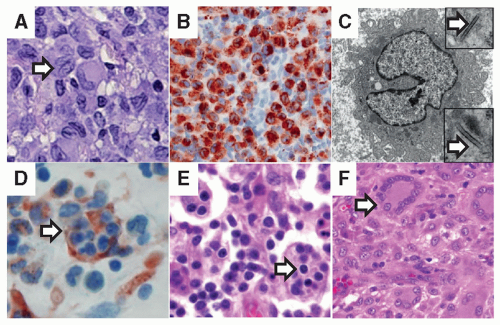
Studies showing clonality in LCH were published in 1994 using polymorphisms of methylation-specific restriction enzyme sites on the X-chromosome regions coding for the human androgen receptor assay (HUMARA), as well as polymorphism for three other loci.
8 Biopsies of lesions from both single system and multisystem disease were found to have a proliferation of LCs from a single clone. Despite clonality, pathologic DCs do not appear dysplastic, mitoses are rarely observed,
7 and chromosomes are typically intact.
9A major discovery in 2010 described recurrent somatic mutations in the
BRAF gene in 57% of LCH lesions.
10 The
BRAF-V600E mutation leads to constitutive activation of the downstream kinases, MEK and ERK, and is observed in a wide spectrum of human cancers (e.g., melanoma, thyroid carcinoma, and hairy cell leukemia), as well as many benign neoplasias (e.g., nevi, colon polyps).
11 In an institutional series,
BRAF-V600E was associated with a twofold increase in risk of recurrence, but otherwise no clinical differences have been associated with the mutation, including age, survival, or clinical risk status.
12 The presence of circulating cells carrying the
BRAF-V600E mutation was associated with high-risk clinical status (liver, bone marrow, spleen involvement) and localized to myelomonocytic lineage (CD11c
+ and CD14
+ fractions). Hematopoietic cell progenitors with the
BRAF-V600E mutation were also identified in patients with high-risk LCH. Therefore, a proposed model of LCH pathogenesis is one in which high-risk LCH arises from pathologic activation of ERK in hematopoietic progenitor cells, and low-risk disease arises from ERK activation in tissue-restricted precursors. This model is supported by the ability of enforced expression of
BRAF-V600E in early myeloid (CD11c
+) cells to drive a LCH-like phenotype in mice.
12 The presence of
BRAF-V600E in hematopoietic progenitor cells in bone marrow and myelomonocytic precursors in circulating blood in patients with high-risk LCH is also consistent with the classification of LCH as a myeloid neoplasia, and suggests that “histiocytosis X” may in fact be a more appropriate nomenclature than “Langerhans cell” histiocytosis.