dose but increase the V5 to the lungs or V4 to the breasts, resulting in a higher risk for secondary cancer in those organs (Figs. 77.3 and 77.4).
With respect to the breasts in women, given the excess risk of secondary breast cancer that exists for doses as low as 4 Gy, it is reasonable to track the breast V4 and keep that volume as small as possible, especially for women <30 years of age.96
Subdiaphragmatic Fields
The classic subdiaphragmatic irradiation field for Hodgkin lymphoma was the inverted-Y, which included the retroperitoneal and pelvic lymph nodes and spleen. Sequential treatment to a mantle and inverted-Y field was referred to as total lymphoid irradiation (TLI); if the subdiaphragmatic field did not include the pelvis, the term subtotal lymphoid irradiation was used.
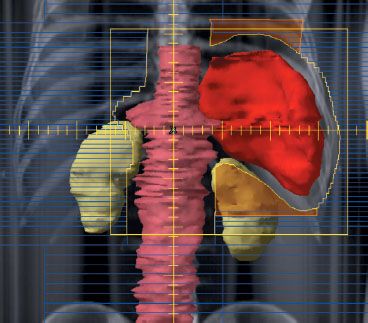
Currently, in the context of combined modality therapy, common radiation therapy fields include the spleen with or without the para-aortic nodes (Fig. 77.7) and unilateral or bilateral pelvic fields (Fig. 77.8). In the two-dimensional design of a para-aortic field, the width of the field generally corresponds to the width of the transverse processes. The spleen may be treated in contiguity with this field. The design of the splenic field requires consideration of respiration and the use of generous (~2 cm) superior and inferior margins or respiratory gating. Three-dimensional planning for this field, especially if the spleen is being irradiated, is essential in order to more accurately localize the spleen and evaluate the DVH for the left kidney (Fig. 77.7). GTV, CTV, and PTV considerations for subdiaphragmatic nodal fields are similar to those for supradiaphragmatic fields, that is, GTV includes prechemotherapy disease on PET or CT, CTV includes 2 to 5 cm proximal and distal, and PTV expansion is 1 cm.
Careful blocking considerations are required when the pelvic region is treated. Because the volume of marrow in the pelvis is substantial, the fields must be shaped carefully to minimize the amount of marrow treated (Fig. 77.8). With two-dimensional planning, the lateral margins are set 1.5 to 2 cm lateral to the widest point of the bony pelvis. Inferiorly, the pelvis field should extend to at least the lesser trochanters, unless there is disease that extends further inferiorly. Gonadal toxicity may also be an issue. In women, the ovaries normally overlie the iliac lymph nodes. To avoid irradiation-induced amenorrhea, an oophoropexy must be performed. This procedure is done by medial or lateral transposition of the ovaries via laparoscopy. The surgeon marks the ovaries with radiopaque sutures or clips and relocates them medially and as low as possible behind the uterine body. A double-thickness (10 half-value layers) midline block is then used; its location is guided by the position of the opacified nodes and transposed ovaries. When the ovaries are at least 2 cm from the edge of this block, the dose is decreased to 8% of that delivered to the iliac nodes.97 Alternatively, one or both of the ovaries can be transposed laterally to a position overlying the iliac wings.
In men, if no special blocking is provided for the testes, the testicular dose may be as high as 10% of the dose delivered to the inguinal-femoral nodes. Use of a double-thickness midline block and a specially constructed testicular shield can reduce this dose to 0.75% to 3.0%, most of which results from internal scatter. The precise dose depends on the position of the testes in relation to the inferior margin of the inguinal-femoral field.
FIGURE 77.7. Example of a para-aortic–spleen field treated with respiratory gating. In this example there was a positron emission tomography+ node at the L1 level, and the inferior portion of the para-aortic field was set at the bottom of L2. The para-aortic nodes are highlighted in light red and the spleen in dark red. Due to the complex shape of this field, it is defined with a combination of multileaf collimators and Cerrobend blocks (shown in light orange) to shield the base of the left lung and the upper half of the left kidney.