Classic (1872)
Elderly men of Eastern, European, or Mediterranean origin
Slow chronic evolution
Localized lesions
Lower extremities
Endemic: three different forms (1914–1950)
Adults (mostly men) but also some children in Central, East, and South Africa
1. Slow evolution as classic KS
2. Usually more aggressive than classic KS with disseminated lesions with lymph node
3. Children form with lymph node
Immunosuppression associated/iatrogenic (1970)
Organ-transplant recipients
1. Chronic
2. Rapid aggressive evolution
3. Regressive in some cases after removal of immunosuppression
AIDS-associated epidemic (1981)
HIV-infected persons Homosexual men in Occident
Heterosexual men and women in Africa
Aggressive
Frequent disseminated lesions
Visceral mucosal involvement
Lymph node
The histological features of the four epidemiological forms of KS are indistinguishable. This complex tumor, of mixed cellularity, is defined histologically by the coexistence at different levels, according to the evolution stage, of the main following features: a vascular proliferation, extravasated erythrocytes, inflammatory infiltration, and the presence of tumor cells (spindle cells) [5]. At the early stage lesion (patch-stage KS), a macular lesion of the reticular dermis is observed with proliferation of small irregular endothelium-lined spaces with a variable inflammatory lymphocytic infiltrate. During the progression of the disease (plaque-stage KS), a small palpable lesion exists with expansion of a spindle cell vascular process in the entire dermis associated with a perivascular inflammatory infiltrate. At a late stage, lesion (nodular-stage KS), nodules, and tumors (skin, lymph node, etc.) are associated with proliferation of spindle cells (sheets, fascicles, etc.). The cellular origin of the spindle cells (the main proliferative elements of KS) remains a matter of debate; however it is suspected that HHV-8 infects circulating endothelial precursor cells driving them toward a lymphatic lineage [6].
2 Discovery of HHV-8
A viral etiology for KS has been suspected for a long time, and several viruses were detected in some KS lesions such as CMV, HHV-6, BK, etc. In the 1990, solid epidemiological studies indicate that an as-yet unidentified infectious agent, transmitted mainly by sexual contact, may cause Kaposi’s sarcoma in persons with AIDS, especially in MSM [7]. In 1994, a new herpesvirus was first identified in a tumor biopsy from an AIDS-KS and called human herpesvirus-8 (HHV-8) or Kaposi’s sarcoma-associated herpesvirus (KSHV) [8]. It is now considered as the causing agent of all forms of Kaposi’s sarcoma. HHV-8/KSHV is a Gammaherpesvirus and the only known Rhadinovirus infecting humans. The 165 kb HHV-8 genome is notable for molecular piracy of several genes homologous to cellular regulatory genes that are likely to contribute to KS pathogenesis of KS [9].
3 HHV-8 Epidemiology, Geographical Distribution, and Modes of Transmission
Diagnosis of an HHV-8 infection is mostly based on the demonstration of specific antibodies directed against latent and/or lytic HHV-8 antigens in the plasma/sera of infected persons [10, 11]. While both assays perform very well in epidemiological studies, the latter are generally considered less specific than the anti-latent ones. This implies that seroprevalences are frequently lower in studies using anti-latent assays. Both ELISA, using different specific peptides, and immunofluorescence assay, using different HHV-8 infected cell lines, are used for serology. Direct detection of fragments of genomic DNA of HHV-8 is performed by specific polymerase chain reaction targeting different HHV-8 genes [11]. Sequencing of such amplicons, especially of the K1 gene (around 900 bp), is very useful for genotype determination and molecular epidemiological studies [12, 13]. Antibody titer determination, as well as viral load quantification, can be useful, especially for biological follow-up of some patients.
HHV-8 is not a ubiquitous virus and the viral global distribution is quite heterogeneous (Fig. 1). Generally speaking, areas of high or very high endemicity correspond mostly to the regions of classic and endemic KS forms. For instance, HHV-8 seroprevalence ranges from 20 to 80 % in the general adult population in sub-Saharan Africa, especially Central and East Africa [14], and in parts of South America [15], parts of China [16], and Melanesia [17]. Mediterranean countries have a global seroprevalence in adults ranging from 5 to 30 %, depending on the areas [18]. The low endemic regions are North America and most of European and Asian countries. However, in these regions, HHV-8 is often endemic in the homosexual male population. Indeed, MSM represent an important HHV-8 endemic population with a seroprevalence level ranging from 15 to more than 50 % in some countries [19, 20].
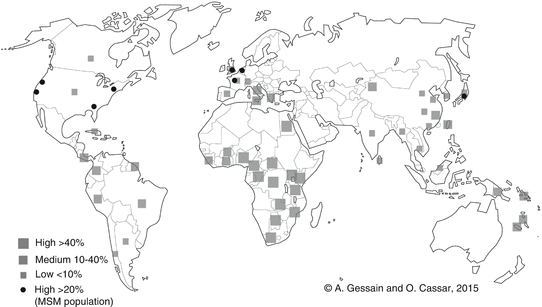

Fig. 1
Geographical repartition of HHV-8 seroprevalence. Relatively few studies were performed either in representative general population or among blood donors. Most studies were conducted to evaluate HHV-8 prevalence among high-risk populations (men who have sex with men (MSM) and HIV individuals) and to study mother-to-child transmission
The epidemiological determinants, including the modes of transmission, are quite different depending on the studied population and the level of endemicity. In highly endemic areas, HHV-8 infection is mainly acquired during childhood and is very probably transmitted during mother-to-child contacts and between young siblings [21, 22] (Fig. 2). Indeed, in endemic population of African origin, studies have demonstrated a high level of familial aggregation, with strong evidence for transmission between children of the same family (especially in siblings with an age difference of less than 5 years) and from mother to child [22, 24]. It is also worth to note that in central, and mostly in East Africa, endemic KS can also occur in children [1, 2]. Interestingly, HHV-8 infection can be unevenly distributed from one region to another, suggesting nonuniform specificities in transmission modes [25].
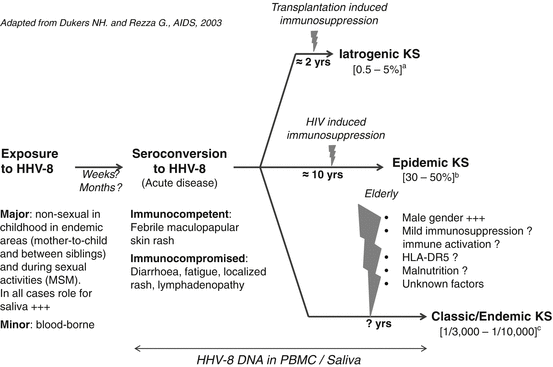

Fig. 2
Natural history of HHV-8 infection and KS development: Determinants and risks (Adapted from Dukers and Rezza [23]). (a) The incidence of KS after organ transplantation depends on the geographical origin of HHV-8-infected individuals (mostly patients of Mediterranean, Jewish, and Arabic ancestry) as well as the type of transplanted organ. (b) The risk of KS among HIV-infected individuals is higher when HHV-8 seroconversion occurs after HIV seroconversion. (c) The risk of KS among immunocompetent individuals over 50 years of age varies according to gender suggesting the existence of sex-related factors. MSM men who have sex with men
In endemic areas, as rural general populations of Central and East Africa, adult HHV-8 prevalence is generally similar in men and women and increases slightly with age. In such populations, nonsexual transmission of HHV-8 is considered as the major mode of viral acquisition. However, little remains known about HHV-8 spread in adult African population. This feature appears to greatly differ to that of industrialized/occidental countries where most of the infection seems to be acquired after adolescence, especially in high-risk groups. Indeed, it is clear that in MSM, HHV-8 is associated with sexual risk factors, such as number or partners and history of sexual transmitted disease [26]. In both high endemic areas, as Central Africa, and in high-risk population, as MSM, saliva is considered as the main vector of HHV-8 infection [27]. Transmission via blood transfusion seems rare, while transmission via solid organ donation has been clearly documented (for review on the different modes of transmission, see [9, 23, 28–30]).
As demonstrated for Epstein-Bar virus, some studies have suggested that HHV-8 prevalence may also be related to the socioeconomic level of the studied populations. In highly endemic areas of East Africa, HHV-8 infection is associated to factors such as low socioeconomic level, common living standard, water supply, and promiscuity [31]. Furthermore, few studies, conducted in another environment, show that populations that kept a traditional way of life show high prevalence for HHV-8. However, these data remain yet scarce, and other studies are necessary to appreciate the different items (environmental cofactors, specificities in ways of life influencing transmission modes, or even genetic features), which can lead to the apparent HHV-8 seroprevalence differences observed in different populations, especially in Africa.
4 Molecular Epidemiology
Molecular epidemiology studies on HHV-8 have mainly focused on the variable K1 region (ORF-K1), a region encoding a membrane protein-expressed during the early lytic phase of the viral cycle [32]. This has led to the identification of five main viral subtypes (A, B, C, D, E) that exhibit a geographical clustering. There is a 15–30 % amino acid difference overall among those subtypes and a 30–60 % amino acid difference within two K1 highly variable regions VR1 and VR2, which encode the areas usually targeted by the immune system on the K1 protein [32]. Subgroup A1–4 and subtype C are predominant among populations of European descent but also in North Africa, in the Mediterranean Basin, and in some regions of Asia. Subgroup B1–4 and clade A5 are predominant in sub-Saharan Africa. The reported subtype D sequences have been described in Southeast Asia (Japan, Taiwan) and in the Pacific (Australia, New Zealand, Wallis, Vanuatu), while subtype E is restricted to the Amerindian populations. Thus, HHV-8 appears to be a very ancient virus infecting the human population [13, 32, 33]. Its genetic variability can be used as a molecular mean to better understand population migrations. HHV-8 has several distinct simian counterparts that have been characterized both in nonhuman primates from the Old World (gorillas, chimpanzees, and macaques) and the New World (squirrel monkeys, marmosets, and tamarins) [34].
5 Other Cancers Associated with HHV-8
HHV-8 has been associated with other tumors such as primary effusion lymphoma (PEL) [35], most multicentric Castleman diseases (MCD) [36], and other rare associated lymphomas.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree