Gene
Relative risk (Ref.)
Absolute risk by 70–80 years of age (%)
Associated tumours
BRCA1
11.4 [3]
75
Breast, ovarian carcinoma
BRCA2
11.7 [3]
76
Breast, ovarian carcinoma
TP53
4.3–9.3 [4]
75
Sarcoma, breast, adrenocortical carcinoma, brain
PTEN
2–5 [5]
39, 43, 49
Breast, thyroid, endometrial cancer
CDH1
6.6 [6]
53
Diffuse gastric cancer
STK11
2–4 [7]
45, 18, 57, 11
Breast, ovarian sex cord-stromal tumours, colon, pancreas,
NF1
2.6 [8]
26
Peripheral nerve sheath, brain
PALB2
5.3 [9]
45
Pancreas
ATM
2.8 [10]
27
Pancreas
CHEK2
29
Lung
NBN
2.7[13]
29
Breast
RAD51C
1.5–7.8 [14]
Breast
RAD51D
6.3[15]
Ovarian carcinoma
BRIP1
2 [16]
3.6–11 [17]
Breast
Ovarian carcinoma
Clinical experience with multigene panel testing of individuals who lacked BRCA1/BRCA2 mutations has reported deleterious mutation rates of 3–11%, most commonly in moderate-risk breast and ovarian cancer genes and Lynch syndrome genes [19–21]. The most common mutations belonging to this category were in PALB2 (23%), CHEK2 (15%) and ATM (15%) genes [19]. Regarding factors that predicted for mutations in genes other than BRCA1/BRCA2, one study did not find any clinical predictors in 1781 patients, even though the age at first BC diagnosis was slightly lower for BRCA1/BRCA2 mutation carriers compared to those with mutations in other BC susceptibility genes [20]. In addition, the risks associated with some genes could be the result of interactions with other genes or environmental interactions. Moreover, it is perfectly possible that different mutations located in the same gene confer a different risk of hereditary cancer. As a result of these limitations, current international initiatives are trying to fill in these gaps in genotype-phenotype correlations, expressivity and penetrance, including the Evidence-based Network for the Interpretation of Germline Mutant Alleles (ENIGMA), the Prospective Registry Of MultiPlex Testing (PROMPT) and the Consortium of Investigators of Modifiers of BRCA1/2 (CIMBA).
The current version (2.2016) of the NCCN guidelines recommends performing regular breast magnetic resonance imaging (MRI) tests in women harbouring germline mutations in ATM, CDH1, CHEK2, PALB2, PTEN, STK11 and TP53 genes based on a >20% risk of BC. Further, risk-reducing mastectomy (RRM) should be considered in CDH1, PTEN, TP53 and PALB2 mutation carriers and risk-reducing salpingo-oophorectomy (RRSO) in MMR, BRIP1, RAD51C and RAD51D mutation carriers [2].
41.2 Hereditary Breast Cancer Immunophenotype
BRCA1 tumours are more likely to be high grade medullary-like subtype with features such as an increased mitotic count, lymphocytic infiltrate, pushing margins, trabecular growth pattern and necrosis [22–24]. Further, frequent basal-like immunohistochemical markers including a lack of oestrogen (ER), progesterone receptor (PR) and human epidermal growth factor receptor-2 and increased tp53, cytokeratin 5/6, cytokeratin 14, cytokeratin 17 and epidermal growth factor would also suggest a BRCA1-related BC. Gene-expression studies have shown the majority of BRCA1-BCs fall into the basal-subtype group [25]. Single-cell analyses of temporal somatic events in BRCA1-BC tissue have revealed loss of PTEN and TP53 mutations as early events in the development of basal-like and luminal tumours, respectively [26].
Breast cancers arising in BRCA2 mutation carriers tend to be more heterogeneous than those arising in BRCA1 mutation carriers. However, sparse studies of detailed tumour pathology information from BRCA2 carriers have revealed that they also exhibit a distinct morphologic and molecular phenotype. Regarding expression of the ER, the majority of early studies had concurred with a similar prevalence of ER-positive BRCA2-associated BC compared with sporadic controls [24, 27]. In contrast, recent studies have demonstrated that BRCA2-associated tumours are predominantly luminal B in terms of gene expression, more likely to be ER positive and with high histological grade, with reduced tubule formation, continuous pushing margins and less expression of basal cytokeratin 5 and HER2/neu protein overexpression compared with sporadic BC [24, 28].
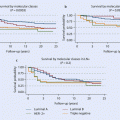
The knowledge of the histopathological features that are characteristic of hereditary BC may be additionally used to prioritize testing of BRCA1 and BRCA2 mutations in BC cases. The most recent initiative using the largest pathology datasets accrued by CIMBA and BCAC has obtained the most robust pathology-based likelihood ratio estimates for prediction of BRCA1/BRCA2 mutation in women younger than 70 years [29]. Therefore, an ER-positive phenotype negatively predicted BRCA1 mutation status, irrespective of grade, whereas ER negativity plus histological grade 3 was more predictive of positive BRCA1 mutation status in women 50 years or older. In contrast, ER positivity plus grade 3 modestly predicted BRCA2-positive mutation status irrespective of age, whereas ER negativity plus histological grade 3 features modestly predicted BRCA2-positive mutation status at 50 years or older. Triple-negative tumour status was highly predictive of BRCA1 mutation status irrespective of age and modestly predictive of positive BRCA2 mutation status in women 50 years or older.
Less is known on the phenotype of other mutated gene-associated BC. PALB2-related BC, the third most frequent cause of hereditary breast cancer, mostly resembles BRCA2-associated tumours [9, 30]. Two recent series of PALB2 mutation carriers have compared PALB2-related and sporadic BC. Both studies reported that 60–74% of tumours were ER positive, and, remarkably, 30–35% of them were ER/PR and HER2 negative (triple negative). Moreover, the largest published cohort of BC in Li-Fraumeni syndrome to date, in 39 women with TP53 mutations, found that 63% of the invasive BC and 73% of DCIS were positive for HER2 and more than three quarters of the tumours were positive for ER. Remarkably, the concurrent expression of the ER and HER2 was present in 49% of the tumours [31].
Finally, recent studies on the genomic profiles of hereditary breast tumours compared with sporadic tumours also suggest distinct genetic pathways in terms of the regions altered. Therefore, loss of 4q, 3p and 12q and loss of 11q and 13q are recurrently seen in BRCA1 and BRCA2 tumours, respectively [32, 33]. Additionally, one study on comparative genomic hybridization array (CGH) analysis of hereditary BC biopsies has identified four major different groups according to the type and amount of genomic alterations showing one group with a significantly inferior survival [34]. This research has also supported the existence of a subset of sporadic tumours that acquire alterations leading to genomic instability similarly to BRCA1– and BRCA2-related tumours with the potential benefits of targeted therapy through the use of agents that lead to DNA double-strand breaks such as PARP inhibitors and platinum agents.
41.3 Risk-Reducing Strategies for BRCA-Associated Breast Cancer
The main goal of managing hereditary BC is to minimize the incidence and mortality of a first or a second tumour. Early intensive surveillance based on the combination of annual MRI and mammography achieves a high sensitivity (80–94%) in the detection of BC at an early stage and a promising low mortality rate at 5 years [35, 36]. The contribution of mammography to screening accuracy in BRCA1/BRCA2 carriers has been recently addressed in a recent individual-patient meta-analysis, which found differences according to age and mutation status. Therefore, whereas in BRCA1 mutation carriers the addition of mammography leads to a 3.9% increase in sensitivity and a 4% loss of specificity regardless of age, a third of tumours in BRCA2 mutation carriers younger than 40 years of age were detected by mammography. These results support the potential consideration of different screening recommendations according to BRCA1 or BRCA2 mutation status [37].
This issue is discussed in more detail in ► Chap. 6
The portfolio of risk-reducing surgical interventions includes risk-reducing bilateral mastectomy (RRBM), prophylactic contralateral mastectomy (PCM) and risk-reducing salpingo-oophorectomy (RRSO). Rates of prophylactic bilateral mastectomy and particularly contralateral mastectomy have been steadily rising over the past 15 years due to multiple factors, including the increased use of preoperative MRI and the advances in immediate breast reconstruction. However, prophylactic surgery is not an inconsequential decision. Informed consent should be based on the individual cancer risk evaluation in the setting of comprehensive pretest and post-test counselling. Moreover, multidisciplinary consultations before surgery are recommended to ensure informed decision-making by the patient.
41.3.1 Risk-Reducing Bilateral Mastectomy in Healthy BRCA1/BRCA2 Mutation Carriers
Updated reports from retrospective and prospective observational studies that compared BC outcomes in women who underwent risk-reducing bilateral mastectomy (RRBM) with surveillance showed a reduction of 90% or more in the risk of subsequent breast tumour development and decreased BC mortality by 81–100% among those women who underwent surgery [14, 38–41]. In line with previous results, the most recent prospective study assessing the efficacy of RRBM compared to surveillance in a cohort of 570 healthy BRCA1/BRCA2 carriers showed significant reductions in all-cause mortality and BC-specific mortality, although confirmation in larger cohorts of patients with a longer follow-up is warranted [41].
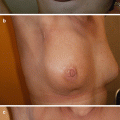
Due to the possible retention of at-risk tissue in the skin flaps and below the areola, the classic subcutaneous mastectomy, defined as the complete removal of all breast tissue while leaving the nipple-areola complex (NAC) intact, has been replaced by total skin-sparing mastectomy (TSSM), a more recent and popular technique among surgical oncologists that preserves the overlying skin over the whole breast and the NAC and enhances the cosmetic outcome. Recent studies in average-risk populations have demonstrated similar oncologic safety of TSSM and simple non-skin-sparing mastectomy, with a locoregional recurrence rate of 2% at 3 years of follow-up [42], and better cosmetic outcomes. Ductal tissue beneath the nipple should be excised and submitted to pathology separately. If DCIS or invasive cancer is identified, then the NAC should be removed. In women with a previous diagnosis of BC, factors that have been associated with nipple involvement are the mammographic distance of the tumour from the nipple and tumour size. In particular, when the tumour-nipple distance is less than 2 cm or tumour size greater than 4 cm, nipple involvement is reported in an average of 50% of cases. For BRCA1/BRCA2 mutation carriers, the concern is that preservation of the NAC skin may confer an unacceptable rate of new cancer development. Available data from two subsets of BRCA1/BRCA2 mutation carriers within overall cohorts of women undergoing prophylactic and therapeutic nipple-sparing mastectomy (NSM) reported no new cancers at a maximum follow-up of 43 months [43, 44]. The outcomes of 413 patients undergoing NSM, including 177 BRCA germline mutation or genetic variants of uncertain significance, at one single institution reported unexpected invasive carcinoma in 1.7% and 17% of prophylactic and therapeutic NSM, respectively [45]. Further, one study compared the oncologic outcome and tumour involvement of the NAC in 53 carriers treated with TSSM and immediate reconstruction for prophylactic (26 patients) or therapeutic indications (27 patients) with age- and stage-matched non-BRCA controls. At a mean follow-up of 51 months, among patients undergoing prophylactic surgery, no new cases of invasive cancer were found. However, one ductal carcinoma in situ was diagnosed in one nipple specimen from one carrier compared with two specimens found in the noncarriers cohort. In patients undergoing TSSM for therapeutic indications, «nipple specimen» analysis found one invasive and one in situ carcinoma in the noncarrier cohort. At a mean follow-up of 37.3 months, there were no local recurrences in the BRCA1−/BRCA2-positive cohort [42].
Data from the largest study of its kind, presented at the Annual Meeting of the American Society of Breast Surgeons 2016, supports NSM approach as being safe and effective in the short term. After a median follow-up of 34 months, none of the 551 nipple-sparing mastectomies performed prophylactically in 348 BRCA1/BRCA2 mutation carriers developed BC at any site. Whereas accruing more patients and longer-term follow-up will be important to confirm the oncologic safety of this approach, NSM may be offered to BRCA-positive women undergoing risk-reducing mastectomy.
Regarding reconstructive techniques, BRCA1/BRCA2 mutation carriers mainly prefer immediate reconstruction with breast implants which may relate to their young age, when autologous donor sites are less likely to be adequate for bilateral reconstructions. Major surgical complications such as infection, flap necrosis and loss of reconstruction occur in about 20% of cases [46]. Psychological effects and health-related quality of life have also been addressed in retrospective [47] and a few prospective studies [48–50]. A large retrospective study from the Mayo Clinic suggested that 74% of women had a diminished level of emotional concern of developing BC and 48% of women did not change their level of satisfaction with their body appearance [47]. Remarkably, one prospective study of 65 Swedish women questioned at baseline and 1 year postoperatively did not detect significant negative effects on anxiety, depression and quality of life. However, women with RRBM reported a negative impact on sexual pleasure and body image although they improved their anxiety and social activities over time [49].
41.3.2 Surgical Approach to BC in BRCA Mutation Carriers
BRCA mutation testing strongly influences surgical decisions in newly diagnosed BC patients. An ongoing prospective cohort study, including 897 women aged 40 years and younger at BC diagnosis, resulted in 30% of women reporting that knowledge or concern about genetic risk had influenced their surgical treatment decisions [51]. Moreover, once a BRCA mutation carrier is diagnosed with BC, she faces the difficult dilemma to choose between breast-conserving therapy (BCT) followed by radiation therapy, unilateral therapeutic mastectomy or unilateral therapeutic mastectomy and prophylactic contralateral mastectomy. Decision-making about her optimal local management would depend on her risk of ipsilateral breast recurrence (IBR), the risk of contralateral BC (CBC), the potential survival benefit of prophylactic mastectomy and the potential factors that modify her risk of IBR and CBC.
The risk of IBR in BRCA mutation carriers compared with noncarriers was analysed in a recent meta-analysis [52] that included ten studies (six cohort and four case-control studies) and reported pooled rates of IBR of 17.3% and 11% for carriers and controls, respectively (p value = 0.07). Only two studies showed a trend for more frequent new primary cancers in BRCA mutation carriers. Based on a subgroup analysis according to follow-up, there was no difference in IBR risk in carriers versus noncarriers in studies with a median follow-up of less than 7 years. However, studies with longer median follow-up showed that carriers had a significantly higher risk of IBR compared with patients with sporadic BC. This finding indicates that radiotherapy in carriers is as effective as in noncarriers, at least in the short term, although the persistence of a higher risk for developing a new primary cancer in the residual breast tissue should be also taken into account. No difference was found in the IBR risk between BRCA1 and BRCA2 mutation carriers. Regarding overall survival (OS) and BC specific survival (BCSS) after BCT between BRCA mutation carriers and noncarriers, two studies revealed no differences in OS, and two other studies found conflicting results in BCSS [52].
The most appropriate surgical management of unilateral BC in BRCA mutation carriers is addressed in one retrospective cohort study that compared both approaches: BCT and mastectomy [53]. Remarkably, despite the higher risk of IBR following BCT compared with mastectomy observed in this study (23.5% vs. 5.5%), no differences in BCSS and OS were detected at 15 years. Most of the IBR in the BCT group consisted of less biologically aggressive new primary cancers than true recurrences [54]. Based on a moderate level of evidence, two factors were protective against IBR, the use of adjuvant chemotherapy (RR 0.51) and undergoing oophorectomy (RR 0.42).
To answer the question regarding the benefit of bilateral mastectomy, eleven studies (seven cohort and four case-control studies) fulfilled the criteria to be included in the aforementioned meta-analysis [52]. BRCA mutation carriers had a 3.5-fold increased risk for CBC compared with noncarriers, and consistently, bilateral mastectomy reduced the risk of CBC in carriers. Regarding the differential risk between both genes, BRCA1 mutation carriers had a higher risk of CBC compared with BRCA2 mutation carriers (21% for BRCA1 and 15% for BRCA2 mutation carriers). Risk factors for CBC among BRCA mutation carriers were investigated in nine studies. Oophorectomy (RR 0.52), older age at diagnosis and the use of adjuvant tamoxifen (RR 0.57) were associated with a decreased risk for CBC. With a low level of evidence, the protective effect of tamoxifen may be stronger in patients not undergoing oophorectomy. Evidence did not support the impact of either adjuvant chemotherapy or radiotherapy on the risk of CBC [55].
Regarding the outcome of patients with a personal history of BC who complete CPM compared to high-risk surveillance, two early studies [56, 57] with a limited number of patients and follow-up found no differences in either BCSS or OS [57]. However, more recent evidence from two retrospective [58, 59] and one prospective observational [41] studies revealed an improved survival after CPM compared with unilateral mastectomy. In the first study, from Canada, despite the differences between the two groups concerning favourable tumour characteristics and more efficient chemotherapy regimens in the CPM group, the 20-year survival was 88% compared with 66% for those patients who chose surveillance [58]. The average time from diagnosis to CPM was 2.3 years. In agreement with these results, a second British cohort study reported a 10-year OS of 89% in the CPM group compared to 71% in the non-CPM group [59]. This benefit in BCSS was consistently found in a prospective Dutch analysis, especially for patients with low risk of primary BC-specific mortality such as age < 40, grade 1 or 2, no triple-negative phenotype and lack of adjuvant chemotherapy.
It is important to note that the discussion for or against CPM should include the risk of recurrence and metastasis as well as the risk of OC and consideration of prophylactic bilateral salpingo-oophorectomy.
41.3.3 Risk-Reducing Bilateral Salpingo-Oophorectomy
Based on the high risk of ovarian and fallopian tube cancer and the lack of effective screening tools for these tumours, current international guidelines recommend healthy BRCA1/BRCA2 mutation carriers to undergo risk-reucing bilateral salpingo-oophorectomy (RRBSO) around the age of 40 upon the completion of their childbearing potential or individualized based on the age of onset of OC in their family [60]. In addition to the reduction of cancer incidence, RRBSO also decreases the all-cause mortality, BCSS and OC-specific mortality according to a meta-analysis of three prospective studies [61, 62]. The procedure must include the visual assessment of the abdomen and pelvis, pelvic washings and total bilateral salpingo-oophorectomy with ligation of the ovarian artery and vein approximately 2 cm proximal to the ovary and tube. Both serous tubal intraepithelial and occult invasive serous carcinomas have been identified in 2–17% of the fallopian tubes of BRCA1−/BRCA2-positive women undergoing RRBSO. Therefore, meticulous processing of the surgical specimen is mandatory to identify occult invasive disease and the coexistence of potential precursors of high-grade serous carcinoma [63]. Based on the recent hypothesis about serous cancers originating from the fallopian fimbria, bilateral salpingectomy with delayed surgical menopause is an option that should not be used outside of clinical trials.
Beyond its use for the prevention of gynecologic cancer, several observational studies have evaluated the effects of RRBSO on BC risk. Five out of seven observational studies and one meta-analysis, using different designs and analytical methods, reported a reduction in the risk of BC of approximately 50% when the surgery was performed before menopause (◘ Table 41.2). Among the major studies, three of them did not find a reduction in the risk of a second primary BC [3, 71, 67]. Minimizing the potential bias of prior studies, the most recent analysis could not find a significant reduction in the risk of second BC after a median follow-up time of 3.2 years. Therefore, when counselling BRCA mutation carriers on a second primary BC risk reduction, caution is warranted until a representative number of patients, specially BRCA2 mutation carriers, and longer follow-up allow us to draw any conclusion [71].
Table 41.2
Breast cancer risk reduction after bilateral salpingo-oophorectomy
Authors | Design | Patients | Follow-up | HR (95%) | |||
|---|---|---|---|---|---|---|---|
RRSO | No RRSO | (FU) | Total | BRCA1 | BRCA2 | ||
Rebbeck et al. [64] | Retrospect/prospect FU | 43 | 79 | RRSO 9.6 year No RRSO 8.1 year | NR | 0.53 (0.33–0.84) | NR |
Rebbeck et al. [65] | Retrospect/prospect FU | 99 | 142 | RRSO 8.2 year No RRSO 8.9 year | 0.47 (0.29–0.77) | NR | NR |
Domcheck et al. [66] | Prospect matched cohort | 155 | 271 | RRSO 3.1 year No RRSO 2.1 year | 0.36 (0.20–0.67) | NR | NR |
Domcheck et al. [38] | Prospect unmatched cohort | 336 | 1034 | 3 year | 0.54 (0.37–0.79) | 0.63 (0.41–0.96) | 0.36 (0.16–0.82) |
Mavaddat et al. [3] | Prospect unmatched cohort | 309 | 679 | 3 year | 0.62 (0.35–1.09) | 0.52 (0.24–1.13) | 0.79 (0.35–1.8) |
Heemskerk-Gerritsen et al. [71] | Retrospect/prospect FU | 346 | 476 | RRSO 5.5 year No RRSO 3.9 year | 1.09 (0.67–1.77) | 1.21 (0.72–2.06) | 0.45 (0.17–1.66) |
41.4 BRCA1/BRCA2 Mutation-Associated Breast Cancer Systemic Treatment
Proteins encoded by the BRCA1 and BRCA2 genes are essential for homologous recombination (HR), the cell’s most efficient and accurate DNA repair pathway for double-strand DNA breaks [68]. According to the Knudson hypothesis [69] and as a result of genomic injury, tumours in patients harbouring germline BRCA1/BRCA2 mutations suffer a somatic loss of the second BRCA allele, thus leaving the tumour tissue with a defective HR repair pathway and in need for alternative DNA repair methods. Single-strand-based base excision repair (BER) is a primary backup system for HR loss in response to BRCA1/BRCA2 mutations [68], and PARP-1 mediates BER by recruiting the scaffolding proteins XRCC1, DNA ligase III and DNA polymerase ß [70]. In BRCA-mutated tumour cells, disruption of both repair pathways leads to cell death.
Advances in the treatment of hereditary BC and OC have been based on the common clinical and pathologic features shared by BRCA mutation-associated tumours, sporadic poorly differentiated high-grade OC and triple-negative BC (TNBC) – referred as «BRCAness» – and the hypothesis that all these tumours might end up being particularly sensitive to the same drugs. Clinical trials encompassing all these tumours have tested either the efficacy and tolerability to DNA-damaging agents alone or in combination with other cytotoxic agents or the synthetic lethality approach through the identification, validation and application of new targets involved in pathways alternative to HR repair of DNA damage such as poly-ADP ribose polymerase (PARP)-1 and PARP family members.
Current phase 2–3 clinical trials in hereditary BC are based on preclinical and a few small retrospective studies suggesting that BRCA1−/BRCA2-mutated BC cells exhibited greater chemosensitivity compared with wild-type BRCA1/BRCA2 cells with regard to agents causing double-strand DNA breaks such as cisplatin and less sensitivity to taxanes [72, 73]. In addition, PARP inhibitors (PARPi) were developed either in combination with other drugs in a range of solid malignancies [74] or in monotherapy in tumours predicted to be highly sensitive to PARP inhibition such as the BRCA mutation-associated breast and ovarian cancers.
41.4.1 PARP Inhibitors in BRCA-Associated Tumours
Earlier evidence from phase 1–2 studies testing five PARPi in monotherapy (olaparib, veliparib, talazoparib, niraparib and rucaparib) in BRCA-associated breast and ovarian cancers demonstrated promising antitumour activity with overall objective rates ranging from 13% to 57% and clinical benefit in 63% of BC patients [75–84], which was comparable with response rates observed in studies of single-agent chemotherapy. Additional studies of veliparib as a single agent or in combination with chemotherapy are currently active [85, 86]. Toxicity profiles appear to be similar to cytotoxic agents but generally manageable. The most frequent grade 1–2 adverse events are nausea, vomiting, diarrhoea, fatigue, headache and anaemia (common to all them) reported in 81% of patients and thrombocytopenia in 35% of patients treated with niraparib [87]. Among grade 3–4 toxicities, nausea, vomiting and haematological toxicity were the most common dose-limiting adverse events found in the phase 1 trials with olaparib [77, 78].
These data guided the development of phase 1 trials with other agents and two clinical trials of PARPi in combination with chemotherapy in the treatment of advanced BC. Based on preclinical data showing the synergic activity between veliparib and temozolomide [88], this PARPi has been further investigated as part of combination schedules in one phase 2 with a response rate of 22% and clinical benefit rate of 50%. Best results in terms of efficacy have been obtained in other phase 1–2 trials of the combination of a PARPi with cisplatin [89, 90], carboplatin [83, 91] and topotecan [92] with a wide range of response rates (25–73%) likely explained by the differences in inhibitory action on PARP among particular inhibitors.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree