Chapter 25 High-Grade Gliomas
Etiology and Epidemiology
Most malignant brain tumors are high-grade gliomas, and most of these are GBM, a World Health Organization (WHO) grade IV tumor. The remainder are WHO grade III tumors such as anaplastic astrocytoma, anaplastic oligodendroglioma, and anaplastic oligoastrocytoma. Men are more commonly affected than women. The peak incidence occurs in the age range of 65 to 75 years, and the median survival time is inversely proportional to age; these findings have prompted a redoubling of efforts in elderly subpopulations.1
There has been concern for cancer development following exposure to electromagnetic fields, but definitive evidence is lacking. More recently, the use of cellular telephones has been studied extensively in Europe,2 but its importance as a risk factor has not been established. In terms of chemical exposure, nitrosamines have long been regarded as culpable, but causality is far from proven.3 Brain tumors have also been linked with previous exposure to therapeutic ionizing radiation.3 However, the absolute risk is low.
Most gliomas are sporadic, but genetic susceptibility is suspected based on the occurrence of multiple brain tumors in families with germline mutation of the TP53 suppressor gene and patients with neurofibromatosis type I as well as the rare patients who have been diagnosed with Turcot’s syndrome. A heritable syndrome contributes to less than 5% of GBMs.4
Prevention and Early Detection
No viable strategy for screening or early detection of glial tumors has been developed. There is also no convincing evidence demonstrating either improved survival when high-grade gliomas are found early, or a clear rationale for prophylactic strategies to reduce the incidence of these aggressive tumors.3
Biologic Characteristics and Molecular Biology
Investigators around the world are searching for the molecular biologic characteristics of gliomas in an effort to improve therapy. For example, work by the Cancer Genome Atlas Research Network5 and others6 suggests that at least three molecular subclasses of GBM exist with potential therapeutic and prognostic implications. A set of nine genes (derived from a larger set of 38 genes) identifiable from paraffin blocks is also under investigation as a stratification factor in an ongoing phase III trial (Radiation Therapy Oncology Group [RTOG] 0825).7 Mouse modeling has also demonstrated the oncogenic importance of abnormalities in receptor signaling (e.g., epidermal growth factor receptor [EGFR] and platelet-derived growth factor receptor [PDGFR]), signal transduction cascades (e.g., RAS and AKT), and cell cycle regulation.8
In the early 1990s, it was recognized that deletion of the short arm of chromosome 1 (1p) and the long arm of chromosome 19 (19q) occurs in most oligodendrogliomas.9 Co-deletion of 1p and 19q is prognostic for longer survival,10,11,12 although controversy continues as to whether this finding should alter therapy.13 It is now recognized that an unbalanced chromosomal translocation underlies 1p/19q co-deletion,14 but the specific genes involved and their mechanism of action remain elusive. More recently, mutations in the isocitrate dehydrogenase 1 and 2 (IDH1 and IDH2) genes, occurring most often in low-grade gliomas but also in a minority of WHO grade III and IV tumors,15,16 are also prognostic for longer survival.12,15,17,18 Methylation of the MGMT promoter (see the section on Chemotherapy) is emerging as both a predictive factor and a prognostic factor in the treatment of newly diagnosed GBMs.
It is not yet clear how to best incorporate molecular data into the treatment of individual patients. Detailed discussions of the biologic characteristics of glioma and their clinical relevance are beyond the scope of this chapter and can be found elsewhere.19
Pathology and Pathways of Spread
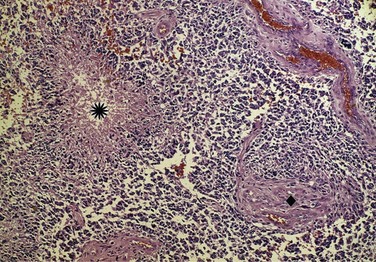
The WHO classification system20 is derived in part from the correlation between pathologic findings and survival rates observed by Bailey and Cushing21 and published in the early 1900s. In current parlance, low grade refers to WHO grade I to II tumors and high grade to WHO grade III to IV tumors (Fig. 25-1). However, the WHO grade I gliomas (e.g., juvenile pilocytic astrocytomas) are biologically different tumors from the others, infrequently occur in adults, and may be amenable to surgical cure. Anaplastic in the context of gliomas refers to WHO grade III tumors such as anaplastic astrocytomas, anaplastic oligodendrogliomas, and anaplastic oligoastrocytomas. GBM is a WHO grade IV astrocytoma, although the most recent WHO classification scheme does account for other rare subtypes, including GBM with oligodendroglial components.
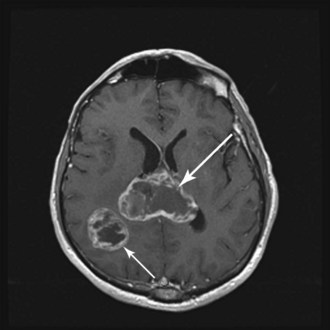
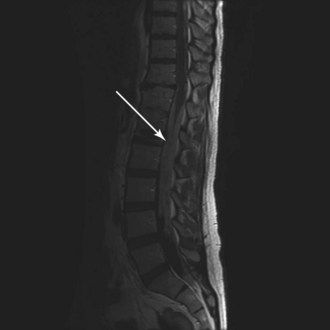
WHO grade II to IV gliomas are characterized by a tendency to directly infiltrate adjacent brain tissue. Lesions with direct access to the corpus callosum may extend across the midline and configure themselves in a classic “butterfly pattern” (Fig. 25-2). MRI underestimates the extent of invasive disease, and the very diffusely infiltrative nature of these tumors makes complete removal of all tumor cells impossible. This “misleading appearance of enucleability” was described over 80 years ago.21 Leptomeningeal spread occurs occasionally (Fig. 25-3). Hematogenous and lymphatic spread are exceedingly uncommon.22
Clinical Manifestations, Patient Evaluation, and Staging
The typical imaging appearance of a GBM is a ring-enhancing or heterogeneously enhancing lesion. The differential diagnosis may include stroke, brain metastasis, primary central nervous system lymphoma, demyelination, and even infectious or other inflammatory diseases. If a brain metastasis is suspected, it is prudent to perform an appropriate extracranial evaluation to identify the primary malignant tumor. If there is a high index of suspicion for primary central nervous system lymphoma, such as in multifocal, periventricular, and/or homogenously enhancing lesions,23 corticosteroids should be withheld preoperatively unless herniation is imminent because their use confounds the histologic diagnosis.
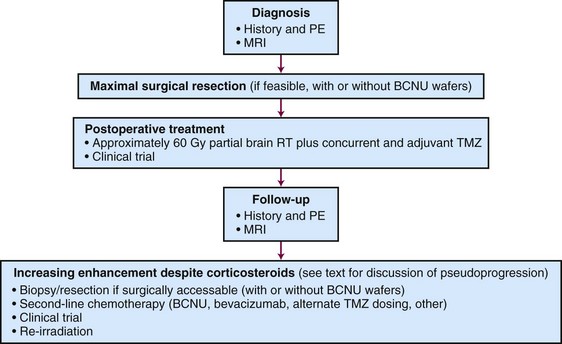
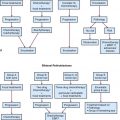
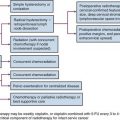
An algorithm for the evaluation and management of patients with GBM is shown in Figure 25-4.
Primary Therapy
Prognostic and Predictive Factors
Historically, all high-grade gliomas were lumped together in most clinical trials, thereby confounding results due to maldistribution of patients with differing prognoses. In 1993, Curran and colleagues24 published a landmark paper describing a prognostic classification scheme based on clinical variables. Data from three RTOG trials that enrolled nearly 1600 high-grade glioma patients from 1974 to 1989 were used. This recursive partitioning analysis (RPA) methodology builds decision trees to model predictors by examining all possible “cut points” for all variables included in the model. Patients were segmented into six distinct groups with different survival outcomes. The key variables included patient age, performance status, histologic tumor type (i.e., anaplastic astrocytoma vs. GBM), mental status, symptom duration antecedent to diagnosis, extent of resection, neurologic function, and radiotherapy dose. Median survival ranged from 4.6 months for class VI patients to almost 5 years for class I patients, underscoring survival heterogeneity. The European Organization for Research and Treatment of Cancer (EORTC) demonstrated the prior RTOG RPA classification remained valid among patients in a more recent phase III trial.25
One of the more controversial factors in the setting of high-grade gliomas has been the extent of surgical resection. Bailey and Cushing observed longer survival following resection in their 1926 publication,21 as did others in the 1960s.26 Numerous series since then also support more complete resection as a prognostic factor.27–31 One of the largest involved over 400 patients at the M.D. Anderson Cancer Center and demonstrated improved median survival (13 months vs. 8.8 months; p < .0001) following at least 98% resection, as defined by postoperative MRI scans.32 One small randomized study of 30 patients older than 65 years demonstrated improved survival rates following resection versus biopsy alone.30
External Beam Irradiation
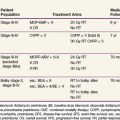
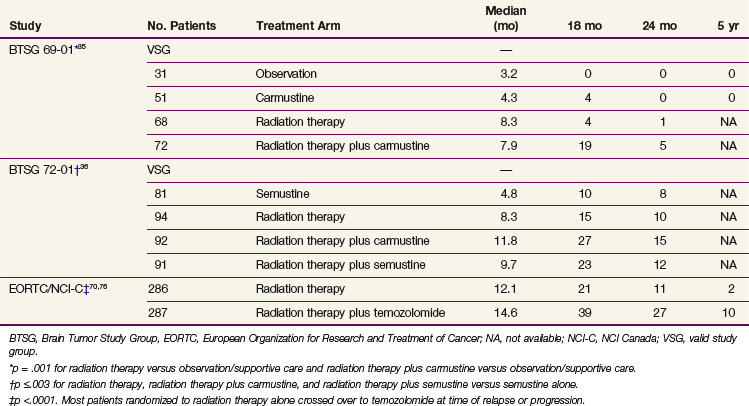
External beam irradiation (EBRT) has historically been the cornerstone of the therapeutic approach to GBM (and anaplastic astrocytoma) for the past half century, and its use in brain tumors was already described by the 1920s.33 In the 1970s and early 1980s, categorical level I data became available from several studies,34 including prospective phase III trials conducted by the Brain Tumor Study Group (BTSG)35,36 (Table 25-1).
TABLE 25-1 Positive Phase III Trials Evaluating the Role of Irradiation, Chemotherapy, or Chemoradiation in the Treatment of Malignant Gliomas
The radiotherapeutic approach to high-grade glioma has evolved. Initially, large opposed lateral fields were employed to cover the entire brain volume. In 1989, Shapiro and colleagues37 published data from Brain Tumor Cooperative Group trial 80-01, in which the randomization was altered during the trial to compare partial brain irradiation with whole-brain radiotherapy. No difference in OS or change in the patterns of failure was seen. Accordingly, whole-brain irradiation is generally not advocated, except perhaps in the scenario of a widespread intracranial process such as gliomatosis cerebri.38
Several lines of evidence have influenced the trend to treat the gross tumor volume along with a margin of approximately 2 cm. In a classic paper published in 1980, Hochberg and Pruitt39 used CT scans to determine that nearly 90% of GBM recurrences occurred within 2 cm of the primary tumor site (although this may be changing with the use of bevacizumab).40 Wallner and associates41 assessed the patterns of recurrence in 32 patients with unifocal malignant gliomas who were treated with primary surgery and postoperative irradiation. Nearly 80% of patients manifested recurrence or progression within 2 cm of the original tumor. Even when 80 Gy of partial brain irradiation was used in a prospective phase I trial,42 90% of patients failed within the high-dose region.
It has been demonstrated on biopsy and autopsy studies that the abnormality detected on T2 or fluid-attenuated inversion-recovery (FLAIR) images harbors microscopic tumor extension. Accordingly, 45 to 50 Gy delivered, in 1.8- to 2-Gy fractions, to the T2/FLAIR abnormality seen on the image, followed by a boost to raise the total dose to 60 Gy based on the T1-enhancing abnormality, is generally prescribed. The MRI abnormalities, however, remain quite nonspecific in terms of histopathologic confirmation, and even when novel strategies such as MRS are used for radiotherapy planning, there can be overestimation or underestimation of the target.43
Rationale for Current Total Irradiation Dose
A pooled analysis of three successive randomized trials conducted by the Brain Tumor Study Group (BTSG 66-01, 69-01, 72-01, respectively) generated data to support doses in excess of 50 Gy.44 A stepwise improvement in survival was observed with doses ranging from less than 45 Gy to 60 Gy, consistent with dose response. A comparison of 70 Gy versus 60 Gy demonstrated no survival or local control advantage for the 70-Gy dose.45,46 These results established 60 Gy as the standard of care.
Dose escalation has remained an important investigational option because there is still a pattern of failure characterized by local progression or recurrence. There are now multiple techniques for dose escalation, including three-dimensional conformal irradiation, radiosurgery, and brachytherapy, but these have not yielded higher rates of disease control or survival. Increasing the radiotherapy dose or reirradiation with radioprotective or antiangiogenic agents may be a useful strategy in the future.47
Altered Fractionation
The RTOG has systematically and rigorously studied hyperfractionation for high-grade gliomas. In trial 83-02, patients were randomized to one of four dose arms (64.8, 72, 76.8, or 81.6 Gy) using twice-daily fractions of 1.2 Gy. Initial results suggested the superiority of 72 Gy,48 but a subsequent phase III trial demonstrated no improvement.49
Prados and colleagues50 used an elegant randomization to assess not only a hyperfractionation schedule of 1.6 Gy twice daily to a total dose of 70.4 Gy, but also to determine the activity of difluoromethylornithine (DFMO), a compound that inhibits sublethal and potentially lethal damage repair. Unfortunately, neither intervention improved survival.
Stereotactic Irradiation
Two provocative small-scale experiences prompted the design of phase III trials to formally evaluate radiosurgery for high-grade gliomas. Loeffler and colleagues51 reported on 37 patients treated with 59.4 Gy fractionated radiotherapy followed by a radiosurgical boost to a median dose of 12 Gy. After a median follow-up period of 19 months, a 76% survival rate was reported. Sarkaria and associates52 described 115 high-grade glioma patients who received conformal radiation therapy and a stereotactic boost. The median survival time was 96 weeks. These results called into question whether they represented a benefit from radiosurgery or simply selection bias.
RTOG 93-05 compared conformal irradiation plus carmustine with or without a radiosurgical boost for newly diagnosed GBM. No differences were observed in terms of OS (median, approximately 13 months in each arm) or quality of life.53
Radiosurgery may still have a role in the treatment of recurrent disease, particularly if a focal region of recurrence can be defined.47
Brachytherapy
Gutin and associates54 suggested a potential survival benefit in a phase II trial. These findings were not reproduced in subsequent randomized trials.55,56
Interest in this modality was rekindled when the GliaSite radiation therapy system (Proxima Therapeutics, Alpharetta, Georgia) received approval by the Food and Drug Administration (FDA) in 2001. This intracavitary device is implanted at the time of tumor debulking, and a solution of iodine-125 (125I) is injected into an expandable closed-catheter balloon. A retrospective study suggested reasonable safety and promising efficacy.57 A phase I study was conducted.58 However, the implant induces changes in imaging that complicate determination of disease progression.59
Chemotherapy: Concurrent with EBRT, Maintenance Chemotherapy, Other
Early randomized trials of chemotherapy were individually negative, but meta-analysis of these trials showed that 15% to 20% of patients treated with radiation therapy (RT) and nitrosoureas survived at least 18 months versus 5% treated with radiotherapy alone.35,36,60,61 Nitrosoureas, especially carmustine, were the most commonly used drugs, although procarbazine was also used extensively.62 The combination of procarbazine, lomustine (CCNU), and vincristine, also called PCV,63 had no clear benefit (yet much greater toxicity) versus carmustine for anaplastic astrocytoma,64 and this regimen has been largely abandoned.
Intratumoral delivery of chemotherapy for residual postoperative disease is most commonly in the form of carmustine-eluting (Gliadel) wafers. Patients undergoing wafer implantation during surgery for recurrent GBM survived approximately 2 months longer than patients without wafers in one study (p = .02).65 Treatment of newly diagnosed disease also yielded a 2-month prolongation of average survival.66,67 However, this was not statistically significant when the analysis was restricted to patients with GBM histology. Of note, wafer delivery of carmustine versus systemic administration has not been compared for safety or efficacy. Gliadel does, however, carry an FDA label for implantation during resection of recurrent GBM and newly diagnosed malignant glioma. Attempts to treat residual visible or microscopic disease with other local chemotherapies delivered through implanted catheters and using convection-enhanced migration of drug have generally failed.68
Currently, the most widely used chemotherapeutic agent is temozolomide. Whether it is more effective than nitrosoureas has not been investigated, but it is unquestionably better tolerated with significant myelosuppression in less than 20% of patients.69,70 Temozolomide was first approved for recurrent anaplastic astrocytomas following a phase II study.71 A randomized study also demonstrated superior efficacy to procarbazine in recurrent GBM.72
Temozolomide for newly diagnosed GBM has been studied both when given before RT73 and when combined with RT in various dosing schedules.74,75 Its role for newly diagnosed GBM was established by Stupp and associates69,70 on the basis of the EORTC 26981/22981 and NCIC CE.3 trial. In this phase III multicenter study, 573 newly diagnosed GBM patients received RT alone or RT with concurrent temozolomide followed by six adjuvant cycles of temozolomide. The patients who received the combined-modality regimen had significantly longer OS and progression-free survival (PFS) rates without significantly more toxicity (see Table 25-1). The 5-year OS was 10% among those receiving temozolomide versus 2% among those receiving radiotherapy alone (p <.0001).69 Patients in the most favorable RPA class had a 5-year OS rate of 28% following combined therapy.69 In a companion paper, Hegi and colleagues76 reported that methylation of the promoter for the O6-methylguanine DNA methyltransferase (MGMT) gene, which encodes the DNA repair enzyme O6-alkylguanine DNA alkyltransferase (AGT or AGAT, but now commonly also referred to as MGMT), was correlated with prolonged survival.
MGMT repairs DNA damage induced by temozolomide, and methylation of the MGMT promoter silences expression of the protein, thereby accentuating the antineoplastic effects of temozolomide. However, the mechanism by which MGMT promoter methylation leads to an improved outcome is more complex. For example, patients with tumors harboring methylated MGMT survived longer following treatment with RT alone than patients who did not have tumors harboring methylated MGMT treated identically.69 Others reported similar findings in GBM77 and other malignant gliomas.78 Moreover, MGMT protein expression by immunostaining does not predict outcome.79 It remains unclear whether MGMT analysis should alter treatment, although several alternate dosing schedules of temozolomide are under investigation80,81 as a means to overcome this and other potential resistance mechanisms.
Anaplastic Gliomas
Anaplastic astrocytomas, anaplastic oligodendrogliomas, and anaplastic oligoastrocytomas represent the most common WHO grade III tumors.1,20 In anaplastic gliomas, resection appears to improve survival relative to biopsy, as it does for GBM.
It is generally accepted that radiotherapy should be administered postoperatively for astrocytomas. In a German study (NOA-04), survival rates were equivalent whether chemotherapy or radiotherapy was used first among patients with anaplastic astrocytomas, oligodendrogliomas, and mixed tumors.12 However, time to progression following RT was longer than after chemotherapy, and initial radiation therapy achieved more complete and partial responses than initial chemotherapy, suggesting the superiority of radiotherapy.82
Combs and associates83 reviewed the outcome of 191 patients with grade III astrocytic tumors treated at the University of Heidelberg with either RT alone or RT in combination with temozolomide during a 20-year period (from 1988 to 2007). In this retrospective study, no significant advantage in rates of OS or PFS could be attributed to the combination. RTOG trial 9813 randomized patients with anaplastic astrocytomas (or oligoastrocytomas) to radiotherapy with concurrent nitrosourea (carmustine or lomustine) or with temozolomide, and results are pending.
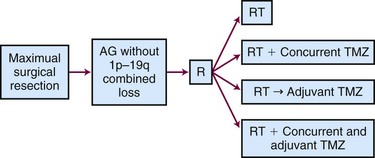
The ongoing CATNON (Concurrent vs. Adjuvant Temozolomide for NON 1p19q co-deleted anaplastic gliomas, also called EORTC 26053-22054) Intergroup trial randomizes patients to receive postoperative RT alone, concurrent temozolomide and RT without adjuvant temozolomide, RT (without concurrent temozolomide) followed by adjuvant temozolomide, or RT with both concurrent and adjuvant temozolomide (Fig. 25-5). Although technically this study allows any WHO grade III glioma without 1p/19q co-deletion, the majority of the tumors will be anaplastic astrocytomas.84
The role of radiotherapy for newly diagnosed anaplastic oligodendroglioma is most controversial because of the tumor’s reported sensitivity to chemotherapy,85 especially tumors with 1p/19q co-deletion.86 In spite of this, two separate randomized studies that compared radiation therapy with radiation therapy plus chemotherapy (RTOG 94-0210 and EORTC 2695111) failed to demonstrate a survival advantage from the addition of chemotherapy. PCV chemotherapy prolonged PFS, and those who failed irradiation were frequently treated with PCV, possibly confounding the results. Both trials provided validation of the prognostic value of the allelic loss of heterozygosity of the 1p and 19q chromosomes.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree