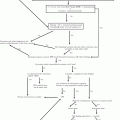
Fig. 26.1
Suggested diagnostic work-up for staging of Kaposi sarcoma
It is now known that the pathogenesis of all clinical variants of KS involves infection with KSHV, a deoxyribonucleic acid (DNA) virus first isolated by Chang et al. in 1994 from the KS lesions of an AIDS patient. It has also been established that although necessary, KSHV by itself is not sufficient for initiation and progression of KS. In HIV-seropositive patients it is postulated that HIV-mediated immune suppression/dysregulation promotes T-helper type-1 cytokines such as TNF-alpha, Interleukin-1b (IL-1b) and IL-6. Production and release of HIV-Tat protein from HIV-infected cells further contribute to release of proinflammatory cytokines, vascular endothelial growth factor (VEGFs) and matrix metalloproteinases (MMPS), which all together facilitate the proliferation of endothelial cells and development of KS. HIV-Tat protein and chronic inflammation also mediate reactivation and replication of latent KSHV, promoting expression of viral gene products implicated in angiogenesis [9, 10].
KSHV Seroprevalence and Mode of Transmission
The seroprevalence of KSHV among the general population varies geographically. The precise mode of its transmission though not clearly understood is believed to include vertical, horizontal through sex or oral shedding, blood transfusion and injection drug use, as well as solid organ or bone marrow transplantation [11]. In areas where KSHV infection is endemic the infection is thought to be acquired in childhood from seropositive family members and seroprevalence rates increase with age reaching as high as 80 %.
Natural Course of HHV8 Infection in Children
In immunocompetent children, HHV8 may be associated with a febrile maculopapular skin rash while in HIV-infected older children a transient angiolymphoid hyperplasia occurs as part of the HHV8 seroconversion syndrome. In children it is believed that KS is a manifestation of primary infection, whereas in older children KS occurs after primary infection.
Clinical Features
Epidemic KS has a wide spectrum of presentation ranging from minimal disease that is discovered incidentally on a routine clinic visit to aggressive growth with significant morbidity and mortality. In general the presentation may be classified into lymphadenopathic, cutaneous, mucosal, visceral and other [9, 11].
Lymphadenopathic KS
This is the most common presentation of disease in children. It tends to occur in younger children with relatively higher CD4 counts likely owing to recent HHV8 infection with a rapid progression to malignancy (since the virus is tropic for lymph nodes during seroconversion) [12]. Lymph node involvement may be the sole presentation of disease. Massive lymph node enlargement and lymphoedema may develop.
Cutaneous KS
Cutaneous lesions vary in size characteristics and number ranging from a small number of isolated lesions to widespread cutaneous involvement. The lesions may be small sub-centimeter macular/popular or large confluent nodules 10 cm or larger in the widest diameter. They are dark, almost black in dark/olive skinned patients and if chronic may appear violaceous and hairy. In some patients they may be arranged linearly and symmetrically along skin tension lines, while in others they are randomly distributed. When large they may become painful otherwise they are usually painless and non-pruritic. There may be associated woody edema. Plaque-like lesions may also occur. These lesions are similar to nodules but are more extensive locally, suggesting the coalescence of multiple nodules. The plaque-like lesions occur on the thighs, calves or soles of the feet and may be exophytic and fungating with breakdown of overlying skin. The lesions are often complicated with lymphoedema, which may occur as a relatively isolated finding and may be out of proportion to the extent of visible cutaneous disease. Plaque-like lesions may ulcerate, bleed or become a focus for secondary bacterial infection.
Mucosal Disease
Disease involving the oral cavity may be the initial presentation of KS. Oral lesions range from flat, red to violet papules to exophytic, ulcerative nodules. Lesions most commonly occur on the palate, oropharynx and gingival, but may involve any part of the mucosal surface including the tongue, tonsillar pillars and floor of the mouth, pharynx or trachea. These lesions may become painful, bleed or ulcerate if traumatized during normal chewing. They may become secondarily infected. When bulky they may interfere with nutrition and speech.
Laryngeal involvement may occur, the most common site being the epiglottis. Presenting symptoms of laryngeal KS may include pain, bleeding, dysphagia speech abnormalities and airway compromise. Indeed laryngeal KS must be excluded when a suspicious lesion in an HIV-infected patient is seen, as it is associated with severe bleeding, airway obstruction and death.
KS involving the sclera is commonly missed. In the early stages of disease it may appear as painless conjunctival injection particularly at the canthi of the eyes and is commonly treated as conjunctivitis. Red or purplish nodular lesions may also be seen involving the tarsal conjuctiva.
Visceral Presentation
Pulmonary involvement
Pulmonary KS must be excluded in the evaluation of HIV-infected patients with respiratory symptoms or abnormal chest X-ray (CXR) findings, especially in the presence of cutaneous KS. Pulmonary involvement usually occurs as a late manifestation of HIV disease, although it may occur at any stage of HIV. Almost all intra-thoracic structures may be involved in this disease including the tracheobronchial tree, pulmonary parenchyma and pleura. The lymph nodes may also be involved. Common presenting symptoms of pulmonary KS include cough, haemoptysis and shortness of breath, pleuritic chest pain and fever. Physical examination may be normal or non-specific with the presence of crackles or wheeze related to the involvement of the upper respiratory tract.
Gastrointestinal involvement
GI involvement may occur in the absence of cutaneous disease and many patients with GI KS remain asymptomatic. Patients with GI lesions may present with nausea, vomiting, abdominal pain, weight loss, upper and lower GI bleeding or intestinal obstruction.
Other organs
KS may involve other organs as well including the heart and pericardium, kidneys, urogenital tract and bone marrow. Involvement of the brain and intraorbital structures is rare likely owing to their lack of lymphatics.
KS Histology and Diagnosis
The diagnosis must be proven on tissue biopsy. Microscopic features of KS include an abundance of proliferating mononuclear inflammatory and spindle cells, ill-defined vascular channels, haemorrhage and oedema.
Initial work-up for staging epidemic KS involves a complete physical examination that includes evaluation of the skin, oral cavity and sclera to determine extent of mucocutaneous involvement.
Staging investigations will establish the presence of visceral involvement and include a Chest X-ray, Abdominal Ultrasound scan and faecal occult blood test.
Other necessary investigations include: a CBC, CD4 count/percentage, HIV viral load if possible, LDH, RFTs and LFTs (Fig. 26.1).
Staging and Prognosis of Epidemic KS
The outlook for HAART-naïve patients with epidemic KS is influenced at least as much by the presence of other AIDS-related problems as it is by the spread of KS. Thus, staging of epidemic KS also considers factors such as how much the immune system is damaged and the presence of AIDS-related infections [11]. In 1988, the AIDS Clinical Trial Group (ACTG) of the National Cancer Institute of Health developed a system that divides patients into good and poor risk groups based on three parameters and it considers three factors:
The extent of the tumour (abbreviated T)
The status of the immune system (I), as measured by the number of certain cells (CD4) or in the case of children less than 5 years, the CD4 percentage present in the blood
The extent of involvement within the body of systemic illness (S)
Under each of these major headings, there are two subgroups identified by either a zero (0 or good risk) or a 1 (poor risk) (Table 26.1).
Table 26.1
ACTG staging system for Kaposi sarcoma
Parameter | Good risk (all of the following symptoms) | Poor risk (any of the following symptoms) |
|---|---|---|
Tumour bulk | Confined to the skin and/or lymphnodes and/or minimal oral disease (non-nodular KS confined to the palate) | Tumour-associated oedema or ulceration |
Extensive oral KS | ||
Gastrointestinal KS | ||
KS in other non-nodal viscera | ||
Immune status | CD4 count >200 | CD4 count <200 |
CD4 percentage>15 % | CD4 percentage <15 % | |
Severity of illness | No history of opportunistic infection or thrush | History of opportunistic infection and/or thrush |
No B symptomsa | B symptomsa present | |
Karnofsky Performance status >70b | Karnofsky Performance Status <70 | |
Other HIV-related illness (e.g. neurologic disease, lymphoma) |
The current prognostic indicators for the staging of epidemic KS, proposed by Nasti et al., include tumour extension (T) and HIV-related systemic illness (S) resulting in good and poor survival depending on the combination of prognostic markers [13]. Patients with a combination of advanced disease and constitutional symptoms (T1S1) have the worst prognosis, while those with minimal disease (T0S0) had the best prognosis with the other patients (T0S1, T1S0) falling in between these extremes.
From his work the following tentative recommendations were made with regard to treatment of epidemic KS (Table 26.2).
Table 26.2
Recommended staging system for Kaposi sarcoma peadiatric HIV
Severity of AIDS-KS | Management approach |
|---|---|
T0S0 (focal disease in the absence of systemic illness) | Watchful waiting, consideration of CD4 count, viral load and active opportunistic infections prior to HAART initiation |
T0S1 (Early but mildly symptomatic KS, e.g. minimal cutaneous disease) | HAART ± local therapy |
T1 S0 (Early progressive AIDS-KS) | HAART |
T1S1 disease | HAART + chemotherapy |
Extensive disfiguring skin lesions | |
Widespread symptomatic cutaneous disease + edema | |
Rapidly progressive disease | |
Symptomatic visceral involvement | |
Obstructive or painful oropharyngeal disease | |
Inadequate response to HAART alone | |
IRIS-associated KS |
Treatment
Due to resource limitations and lack of evidence-based guidelines, practice in sub-Saharan Africa for paediatric KS is variable.
Management of epidemic KS is not aimed at cure rather it aims at palliation and control of KS progression and HAART is an integral component of this process.
HAART is the mainstay of treatment because the resultant immune restoration may be sufficient to induce remission. ART should be started promptly prior to referral to a paediatric oncology unit for staging and definitive management.
ART should be given to HIV-infected children, but there is no evidence to indicate whether ARTs should be started before, at the same time or after chemotherapy. It is also not determined if chemotherapy should be given to limited KS.
It is important to note that the advent of HAART has somewhat affected the prognostic significance of the ACTG staging system [13]. It was observed that patients on HAART who develop KS have less severe forms of disease compared to HAART-naïve patients at the time of KS diagnosis; i.e. severity of immunosuppression reflected in CD4 count is not an independent prognostic indicator for staging epidemic KS. HAART may be NNRTI or PI based. Although protease inhibitors are thought to have specific antiangiogenic effects, the choice of HAART regimen does not appear to influence protection against epidemic KS.
Local Therapy
Local therapy is safe, easy to administer and 1 for limited, asymptomatic mucocutaneous lesions of epidemic KS. It may also be considered when HAART is unavailable; response to HAART is less than optimal or as a palliative measure in patients with rapidly progressive mucocutaneous lesions causing pain, aesthetic concerns or interference with function. The local therapies available include
Cryotherapy with liquid nitrogen for focal skin lesions.
Surgical excision for focal superficial mucocutaneous lesions.
Sclerotherapy.
Intralesional therapy with vincristine or vinblastine. The procedure is painful and there may be necrosis if healthy tissue is injected; however, the effect lasts about 4 months. Other intralesional agents include bleomycin or alpha interferon. It is usually not recommended in children.
Radiotherapy is effective management of local or regional KS causing pain, bleeding or oedema, but it is not always available in all developing countries and if it is available it usually is not a priority for the treatment of KS in children.
Laser ablation therapy has been used particularly in lesions affecting the face or oral cavity.
Topical therapy with 0.1 % alitretinoin, imiquimod 5 % cream. The alitretinoin gel must be applied two to five times daily as tolerated and therapeutic response may take up to 3 months to be registered. It is also expensive and may be accompanied by skin reactions.
Systemic Therapy
Chemotherapy
The main drugs used in the treatment of KS in children are the following: vincristine, bleomycin, doxorubicin, etoposide, cyclophosphamide and paclitaxel.
The protocols described in the literature include monotherapy with vincristine, 2 drugs (most used are the vincristine and bleomycin), or 3 drugs (adding anthracycline—doxorubicin) (Table 26.3).
Table 26.3
Protocols
Single agent regimens (Monotherapy) |
Oral:Etoposide 100 mg/m2 po three times a week (may be increased to 200 mg/m2)—not recommended |
Intravenous: Vincristine 1.5 mg/m2 weekly for 3 weeks (six courses) |
Two agent regimens |
Vincristine 1.5 mg/m2 IV and bleomycin 15 iu/m2 IV or IM weekly every 3 weeks (6 courses) |
Three agent regimens |
ABV |
Day 1 and 15 doxorubicin 25 mg/m2 IV in 50 mL 5 % DW over 30 min; bleomycin 10 iu/m2 IV in 0.9 % saline over 15 min; vinblastine 6 mg/m2 IV bolus (4–6 courses) |
Single doses of vincristine or actinomycin should not exceed 2 mg; Cumulative doses of doxorubicin should not exceed 300 mg/m2 |
When anthracyclines are given, a baseline echocardiogram should be done and repeated after a total cumulative dose of 200 mg/m2 |
A: Adriamycin (doxorubicin); B, bleomycin; V, vincristine; Vinblastine can be replaced with vincristine at a dose of 1.5 g/m2 |
Adult guidelines recommend first-line liposomal doxorubicin, an expensive chemotherapeutic agent not readily available or sustainable in resource-limited settings, not approved as a first-line agent by the United States Food and Drug Administration (FDA), and yet to be approved by the FDA for paediatric patients [14, 15].
No evidence is available on the use of liposomal doxorubicin in paediatric HIV-associated KS. Therefore, alternative, more available and less expensive primary regimens consisting of adriamycin, bleomycin and vincristine (ABV) are utilized in resource-limited settings [12, 16].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree