The vast majority of invasive yeast infections in organ transplant recipients and patients with malignancy are caused by Candida species. However, a variety of other yeasts are important opportunistic pathogens for these patients and are reviewed in this chapter. This includes the Cryptococcus neoformans–Cryptococcus gattii complex, which is the second most common cause of invasive yeast infections in this cohort and an important cause of fungal meningoencephalitis and pneumonia. It also includes a collection of other yeasts that as a group cause a small minority of invasive fungal disease. Disease caused by these pathogens can be difficult to treat because of anti-fungal resistance and is often associated with a high mortality.
Cryptococcosis
Background
Cryptococcus neoformans and Cryptococcus gattii are encapsulated basidiomycetous yeasts that are responsible for the syndrome of cryptococcosis. As these two species share many biologic characteristics and clinical presentations, they are often discussed together as the C. neoformans– C. gattii complex. However, there are notable differences in the epidemiology, risk factors, and clinical presentations of the 2 species. As such, throughout the chapter there is discussion that is generalizable to both species of the C. neoformans and C. gattii complex with distinction between the species highlighted . Although these pathogens can be opportunistic to a wide range of immunocompromised hosts, the focus is on pediatric oncology patients and recipients of solid organ or hematopoietic stem cell transplantation. Diseases secondary to other cryptococcal species (i.e., C . albidus and C. laurentii ) are much less commonly reported and are not discussed further.
C. neoformans and C. gattii exhibit several virulent traits, which have been hypothesized to have evolved as an adaptive response to environmental stressors. The polysaccharide capsule of Cryptococcus plays an essential role in promoting disease by helping the organism evade and limit the host inflammatory response. The capsular material is actively shed during infection and detection of this polysaccharide in bodily fluids serves as an important diagnostic tool. Antibodies to the capsular polysaccharide can be used to serotype the organism. A variety of other cryptococcal virulence factors have been elucidated, including melanization, urease production, ability to grow at 37°C, and phenotypic variation.
Epidemiology and risk factors
In the environment , C. neoformans is often localized to areas contaminated by pigeon droppings and decaying trees. The ubiquitous nature of this pathogen makes it a possible pathogen regardless of geographic location. C. gattii has been classically associated with the red gum eucalyptus tree, which results in a more focused geographic distribution of this pathogen. Until recently, C. gattii disease was primarily restricted to the tropical and subtropical areas, especially Australasia, where the disease is endemic. Beginning in the late 1990s, an outbreak of C. gattii disease was recognized in British Columbia. This outbreak quickly spread to other areas of the Pacific Northwest, including parts of the Northwest United States. Sporadic disease has also been reported in Europe. The regional distribution of the pathogen can help quantify a patient’s risk for C. gattii ; however, clinicians should be aware that prior travel to these regions can place a patient a risk.
Exposure to both species is thought to be via the inhalation of aerosolized organisms from the environment. Subsequent to exposure, a person can develop either a primary progressive symptomatic infection or have an asymptomatic immunologic response. In the latter scenario, the organism can establish latency with risk for reactivation later in life. Person-to-person spread is not thought to occur. Symptomatic disease is uncommon in young children even though serologic studies suggest that exposure occurs in most children by 3 years of age. This increasing risk for symptomatic disease with increasing age is highlighted in specific immunocompromised populations. For example, in children with AIDS, cryptococcosis is more common in adolescents and preadolescents than younger children.
Cryptococcosis can occur in both immunocompetent and immunocompromised children. Interestingly, C. neoformans is much more common in children with defects in immunity, especially cellular immunity; whereas C. gattii tends to primarily cause disease in healthy individuals, although subtle defects in the host immune response may be present such as auto-antibodies to granulocyte-macrophage colony-stimulating factor, corticosteroid use, and chronic lung disease. As C. neoformans is a more ubiquitous pathogen, the epidemiologic data for cryptococcosis are primarily dependent on reports focused on this species . There was a sharp rise in the incidence of cryptococcosis in association with the human immunodeficiency disease epidemic, but this incidence has dramatically waned with the advent and availability of highly active antiretroviral therapy (HAART). C. neoformans remains an important cause of disease in children with primary and secondary immunodeficiencies, especially children who have undergone organ transplantation.
Transplant recipients
Cryptococcosis is significantly more common among solid organ transplant (SOT) recipients compared with stem cell transplant recipients for whom this infection is relatively rare. Among SOT recipients it is the third most common invasive fungal disease (after candidiasis and aspergillosis). Cryptococcosis accounts for approximately 7% of fungal infections in adult SOT recipients, with mortality rates on the order of 30%. In SOT recipients, cryptococcosis typically occur several months to years after transplantation and may result from primary infection or from reactivation of a latent infection. Infections within 30 days of transplantation are well described and appear to be caused by reactivation of a latent infection in the transplanted organ. The majority of SOT recipients have disseminated cryptococcosis, although as many as one-third have isolated pulmonary disease.
Malignancies
A range of hematologic malignancies, including chronic lymphocytic leukemia, acute lymphoblastic leukemia (ALL), acute myeloid leukemia (AML), and lymphoma, have been reported in association with cryptococcosis. Among adults, cryptococcosis is most common in patients with chronic lymphocytic leukemia and lymphoma. In contrast to invasive candidiasis and aspergillosis, neutropenia is generally not considered a risk factor for cryptococcosis. Instead, risk factors include advanced disease, lymphopenia, and prior chemotherapy. Infection most commonly presents as disseminated disease. In children with soft tissue malignancies, C. neoformans pulmonary disease may be detected as a result of imaging done to exclude pulmonary metastasis.
Other risk factors for cryptococcosis
A variety of immunosuppressive regimens, (i.e., corticosteroids, chemotherapeutics) have been associated with an increased susceptibility to cryptococcosis. Biologic agents can also be a risk factor for cryptococcosis. This is especially true of tumor necrosis factor antagonists and T-cell–depleting therapies. More recently several cases of cryptococcosis have been reported in patients receiving ibrutinib, a Bruton’s tyrosine kinase inhibitor. As the number of novel chemotherapeutic and biologic agents available for use increases and their indications for use broadens, clinicians need to be aware that the populations at risk for cryptococcosis will also increase.
Clinical manifestations
The C. neoformans-gattii complex can cause disease in most organ systems, although the most common presentations are meningoencephalitis, disseminated disease, and pneumonia. These organisms are highly neurotropic and can enter the central nervous system (CNS) via several different mechanisms. Compared with C. neoformans , C. gattii more commonly causes pulmonary disease without CNS involvement. Furthermore, C. gattii disease is more likely to be characterized by the presence of cryptococcomas, which are mass-like lesions containing large number of organisms.
Meningoencephalitis.
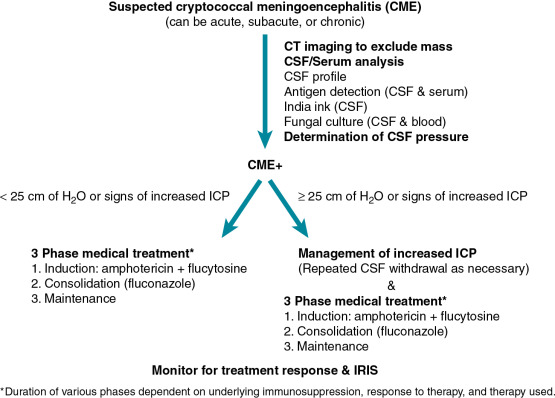
Cryptococcal meningoencephalitis (CME) is a subacute to chronic disease ( Fig. 27.1 ). The precise manner of presentation depends both on the underlying immune status of the host and the infecting cryptococcal species/strain. Symptoms are nonspecific but most commonly include headache, neck stiffness, and fever, which can last for weeks to months before diagnosis. Personality changes, lethargy, along with cranial nerve palsies, nausea, vomiting, and visual changes, may also occur. Meningismus is not a reliable sign and is absent in the majority of patients. Parenchymal brain lesions occur in the minority of patients, but when present are associated with higher mortality. Increased intracranial pressure (ICP) is a hallmark feature of CME. The basis of increased ICP is not completely understood, but it may be related to impaired cerebrospinal fluid absorption caused by the viscous capsular polysaccharide that is shed by the organism. Increased ICP is an important contributor to the acute morbidity and mortality of CME and should be managed aggressively.
Pneumonia.
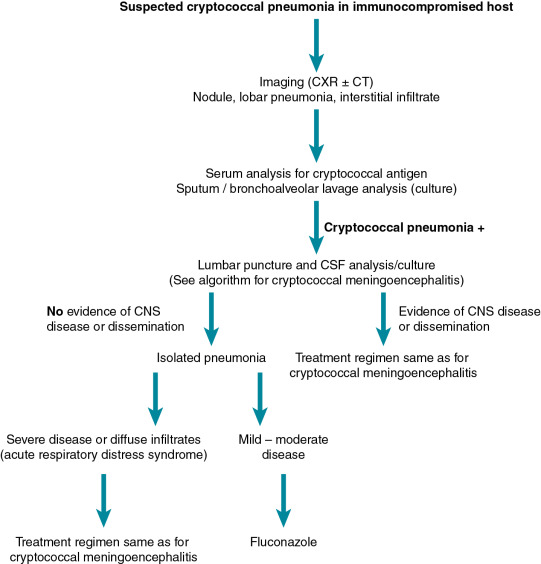
Pulmonary cryptococcosis can occur alone or in the context of CME ( Fig. 27.2 ). Concomitant CNS disease occurs more commonly in patients with AIDS compared with SOT recipients, which is believed to be due to the anti-cryptococcal activity of calcineurin inhibitors. Nonetheless, any patient with cryptococcal lung disease and an underlying immunocompromising condition should be evaluated for evidence of dissemination, which would include a lumbar puncture, fungal blood culture, serum antigen testing, and physical examination. Pulmonary disease is often asymptomatic and may be only recognized as a result of imaging studies done for other purposes. Symptoms, when present, are nonspecific and include cough, dyspnea, increased sputum production (in older children), hemoptysis, and pleuritic chest pain. Rarely, pulmonary cryptococcosis can present with rapid respiratory decompensation. The imaging findings of pulmonary cryptococcosis are variable and include singular and multiple nodules, consolidation, and masses. Interstitial infiltrates have also been described and are often the result of secondary dissemination from other sites of lung involvement. Finally, cavitation and pleural effusions have been described, although they are not common.
Other forms of disease.
Skin involvement in cryptococcosis is typically the result of hematogenous spread and may be the initial clinical sign of disseminated disease. Because skin disease rarely results from direct inoculation, all patients with skin manifestations of cryptococcal disease should be evaluated for disseminated disease and CME. Skin manifestations include papules, nodules, molluscum, acneiform rash, and cellulitis. Because of the high variability and nonspecificity of skin disease, a high degree of suspicion is needed to make the diagnosis (see later text). Cryptococcosis can cause disease in most organ systems, including bones, joints, eyes, prostate, liver, kidneys, and spleen.
Diagnosis
The diagnosis of cryptococcosis can be made by growing the organism in culture. The same diagnostic assays can be used for both C. neoformans and C. gattii infections . Most colonies are white to cream colored and may have a mucoid appearance owing to the capsule. Diagnosis is also made by visualization using microscopy or detection of the cryptococcal polysaccharide in body fluids. The utility of these techniques varies depending on the host and organ system involved. Growth in specialized media and matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF) can be used as necessary to distinguish C. neoformans from C. gattii. For CME, cerebrospinal fluid (CSF) examination generally reveals a lymphocytic pleocytosis, although the actual cell number may be low or normal in patients with severe immune dysfunction. CSF protein levels tend to be elevated, whereas glucose levels are depressed. Cryptococcus can be readily isolated from the CSF by standard fungal culture, although growth may take several days. India ink staining of the CSF allows for rapid detection of the organism because the capsular polysaccharide excludes ink, which allows the unstained organisms to be visualized. The sensitivity of this stain depends on the cryptococcal burden, which is related to the underlying immune status of the host. In SOT recipients, the sensitivity may be as low as 50%.
Several different antibody-based methods are available for polysaccharide antigen detection, including latex agglutination, enzyme-linked immunosorbent assay (ELISA), and a lateral flow assay. The sensitivity of these assays in CSF testing has been reported to range between 93% and 100% (reviewed in Perfect and Bicanic ). The sensitivity of serum antigen testing in patients with CME is lower than CSF testing and in SOT recipients has been reported to be 90%. False-negative reactions for these tests are very uncommon, but can be seen early in disease and with extremely high antigen levels when the latex antigen assay is used. False-positive reactions are also very uncommon, but cross-reactivity with the polysaccharides of other organisms, such as Trichosporon and Capnocytophaga , can occur. Antigen detection assays provide semi-quantitative measurements of polysaccharide antigen within the CSF and serum. High initial CSF antigen levels are generally associated with higher fungal burdens and a worse prognosis. Unfortunately, antigen detection assays have limited utility in measuring response to therapy. Cryptococcal polymerase chain reaction (PCR) assays as part of multiplex meningitis assays are also commercially available, but experience with these diagnostic assays is limited.
Cryptococcal pneumonia can be diagnosed by culture of the sputum and bronchoalveolar lavage fluid. Blood cultures and serum cryptococcal antigen test results are typically negative, especially with isolated pulmonary disease. Latex agglutination testing of bronchoalveolar lavage fluid has been described, although the assay has not been formally studied, nor is it licensed for this purpose. Evaluation for CME including a lumbar puncture should be done in all immunocompromised patients with cryptococcal pneumonia. A complete physical examination along with serum cryptococcal antigen and fungal blood cultures should be performed to evaluate for dissemination. For pulmonary nodules and masses a biopsy may be necessary to establish a diagnosis. Mucicarmine staining highlights the capsule of the organism and helps distinguish this organism from other yeasts.
In skin disease, biopsy and culture of the affected area are generally needed to make a diagnosis. Skin lesions are generally a manifestation of disseminated disease and affected patients often have elevated serum antigen levels. All immunocompromised patients with skin disease should have a complete evaluation to exclude dissemination, including a lumbar puncture to rule out CNS involvement.
Treatment
Because of the rarity of disease in children, many of the recommendations regarding pediatric cryptococcosis are based on extrapolations from adult data, in particular from adults with human immunodeficiency virus/AIDS. Treatment approaches must consider the underlying immune status of the host as well as the organ system involved . In addition to antifungal therapy, attempts should be made to lessen immunosuppression as possible.
The treatment of CME as established by the Infectious Diseases Society of America consists of 3 consecutive phases: induction, consolidation, and maintenance. For the initial induction phase, patients are treated with a combination of amphotericin and flucytosine. Most of the original treatment studies were performed with conventional amphotericin. However, recent studies suggest that lipid preparations of amphotericin are therapeutically equivalent and may be better tolerated in individuals with or at risk for renal disease. Thus strong consideration should be given to use of lipid or liposomal preparations in patients who are at risk for renal dysfunction, including transplant recipients who are receiving calcineurin inhibitors, and for those who cannot tolerate conventional amphotericin.
The duration of induction is typically 2 weeks. Longer durations (i.e., 4 to 6 weeks) should be considered in the following circumstances: (1) in patients who did not receive flucytosine as part of their initial therapy; (2) in those with complicated neurologic disease (including those with cryptococcomas), and (3) failure to sterilize CSF at 2 weeks. Induction therapy is followed by an 8-week consolidation with fluconazole and concluded with a maintenance course of fluconazole . In patients with AIDS the minimal duration of total antifungal therapy is 12 months. After this time, discontinuation of maintenance fluconazole can be considered in the context of immune reconstitution (in adults >100 CD4 + T cells/μL) and undetectable DNAemia. For SOT recipients the maintenance phase of fluconazole should be 6 to 12 months.
The treatment of pulmonary cryptococcosis depends on the extent of disease and underlying immune status of the host. Patients with severe pulmonary disease (i.e., acute respiratory distress syndrome) or with evidence of dissemination should be treated as those with CME. For patients with localized pulmonary disease (i.e., extrapulmonary disease has been excluded), fluconazole therapy for 6 to 12 months is recommended.
Management of increased intracanial pressure
Increased ICP often accompanies CME and plays a significant role in the early morbidity and mortality of this disease. According to Infectious Diseases Society of America guidelines, CSF pressure should be lowered by CSF drainage to less than 20 cm of H 2 O or decreased by 50 % if very elevated. CSF pressure should be monitored daily until CSF pressure has normalized for 2 consecutive days. Mannitol is not helpful in the management of increased ICP in CME and corticosteroids (in the absence of IRIS and cryptococcomas) are generally contraindicated. Some authors have reported the utility of corticosteroid in the treatment of CME caused by C. gattii , although this literature is limited to case reports and series.
Immune reconstitution inflammatory syndrome
Immune reconstitution inflammatory syndrome (IRIS) was first recognized in AIDS patients with a variety of opportunistic infections, for whom disease symptoms recurred with improvement in immune function secondary to HAART. For patients with CME, IRIS results in an enhanced, but detrimental inflammation, often within the brain. This inflammation is characterized by an exaggerated proinflammatory response (i.e., Th1 and Th17 immunity) that begins days to weeks after the initiation of HAART. Cryptococcus -associated IRIS has also been reported to occur in 5% to 14% of SOT recipients, typically occurring several weeks after initiation of antifungal therapy. IRIS in this cohort may result in the loss of graft function. For these patients, cessation of calcineurin inhibitors has been identified as a risk factor. The manifestations of Cryptococcus -associated IRIS include a paradoxical worsening of CNS symptoms (i.e., new brain lesions, recurrent meningitis) and the unmasking of disease in organs not previously recognized to be affected (i.e., adenitis). Cryptococcal IRIS also occurs in SOT recipients and can result in the loss of allograft function. IRIS must be distinguished from failure of antifungal therapy. Both entities result in a recrudescence of symptoms, although treatment failure is associated with microbiologic failure, whereas with IRIS cultures are generally sterile. Some manifestations of CME-associated IRIS may resolve on their own. For more severe symptoms (including increased ICP and persistent symptoms), the addition of corticosteroids to antifungal therapy is recommended with a 2- to 6-week taper.
Disease prophylaxis/prevention
Owing to the relatively low incidence of cryptococcosis in transplant recipients and children with malignancies, neither primary antifungal prophylaxis based on CD4 cell count nor screening with serum antigen testing is recommended to prevent disease. Given the ubiquitous nature of the pathogen, it is difficult to prevent exposure; nonetheless, owning and/or breeding birds (e.g., pigeons, canaries, parakeets) may pose additional risk.
Other invasive yeast infections
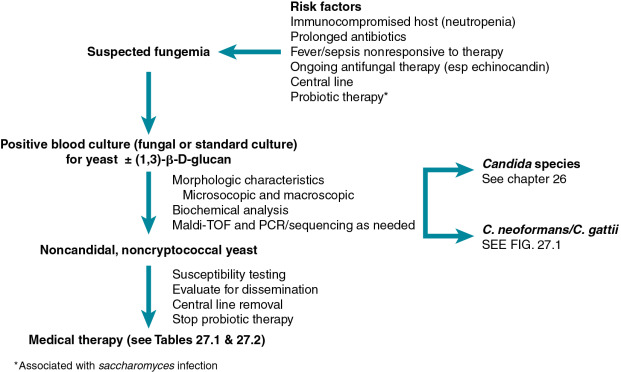
In addition to Candida and Cryptococcus spp., a variety of other yeasts have been found to cause serious, invasive disease in immunocompromised individuals. Because of their rarity, the precise incidence of these infections is difficult to define, but it is generally considered to represent less than 5% of fungemias. Likewise, the description of these infections (especially for children) is limited, consisting of case reports, case series, and systematic reviews of these series. Thus many of the features and treatment recommendations for these infections represent extrapolations from adult literature. These yeasts and the disease they cause tend to share several important epidemiologic and clinical characteristics ( Fig. 27.3 ). These organisms are commonly found in the environment but also colonize humans, causing disease in the appropriate clinical context. Risk factors for these infections tend to be similar to those for invasive candidiasis including underlying immunosuppression, neutropenia, and the presence of a central line. Because of these epidemiologic similarities and their shared morphologic appearance, initial misidentification of these yeasts is common. The mortality from these invasive infections is generally high. Importantly, these infections often occur in the context of ongoing antifungal therapy (especially echinocandins), which should alert clinicians to the possibility of a noncandidal infection and antifungal resistance. Owing to the rarity of these infections, there are no randomized, controlled treatment studies. Removal of potentially infected central lines should be considered early in the course of disease. Initial therapeutic recommendations ( Tables 27.1 and 27.2 ) are generally based on reports in the literature and historic patterns of susceptibility. Therapy is further complicated by the absence of standardized susceptibility cutoff values, and the observation that in vitro susceptibilities may not correlate with clinical response.