Histoplasmosis, blastomycosis, and coccidioidomycosis are the most common endemic mycoses in North America. Their geographic distribution encompasses close to two-thirds of the United States and parts of Canada, Mexico, Central and South America, and Africa. Histoplasma capsulatum , Blastomyces dermatitidis , Coccidioides immitis , and C. posadasii are dimorphic fungi that grow as yeast in the human body and as a mycelial form in the environment. Infections occur as a result of exposures to contaminated environments. Although most infections result in a subclinical process, higher-inoculum exposures and infections in immunocompromised hosts can result in severe life-threatening conditions. Most immunocompromised patients affected by these pathogens are adults. Limited pediatric data are available for various aspects of the epidemiology, risk factors, clinical manifestations, and treatment. Recommendations have been mostly extrapolated from adult studies and anecdotal clinical experiences.
Epidemiology of endemic mycoses
Most infections involve the respiratory tract. Symptomatic extrapulmonary disease is more likely in immunocompromised individuals, which is more likely to lead to hospitalization. A retrospective cohort using a nationwide database of hospital admissions for 2002 estimated that 332 pediatric patients with symptomatic endemic mycoses were hospitalized in the United States, at a rate of 4.6 cases per 1 million with a mortality rate of 5%, compared with 6003 adults at a rate of 28.7 cases per million population and a mortality rate of 7%. Hospitalized children with histoplasmosis were more likely to have an immunodeficiency than adults: 32% versus 14%, respectively. The database provided geographic information confirming prior observations that coccidioidomycosis is most likely to be observed in the West, whereas blastomycosis and histoplasmosis usually occurs in the Midwest and southern states. Although distinctive geographic regions are associated with specific endemic mycoses, in recent years many cases have appeared in areas outside their usual regions, such as blastomycosis in Oregon and Colorado; histoplasmosis in Idaho, California, and New Mexico; and coccidioidomycosis in Washington State. Clinicians will need a greater index of suspicion when evaluating patients with signs and symptoms compatible with endemic mycoses, even in the absence of travel to or residence in traditional endemic regions. When considering a diagnosis of endemic mycoses in a patient with pulmonary disease, several factors need to considered, such as residence or travel to an endemic region, occupation, hobbies and leisure activities, the presence of birds and bats in dwellings, demolition of old structures, excavation or soil tilling, and animal exposures.
A retrospective study of 30 transplant patients with histoplasmosis or blastomycosis at 3 midwestern medical centers between 1996 and 2008 demonstrated a cumulative incidence of infection of 0.5%. A majority (73%) had undergone renal transplantation and were receiving multiple immunosuppressive agents, including corticosteroids. The median time from transplant to infection was approximately 10 months. As expected, the lungs were the most common site of infection. Sixty percent of patients had disseminated disease. A mortality rate of 13% was attributable to infection. Among the 22 patients with histoplasmosis, the median time from onset of symptoms to diagnosis was 30 days (range 4 to 42 days), whereas for blastomycosis it was 14 days (range 3 to 90 days). Graft loss was reported in 27% of patients.
Endemic mycoses are often overlooked as a cause of community-acquired pneumonia. A delay in diagnosis and treatment may result in severe disease in an immunocompromised host such as those affected by human immunodeficiency virus (HIV)/AIDS. In the case of blastomycosis, pulmonary findings on chest radiographs may resemble a bacterial etiology because lobar consolidation is a common feature. Nodular infiltrates may be seen in severe cases. Mediastinal or hilar adenopathy and pleural effusions are less common in blastomycosis. Diffuse reticulonodular or miliary infiltrates along with mediastinal and hilar adenopathies and eventual calcifications are seen in disseminated or severe histoplasmosis, as well as in tuberculosis.
An increased risk of fungal and mycobacterial infections is reported in patients receiving tumor necrosis factor (TNF) inhibitors. Histoplasmosis represents 60% of reported fungal infections in this group, and coccidioidomycosis and blastomycosis 10% and 4%, respectively.
Endemic mycoses are rare in solid organ transplant (SOT) patients. In one study, infection developed in only 33 patients among 16,806 patients who had undergone an SOT; the most common infection was histoplasmosis. However, invasive endemic mycoses can be a major cause of morbidity and mortality in kidney transplant patients, resulting in 41% graft failures and a fatality rate of 19%. Coccidioidomycosis was responsible for graft failures in 67% of patients. The use of antithymocyte globulin, diabetes mellitus, and age were also risk factors for infection. The risk of infection may persist for years after transplantation.
Histoplasmosis
Epidemiology and risk factors
Histoplasmosis is the most common pulmonary and systemic mycosis in humans and affects millions of people. The highest incidence was found in residents of the Ohio-Mississippi-Missouri, the St. Lawrence, and the Rio Grande river valleys. From 2001 to 2012, histoplasmosis-associated hospitalizations in the United States were estimated to be 50,778. Infection rates were lowest in persons younger than18 years. The significance of H. capsulatum as a cause of opportunistic infection has escalated in proportion to the increasing numbers of individuals who are immunosuppressed. An increase in hospitalizations has been observed in transplant recipients and in those receiving biologic agents.
The optimal conditions for H. capsulatum var. capsulatum to survive in the environment are moist, nitrogen-rich soils at temperatures of 37°C or higher. H. duboisii, the cause of African histoplasmosis, is a variant of H. capsulatum. Infections caused by H. capsulatum var. duboisii have been described across central and western Africa .
The majority of illnesses occur sporadically and are not associated with exposure to a specific site or specific activity. Infection rates are higher in summer months when persons are more likely to be outdoors in conditions that favor the aerosolization of spores. Asymptomatic infections are common in children and are usually clinically unrecognized. Regions with high rates of infection are considered endemic. Implicated sites have included blackbird and pigeon roosting areas, chicken houses, bat-infested caves, attics, chimneys, old structures, and decaying woodpiles and trees. Activities that disturb these areas have been implicated in localized outbreaks. Infections in children have been associated with exploring caves, playing in barns or hollow trees, silos, cleaning abandoned buildings, cutting firewood or decayed tree stumps, renovation of older homes, and digging in contaminated sites. Contact with these areas must be avoided by the immunocompromised person.
In a large outbreak in Indianapolis, Indiana, affecting an estimated 100,000 individuals, illnesses were identified in 435 individuals (including 49 children <15 years of age), and disseminated disease developed in 46. In reported outbreaks, 51% of cases occurred among children with a majority associated with common source exposures in schools. Rarely, infections occur in nonendemic areas and may result from reactivation of quiescent infection in patients who become immunosuppressed.
Mother-infant transmission with resulting dissemination has been documented after exposure to TNF-α blocker therapy. Transmission has been confirmed in the recipients of 2 cadaveric organs from a donor who had resided in an endemic area.
Antibodies against the organism are measurable approximately 1 month after infection, but these play no major role in controlling infection. Cell-mediated immune response is key in controlling fungal growth and provides a degree of protection against reinfection. H. capsulatum replicates within macrophages until T lymphocytes are activated. Granuloma formation occurs in response to infection of macrophages. The release of proinflammatory cytokines and chemokines is required for the development of a protective immune response. Reinfection usually results from reexposure; recurrences have shorter incubation periods and are generally milder than primary infections. Recrudescence of latent infection has been documented in recipients of transplanted organs from infected donors, and in people receiving corticosteroids or TNF-α inhibitors.
The length of the incubation period of histoplasmosis varies inversely with the size of the inoculum, the integrity of the host immune response, and the presence of immunity from previous infection. The range of incubation periods is reported to be 1 to 3 weeks in nonimmune hosts. Because most infections occur sporadically and are either asymptomatic or result in nonspecific and self-limited flu-like illnesses that are not diagnosed, the upper range may be longer. In patients who retain specific protective T-cell immunity from previous infection, reexposure results in milder symptoms and shorter incubation periods. A proportion of individuals with primary or acquired cellular immune dysfunction are more likely to experience symptomatic illness after exposure.
Granulomas appear after the development of an effective acquired immune response. Inflammation ultimately progresses to fibrosis and often is accompanied by calcification. The rate of calcification is age dependent, and it may occur within months in children and over several years in adults. Exuberant granulomatous inflammation or fibrosis or both can result in obstruction or dysfunction of adjacent mediastinal or, less commonly, abdominal structures. In areas endemic for histoplasmosis, old granulomas in the lung, bone marrow, or other sites may be seen as incidental findings. In patients with disseminated histoplasmosis, especially those with preexisting cellular immune dysfunction, or in otherwise healthy infants, the inflammatory response is impaired and granuloma formation is poor, leading to extensive parasitization of macrophages by yeasts. Many organ systems are often involved.
The use of antithymocyte globulin as part of a rejection prevention regimen is associated with severe histoplasmosis in kidney transplant patients.
Clinical manifestations
Histoplasmosis begins as an acute inflammatory pneumonitis and undergoes self-limited or progressive dissemination. Aside from patients with known preexisting conditions or those receiving therapy that impairs immune function, all patients with serious disseminated disease, persistent antigenuria after completion of therapy, relapse, or recurrent infections should undergo comprehensive assessment of immune function. Most symptoms of acute primary histoplasmosis are mild, self-limited, and undifferentiated resembling a flu-like illness with cough, myalgia, headache, and variable low-grade fever, with a resolution in 3 to 5 days. After a more significant fungal exposure, fever, myalgia, chills, persistent cough, and nonpleuritic chest pain may last as long as 2 weeks. Fatigue and weight loss improve slowly after the fever resolves. In about 5% of children, symptoms are subacute, persisting longer than 2 weeks.
Pericardial effusion, hypercalcemia, and mediastinal and abdominal manifestations resulting from irritation, compression, or destruction of structures adjacent to infected lymph nodes and granulomas are well described. Osteomyelitis and central nervous system (CNS) infections are unusual manifestations of histoplasmosis in healthy, older children. Hepatosplenomegaly occasionally is present, although its occurrence should raise suspicion of the early onset of progressive dissemination. Rheumatologic manifestations, including erythema nodosum, erythema multiforme, and polyarthropathy, can occur.
Acute primary infections after a high-inoculum exposure results in a diffuse pneumonitis associated with severe symptoms, particularly dyspnea or adult respiratory distress syndrome (ARDS) early in the infection. This may lead to dissemination with a high risk of progression. This presentation resembles the clinical picture observed in immunocompromised hosts with severe disease.
The appearance of intrathoracic and, less commonly intraabdominal, lymphadenitis can mimic malignant tumors . These are mostly seen when acute primary infections are accompanied by fever, weight loss, and masses visible on chest radiograph (mediastinal adenitis) or computed tomographic (CT) scans. The presence of mediastinal lymphadenopathy in the absence of any recognized clinical symptoms often requires definitive diagnosis to exclude a neoplasm, especially lymphoma. Complications of acute primary histoplasmosis are mostly seen when granulomatous lymphadenitis results in inflammation, compression, or obstruction of contiguous structures within the thorax, such as trachea, bronchi, pulmonary vasculature, vena cava, nerves, and lymphatics (mediastinal granuloma). In rare instances, this granulomatous inflammation may progress to the formation of a dense irreversible fibrosis, resulting in stenosis, obstruction, or malfunction of contiguous critical mediastinal structures. Fibrosing mediastinitis does not respond to antifungal therapy or antiinflammatory agents. This condition is rare in children. Constrictive pericarditis and cavitary histoplasmosis are also rarely observed in children.
Disseminated histoplasmosis
Fungal dissemination that occurs early in infection is almost always self-limited in normal individuals. The term “progressive disseminated histoplasmosis” is applied to instances of continued and overwhelming reticuloendothelial infection and is fatal if untreated. The clinical entity is defined as an illness that is accompanied by active replication of H. capsulatum in multiple organ systems. This manifestation often suppresses cellular immune function in previously immunocompetent hosts and is a common opportunistic infection in individuals with acquired or congenital cellular immune dysfunction.
Disseminated histoplasmosis may result from exogenous exposure of a susceptible or immune-impaired host or from reactivation of endogenous quiescent foci of infection. Although reactivation of infection may occur in an immunosuppressed host, epidemiologic data in immunosuppressed individuals who reside in areas highly endemic for histoplasmosis favor a new episode of exogenous exposure as the most common mechanism. Rates of disseminated histoplasmosis in immunocompromised patients increase only during periods in which infection rates increase in the general population and do not increase in interepidemic periods.
In a single-center retrospective analysis of pediatric patients with histoplasmosis, 16 patients (22%) were immunocompromised, with 5 affected by malignancy receiving chemotherapy, whereas others were receiving TNF-α inhibitors and corticosteroids. Disseminated and pulmonary disease affected 56% and 44% of patients, respectively. Cough, fever, fatigue, shortness of breath, and weight loss were common complaints. Immunocompromised patients had longer hospitalizations when compared with non-immunocompromised patients. Children with disseminated disease were also more likely to have antigenemia and antigenuria. Pneumonia with a rapid progression to marked hypoxemia and an ARDS-like picture is not uncommon in the immunosuppressed host with severe histoplasmosis. Fevers, chills, fatigue, anorexia, weight loss, and hepatosplenomegaly are features suggestive of disseminated disease.
CNS involvement is well recognized in patients with disseminated histoplasmosis. Clinical manifestations are varied and include chronic meningitis and arachnoiditis, hydrocephalus, focal parenchymal lesions, cerebellar ataxia, cranial nerve neuropathy, vasculitis, stroke, and/or diffuse encephalitis.
Unusual manifestations of histoplasmosis, especially in patients with disseminated disease, include skin and oral lesions, terminal ileitis, colonic ulcerations, adrenal involvement with insufficiency, endocarditis, genitourinary ulcerations, arthritis, osteomyelitis, sepsis-like syndrome, and superior vena cava syndrome.
Histoplasmosis-induced hemophagocytic syndrome is a well-recognized complication. It is a lethal complication of histoplasmosis observed in immunocompromised patients. Cytopenias, splenomegaly, and hyperferritinemia are clinical markers of the disease. Response to therapy can be measured by a reduction in ferritin levels.
Histoplasmosis in oncology patients
A retrospective review of 57 children with acute histoplasmosis at a pediatric oncology center provides a comprehensive view of the clinical spectrum of disease in children with cancer. A majority of patients had acute lymphocytic leukemia (64%). Ten patients were identified with acute pulmonary disease, and 23 (with 26 episodes) with disseminated disease. Most of the children with acute pulmonary histoplasmosis were not neutropenic at the onset of symptoms. Fever was the most common clinical feature with acute pulmonary and disseminated histoplasmosis, present in 60% and 96% of patients, respectively. Bilateral lung disease was observed in 9 of 13 patients. Nodular infiltrates were present in one-half of the patients with chest radiographs, whereas isolated hilar adenopathy or masses were observed in only two patients. Chest CT demonstrated parenchymal lung disease in 8 and hilar adenopathy in 5. Findings were not suggestive of invasive aspergillosis. The diagnosis of histoplasmosis was based on clinical findings and histopathologic features of lung biopsies in 60% of patients. Liver enzyme and serum lactate dehydrogenase levels were elevated in one-third of patients. No deaths were attributable to histoplasmosis. However, cancer therapy had to be delayed in several of the patients.
In the children with disseminated disease, clinical features, epidemiology, and laboratory findings were similar to those with acute pulmonary disease. Most patients were not neutropenic at the time of the diagnosis. One-third of patients had mediastinal adenopathy and masses compatible with granulomatous disease. Fifteen of 26 patients had positive blood culture results for H. capsulatum using lysis centrifugation tubes. In addition, detection of antigen in urine and histopathologic findings in bone marrow and tissue helped support the diagnosis. Of interest, in only 8 of 26 patients was histoplasmosis suspected before diagnosis. The mean time between onset of symptoms and diagnosis was 18.6 ± 8.2 days. The overall mortality for patients with disseminated disease was 26%. Inactive histoplasmosis was found in a group of children with solid tumors. In a cohort of patients with newly diagnosed solid tumors, inactive histoplasmosis was found in 48% of them at the time of diagnosis.
Histoplasmosis in transplant patients
Infections with endemic mycoses are rare in children who have undergone SOT; no histoplasmosis infections were documented among 584 children with SOTs during a 13 year-study period. Histoplasmosis usually occurs in the first 18 months after transplant. In organ transplant patients, the most common clinical feature of histoplasmosis is prolonged fevers.
In a study at three medical centers in the midwestern United States, 22 adult transplant patients were affected by histoplasmosis. Sixty-four percent of patients had a renal transplant, whereas 36% had liver transplant, and 14% had pancreas transplant. Some patients had multiple organs transplanted with some receiving more than one organ. Ninety-five percent of patients were receiving two or more immunosuppressive agents. Corticosteroids were administered to 73% of patients. The majority of patients (77%) had pulmonary disease, with disseminated disease developing in 64%; a majority (95%) had positive culture result, most often from bronchoalveolar lavage (BAL) or blood. Urine antigen assays were positive in 91% of patients. Two patients (9%) died from the infection, whereas 5 (23%) had graft loss.
Between 2001 and 2006, the Transplant-Associated Infection Surveillance Network identified 52 transplant patients with histoplasmosis. A majority had an SOT, mostly kidney or liver. Disseminated disease was recognized in one-third of patients, whereas pulmonary plus dissemination was documented in another one-third. Disease limited to the lungs was noted in 36% of patients. A majority of patients were receiving 2 or more immunosuppressive agents, including corticosteroids. Fifty-three percent of patients had positive blood culture results, whereas 35% of BAL/sputum specimens were positive. Of interest, serum and urine antigen assays were used to make the diagnosis in 25% and 57% of patients, respectively.
Histoplasmosis is a serious complication of kidney transplantation. Among infected patients documented between 1994 and 2014, graft failure was documented in 21% of patients.
In another study, moderate-to-severe disease was documented in 96% of transplant patients. Fever, cough, and diarrhea were present in 87%, 39%, and 35% of patients, respectively. Urine Histoplasma antigen assay results were positive in 95% of patients. Interstitial and alveolar infiltrates were documented in 63% of patients, with a miliary pattern was observed in only 6% of transplant patients, in contrast to recipients of TNF-α inhibitors, in whom a miliary pattern was documented in 44% of patients. The overall mortality rate in transplant was 9%.
A study of SOT patients from 24 institutions determined that 81% of recipients had disseminated disease, with 28% requiring admission to intensive care units. Of 152 patients with histoplasmosis, 10% died, with 72% of deaths occurring in the first month of diagnosis. The median time from transplant to diagnosis was 27 months, with disease diagnosed in 34% of patients in the first year after transplant.
At another institution, disseminated histoplasmosis was documented in six children with kidney transplants, with one-third of patients presenting in the first year after transplantation. No deaths resulted from these infections. In five of the patients, cytopenia was evidence of dissemination. Five of the patients had received induction with thymoglobulin and steroids.
Diagnosis
The early diagnosis of histoplasmosis in an immunosuppressed host can be achieved only if there is high clinical suspicion of the condition. A thorough consideration of the epidemiology preceding the onset of symptoms and key clinical features are essential in making a diagnosis. Distinguishing histoplasmosis from other fungal infections can be challenging. In patients with disseminated disease, elevated lactate dehydrogenase levels, liver enzymes, especially alkaline phosphatase, and erythrocyte sedimentation rate are commonly present. An elevated aspartate aminotransferase/alanine aminotransferase ratio is suggestive of disseminated histoplasmosis. A markedly elevated ferritin level and pancytopenia are frequently present in disseminated disease.
Medical imaging
The radiographic findings seen most commonly in children with histoplasmosis are not pathognomonic and may mimic the findings seen in tuberculosis or other granulomatous processes and, in some instances, neoplastic conditions, especially lymphoma. CT is highly sensitive and likely to reveal parenchymal infiltrates that are not visualized in plain radiographs. The most common pulmonary parenchymal changes are “soft” single or multiple, poorly defined areas of airspace consolidation often found in the basilar portions of the lungs. Immunocompromised hosts with evidence of dissemination are likely to have a diffuse pneumonitis pattern ( Fig. 28.1 ).
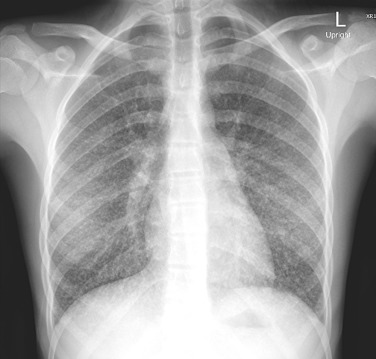
The appearance of enlarged hilar/mediastinal nodes, either in association with pulmonary infiltrates or as isolated findings, also is a common radiographic finding of acute pulmonary histoplasmosis. Low signal intensity within nodes is often observed. Infected nodes may enlarge and compress or obstruct adjacent structures. Isolated calcifications may be seen in the spleen or liver months to years after infection.
Histopathology
In clinical and epidemiologic settings compatible with histoplasmosis, observation of 2- to 4-μm typical yeast forms ( Fig. 28.2 ) in histopathologic specimens demonstrating granulomatous inflammation is strong supportive evidence of histoplasmosis. Caution is merited because intracellular pathogens such as Toxoplasma gondii , B. dermatitidis , yeast forms of Cryptococcus neoformans , and spherules of C. immitis may resemble the yeast forms of H. capsulatum . Both Giemsa- and hematoxylin-eosin–stained specimens may reveal intracellular yeasts in sputum, blood smears, bone marrow aspirates, and biopsy specimens. The Gomori methenamine silver stain is the most sensitive reagent.
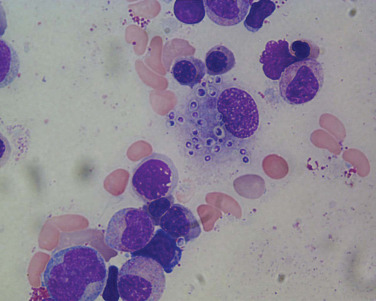
Culture
Recovery of H. capsulatum from a clinical specimen obtained from a symptomatic patient with a compatible illness confirms the diagnosis of active histoplasmosis. Normally, sterile specimens and minced or homogenized tissue can be inoculated onto suitable media, usually Sabouraud glucose (dextrose) agar. Mycelial growth of a white-to-tan mold has the highest specificity, with confirmation by use of DNA probe. Along with cultures, histologic examination of infected tissue can be a complementary test in the diagnosis of histoplasmosis. The optimal method for recovery of H. capsulatum from blood is the lysis-centrifugation technique. Culture results are positive in 75% to 85% of patients with disseminated disease. In the latter patients, sites from which H. capsulatum commonly is recovered include the lower respiratory tract, blood, bone marrow, cerebrospinal fluid (CSF), liver, spleen, skin lesions, and synovium of affected joints. The highest yield is from bone marrow. In adults with disseminated histoplasmosis, rates of positive lysis-centrifugation cultures of peripheral blood are 90% to 100% in acute dissemination.
In children, culture plus staining of BAL fluid has been rewarding in diagnosing high–fungal burden infections in patients with HIV, but it is less sensitive than lung biopsy in other immunocompromised patients, especially those receiving chemotherapy for reticuloendothelial malignancy.
Serology
Serologic methods are frequently used to make the diagnosis of histoplasmosis. The detection of antibodies by immunodiffusion (ID) and complement fixation (CF) have equal sensitivity (75% to 85%); however, ID is slightly more specific (>95% vs. 85% to 90%). In immunocompetent patients, either or both test results are positive in 95% of patients with acute primary pulmonary infection. CF titers often become positive 2 to 4 weeks earlier, usually within 4 to 6 weeks after exposure. When the ID test is reactive, however, it remains so for a longer period of time. In addition to the 4- to 6-week lag in developing elevated titers, an important limitation of both serologic assays is their reduced sensitivity in immunosuppressed patients. Only 50% of immunosuppressed children and adults with disseminated histoplasmosis are seropositive.
A 4-fold increase between acute and convalescent sera provides the best serologic evidence of recent infection. The individual yeast (CF-Y) and mycelial (CF-M) phases are measured. The CF-Y phase is more sensitive than the CF-M phase when performed in a patient with a recent or active infection. In highly endemic areas, background low-titer CF serologic reactions may be present. The titer of antibody by the complement fixation test is directly proportional to severity of illness and degree of exposure.
The ID method detects precipitins (reported as bands) against the H and M glycoprotein antigens of H. capsulatum. The H band is present infrequently in patients with histoplasmosis; when seen, it is transient and its presence suggests active infection. In patients with active pulmonary histoplasmosis, one-half to three-fourths have an M band alone. The H band is present in only 10% to 20% of acute infections, and only 10% of individuals have both M and H bands present. The presence of both is highly suggestive of active histoplasmosis. Only approximately 50% of patients with disseminated disease have a positive M band.
A newer enzyme immunoassay that measures Histoplasma immunoglobulin (Ig) M and IgG antibodies appears to have demonstrably higher sensitivity than CF and ID.
Cross-reactivity with other fungal antigens affects CF and ID assays. CF cross-reactivity occurs most commonly with B. dermatitidis (40%) and C. immitis (16%). Cross-reactions also occur, albeit rarely, with candidiasis, tuberculosis, aspergillosis, and cryptococcosis. Single titers of 1: 32 or higher performed by an experienced laboratory are strong supportive evidence of acute or recent infection, especially when the accompanying clinical symptoms are compatible. However, patients with non- Histoplasma febrile pneumonia may have false-positive CF titers. However, other laboratory and clinical data should be considered when making the diagnosis of histoplasmosis. A low CF titer of a person living in an endemic region is not diagnostic.
The serologic diagnosis of patients with isolated meningitis caused by H. capsulatum often is problematic because no single test exhibits high sensitivity. CF and ID results can be positive in CSF, but one-half of patients with other chronic fungal meningeal infections may show false-positive results. CF-M antibody appeared to be the most sensitive and specific test for the diagnosis of meningitis caused by histoplasmosis. However, a recent study demonstrated that the combined use of Histoplasma antigen assay and anti- Histoplasma IgG or IgM antibody assay detected 98% of cases of Histoplasma meningitis.
Antigen detection
Antigen detection is most sensitive in infections accompanied by high fungal burdens. Antigen detection assays on serum, urine, or other selected body fluids provide a rapid, accurate, noninvasive diagnostic result for the most serious manifestations of disease. It is especially useful for the evaluation of infections in immunocompromised hosts, in whom serologic method results often are negative. In immunocompromised hosts and patients with primary pulmonary infection, the detection of antigen reflects the early hematogenous dissemination that occurs before being aborted by an effective cellular immune response.
The sensitivity of urine antigen detection in patients with acute primary pulmonary infection surpasses 75%, with the highest rates seen in patients tested within a few weeks of exposure, in those with large inoculum exposure, and in patients with extensive pulmonary involvement. The sensitivity of antigen detection is very high in patients with disseminated disease, where antigen is detected in 91% of immunosuppressed patients. In children with disseminated disease, urinary antigen testing was positive in 100 percent. Although Histoplasma antigen can be detected in serum, the sensitivity is substantially less than that of urine. Antigen often is found in BAL fluid of patients after high-inoculum exposure to histoplasmal spores and in immunocompromised patients with hematogenous dissemination and lung involvement. Inactive histoplasmosis consisting of calcified hilar nodes or liver granulomas universally have negative antigen assay results, whereas patients with untreated severe disseminated disease always have positive urine and serum antigen assay results. In a group of immunocompromised children, higher rates of antigenemia and antigenuria were observed compared with non-immunocompromised children, along with longer durations.
A multicenter study of an H. capsulatum antigen detection assay demonstrated a higher sensitivity in immunocompromised hosts compared with immunocompetent patients. Antigenuria was detected in approximately 92% of patients with disseminate histoplasmosis, approximately 84% of patients with acute histoplasmosis, and approximately 30% of patients with subacute histoplasmosis. Antigenemia was present in 100% of patients with disseminated histoplasmosis. Cross-reactivity was observed in 90% of patients with blastomycosis. Specificity of the assay was 90%.
Variations in sensitivity have been reported between commercially available antigen detection assays. These are not considered interchangeable as they are not comparable.
Cross-reactivity between the various endemic mycoses is well recognized; therefore epidemiology, clinical features, and geographic exposures should be factors in determining which mycosis is most likely.
In addition to its usefulness in diagnosis, an antigen assay provides a quantitative parameter with which to assess the pace and adequacy of response to therapy and, thereafter, a sensitive monitor that promptly detects relapse in patients who are at high risk for recurrence. Antigen concentration decreases during effective therapy. Failure of the antigen concentration to decline or documentation of progressive increase may indicate treatment failure. Persistent but decreasing concentrations of antigenuria may be present after completion of an appropriate and effective course of antifungal therapy. Patients who have completed appropriate courses of therapy for serious infections and have had resolution of their clinical symptoms yet demonstrated persistent but decreasing concentrations of antigenuria have fared well after the completion of the planned course of antifungal therapy. Residual excretion of urine antigen after adequate treatment continues to decrease and eventually ceases; monitoring is recommended to confirm resolution of antigenuria. The persistence of moderate antigenuria has been associated with a risk for recrudescence.
If urine concentration is too high and unable to quantitate, monitoring is more informative if antigen concentrations of serum are initially followed because concomitant serum concentrations are lower than those of concomitant urine samples and more likely to be within the range in which differences in concentration could be measured accurately. Thereafter, when antigenemia is resolved, urine concentrations can be followed with the quantitative assay. Testing should be performed routinely at 3- to 4-month intervals during treatment, at the end of therapy, if symptoms recur, and periodically for another year to monitor for relapse.
Molecular testing
Polymerase chain reaction (PCR) methodology has been evaluated for identifying H. capsulatum in tissue and body fluids, but false-negative results were encountered in one-third of specimens. PCR methodology used with clinical specimens (urine, serum, BAL, CSF) containing urine antigen showed specificities of 80% to 100% but sensitivities of only 0 to 22%. In situ hydridization of tissue appears to be a valuable tool in evaluating cutaneous lesions in patients with endemic mycoses. Real-time PCR can detect the presence of B. dermatitidis and H. capsulatum in cultures and clinical specimens with high specificities, 99% and 100%, respectively. Sensitivities are somewhat lower: 73% for H capsulatum , and 86% for B. dermatitidis .
Other assays
In cancer patients undergoing stem cell transplantation and found with pulmonary nodular opacities, false-positive galactomannan assay results may result in delays in initiating effective therapy for disseminated histoplasmosis. Clinicians must be aware of this problem, especially if caring for patients in endemic regions.
Testing of patients with histoplasmosis and blastomycosis may result in a positive (1,3)-β-D-glucan assay. However, caution is merited if this assay is used as a screening test because many patients with blastomycosis are likely to have a negative assay result. However, even though the assay appears to reliably detect individuals with disseminated histoplasmosis, H. capsulatum –specific antigen assays are more sensitive and specific and can be used reliably to assess response to therapy. (1,3)-β-D-Glucan was measured in the CSF of patients with CNS histoplasmosis. The sensitivity of the assay was found to be approximately 53% with a specificity of approximately 87%. Compared with other CNS fungal infections, the specificity was just 46%.
Treatment
Immunocompromised patients with histoplasmosis require antifungal therapy. They are more likely to have symptomatic and disseminated disease. Incidental findings of calcified granulomas in lungs, mediastinum, and/or liver-spleen represent inactive (“old”) histoplasmosis and will not benefit from antifungal therapy. Evidence-based, consensus practice guidelines for treatment of histoplasmosis have been published and include recommendations for treatment of children ( Table 28.1 ).