Amplification of the human epidermal growth factor receptor type 2 (HER2 or ERBB2) gene in human breast cancers is an example of an acquired molecular alteration on which human breast cancer cells with this alteration depend for maintenance of a fully malignant phenotype and which has come to represent a molecular subtype of the disease. HER2/ERBB2 (also known as
neu oncogene) amplification with resultant HER2/ERBB2 protein overexpression has been shown to play a role in sustaining multiple cancer pathways, including self-sufficiency in growth signals, sustained angiogenesis, increased cell division, and enhanced invasion (
1,
2). Inhibition of HER2/ERBB2 membrane signaling in these cancer cells through administration of humanized anti-HER2/ERBB2 antibodies (trastuzumab [Herceptin], pertuzumab [perjeta], Trastuzumab emtansine) or administration of small molecule inhibitors of HER2/ERBB2 tyrosine kinase activity (lapatinib [Tykerb]) is associated with improved patient outcomes for women with both primary and metastatic disease (
3,
4,
5,
6 and
7). Because improvements in outcome are only documented in women whose breast cancers have alterations in the HER2/ERBB2 gene or protein product, accurate clinical testing for HER2/ERBB2 amplification or overexpression has become an important clinical consideration. The importance of this issue is reflected by the fact that the American Society of Clinical Oncology (ASCO) recommends routine testing of only three predictive markers: estrogen receptor (ER), progesterone receptor (PR), and HER2/ERBB2 in women diagnosed with primary, invasive breast carcinomas.
ASSOCIATION OF HER2/ERBB2 AMPLIFICATION WITH HER2/ERBB2 OVEREXPRESSION
The HER2/ERBB2 gene encodes a 185 kDa monomeric protein also known as phosphoprotein 185 (p185
HER2/ERBB2). The HER2/ERBB2 protein is a receptor tyrosine kinase that is classified as a member of the epidermal growth factor receptor (EGFR) family of tyrosine kinases based on significant homology to EGFR (
8,
9). HER2/ERBB2 is a membrane protein expressed at low levels in all epithelial cells in normal fetal and adult tissues (
10).
The HER2/ERBB2 gene is amplified, or increased in copy number, in approximately 25% of human breast cancers (
11,
12) as well as a variable percentage of ovarian (
12), bladder (
13), endometrial (
14), salivary gland (
15), esophageal, and gastric cancers (
16). HER2/ERBB2 gene amplification has been associated with pathologically increased levels of expression of HER2/ERBB2 messenger RNA (mRNA) and p185
HER2/ERBB2 protein product (
12). Although HER2/ERBB2 gene amplification status and expression level were originally found to be closely associated in 90% of frozen breast cancer specimens (
12), subsequent work by the same investigators has demonstrated a near complete concordance between the HER2/ERBB2 amplification status and expression status in the same tissue samples (
17,
18). The 10% of breast cancers that were originally discordant cases were predominantly stromal-rich breast cancers that were classified as
nonamplified, overexpression breast cancers. HER2/ERBB2 gene amplification status had been originally determined by Southern hybridization in these stromal-rich breast cancers and dilution of tumor DNA by more abundant normal DNA resulted in Southern blots that failed to show HER2/ERBB2 gene amplification, owing to the dilution of tumor DNA by the more abundant normal DNA. Reanalysis of these same cases by fluorescence
in situ hybridization (FISH) permitted a nucleus-by-nucleus evaluation of the HER2/ERBB2 gene copy number and demonstrated HER2/ERBB2 gene amplification in the tumor cell nuclei of these cases that were previously considered not to be amplified (by Southern blot), but had overexpression by Northern hybridization, Western Immunoblot, and frozen section immunohistochemical assay (
12). Therefore, one can conclude, when working with frozen tissue specimens, there is a close association between HER2/ERBB2 gene amplification status and overexpression status. That is, when the HER2/ERBB2 gene is not amplified, then the products of the gene are not increased and overexpression is not observed. Similarly, when HER2/ERBB2 gene is amplified, overexpression is consistently observed. Although this close association can be demonstrated in frozen tissue samples, tissue fixation and paraffin embedding of these same specimens lead to difficulties in analysis of protein expression, especially by immunohistochemistry (
12,
19,
20 and
21). This problem is addressed subsequently in this chapter when we discuss clinical assay methods for assessment of HER2/ERBB2 status.
IDENTIFICATION OF FUNCTIONALLY ACTIVE MUTATIONS IN HER2/ERBB2
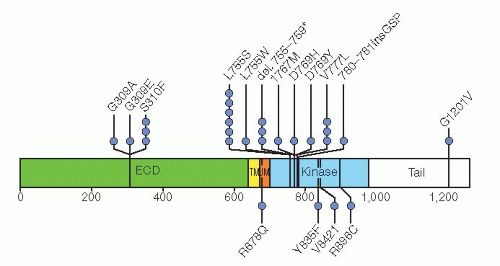
Genome-wide DNA sequence data from eight different studies encompassing 1,499 subjects demonstrated 25 breast cancers with mutations in the HER2/ERBB2 gene, seven of which are activating mutations (
22). These seven activating mutations (G309A, D769H, D769Y, V777L, P780ins, V842I, and R896C;
Fig. 27-1) represent an alternative mechanism for activation of HER2/ERRB2 in addition to gene amplification. Only one of the mutations (V777L) has been identified in breast cancers with HER2/ERBB2 gene amplification. A HER2/ERBB2 in-frame deletion 755-759, which is homologous to EGF receptor (EGFR) exon 19 in-frame deletions, had a neomorphic phenotype with increased phosphorylation of EGFR or HER3. These HER2/ERBB2 somatic mutations are estimated to be present in approximately 1.6% of breast cancers. While some of these mutations are resistant to lapatinib, all were sensitive to the irreversible HER2/ERBB2 tyrosine kinase inhibitor, neratinib. A clinical trial is in progress to evaluate this approach as a treatment strategy in women whose breast cancers lack HER2/ERBB2 gene amplification.
CLINICAL IMPORTANCE OF HER2/ERBB2 GENE AMPLIFICATION AND OVEREXPRESSION STATUS
HER2/ERBB2 gene amplification or overexpression is a prognostic marker of poor outcome in the absence of adjuvant treatment and an important predictive marker of responsiveness to certain treatments. The HER2/ERBB2 alteration has been associated with an increased rate of metastasis, decreased time to recurrence, and decreased overall survival (
11,
12,
23). HER2/ERBB2 gene amplification is significantly associated with shorter disease-free survival and shorter overall survival in primary, invasive, node-negative breast cancer patients treated with surgery alone, without chemotherapy, without hormone therapy, and without radiation therapy in the adjuvant setting (
24). HER2/ERBB2 is a prognostic marker independent of nodal status, tumor size, grade, and hormone receptor status (
24).
As a predictive marker, HER2/ERBB2 amplification or overexpression has been correlated with responsiveness to
anthracycline-based chemotherapy (
25,
26), paclitaxel-containing chemotherapy (
27), and, most importantly, trastuzumab (Herceptin) and lapatinib (Tykerb) anti-HER2 targeted therapies. Currently, patients with HER2/ERBB2-positive tumors are treated with therapy directed against the HER2/ERBB2 protein. Humanized antibodies directed against the extracellular domain of the HER2/ERBB2 membrane protein (trastuzumab [Herceptin], pertuzumab [Perjeta], and trastuzumab emtansine [T-DM1]), and a dual tyrosine kinase inhibitor of EGFR and HER2/ERBB2 (lapatinib [Tykerb]), are currently approved by the U.S. Food and Drug Administration (FDA) for use in the treatment of HER2/ERBB2-positive invasive breast cancers. Trastuzumab is approved for use in the treatment of both metastatic and primary, invasive HER2/ERBB2-positive breast cancer. Pertuzumab and lapatinib are approved for the treatment of HER2/ERBB2-positive metastatic breast cancer (
28). Clinical trials are in progress to evaluate utility in primary breast cancer with HER2-targeted agents such as lapatinib, pertuzumab, and T-DM1, and newer drugs such as neratinib are being actively tested in the metastatic setting. Several other biological agents directed at HER2/ERBB2 or the pathway are in development. Each of these therapeutic agents requires the use of a companion diagnostic test to select the most appropriate patients for treatment (
Table 27-1).
Inaccurate assessment of HER2/ERBB2 status can lead to the inappropriate treatment of breast cancer patients in both the adjuvant and metastatic settings and subject patients to unnecessary risk. With current practice guidelines, falsenegative HER2/ERBB2 status is a serious concern because these patients are denied the substantial benefit of HER2/ERBB2 targeted therapies. On the other hand, false-positive HER2/ERBB2 status is also important because cardiac toxicity is a significant risk for patients who are treated with combinations of trastuzumab and anthracycline-containing chemotherapy. For these reasons, accurate determination of HER2/ERBB2 alterations in breast carcinomas, if present, are of critical importance.
DETECTION OF HER2/ERBB2 AMPLIFICATION AND OVEREXPRESSION IN CLINICAL PRACTICE
In 1998, the humanized mouse 4D5 monoclonal antibody trastuzumab (Herceptin), directed against the human epidermal growth factor receptor type-2 (HER2/ERBB2) (
7,
29), was approved for the treatment of HER2/ERBB2-positive metastatic breast cancer. These patients had been selected for entry to the registration clinical trials of trastuzumab with an immunohistochemistry assay method, known as the
Clinical Trials Assay (CTA) (
7,
30,
31 and
32). The CTA used two different antibodies: 4D5 (the mouse monoclonal antibody used to produce humanized trastuzumab) and CB11 (a mouse monoclonal antibody). Antigen retrieval techniques were used for both antibodies in the CTA. However, because the CTA was not considered appropriate for commercialization, a second immunohistochemistry (IHC) assay method, the Dako HercepTest, was developed for commercial testing of HER2/ERBB2 status to select women for treatment with trastuzumab. This companion IHC assay method to assess HER2/ERBB2 membrane staining was approved by the FDA based on a 79% concordance rate (95% confidence interval [CI], 76%, 82%) between the immunostaining results of the CTA and the immunostaining results of the HercepTest for 548 breast cancer specimens from the National Cancer Institute Cooperative Breast Cancer Tissue Resource, a group of tumors that lacked clinical outcome information. Subsequently, a fluorescence
in situ hybridization assay method was also approved by the FDA to identify women whose breast cancers had HER2/ERBB2 gene amplification for selection to trastuzumab therapy. This approval was based on a blinded, retrospective analysis of archival tissue sections from the breast cancers of women entered in the H0648g and H0650g registration trials (
33). This analysis demonstrated that women entered in the H0648g trial
of trastuzumab for metastatic disease whose breast cancers were IHC 2+ or 3+ but lacked HER2/ERBB2 gene amplification by FISH did not show any incremental benefit from the addition of trastuzumab to the chemotherapy regimen (
33). Those women whose breast cancers were HER2/ERBB2 amplified by FISH and received trastuzumab with chemotherapy had a significantly improved overall survival compared with women whose breast cancers were HER2/ERBB2 amplified and were treated with chemotherapy alone. The separation of the outcomes was greater in this comparison than in the original efficacy population
(p = .009) (
33).
Three FISH assays, two of which are commercially available (PathVysion by Vysis, Inc., now Abbott-Molecular, Inc., and pharmDx by Dako, Inc.), four IHC assay methods (HercepTest by Dako, Pathway by Ventana Medical Systems/Roche, InSite by Biogenex, and Oracle by Leica), and three chromogenic, silver, or dual (bright field microscopic)
in situ hybridization (INFORM-HER, originally a FISH assay by Oncor, Inc., is now a bright-field ISH owned by Ventana Medical Systems, Inc.; pharmDx kit by Dako, Inc.; and SPOT-Light by Life Technologies, Inc.) assay methods are approved by the FDA to select women for trastuzumab therapy (
Table 27-1).
Because HER2/ERBB2 gene amplification is directly correlated with HER2/ERBB2 expression levels at the mRNA and protein levels, determination of HER2/ERBB2 status could potentially be made at any of these levels and should correspond to the HER2/ERBB2 status determined with any of the other measures. Although this is approximated when frozen tissues are used, the use of formalin-fixed, paraffin-embedded tissues for these determinations introduces practical problems that result in some errors in HER2/ERBB2 characterization, especially when IHC is used to assess HER2/ERBB2 status in formalin-fixed paraffin-embedded tissues. We briefly review the various methods used to assess HER2/ERBB2 in breast cancer specimens, and then compare and contrast the results obtained with different methods of analysis, especially between IHC and FISH. A summary of these techniques is provided in
Table 27-2.
CLINICAL ASSAYS FOR ASSESSMENT OF HER2/ERBB2 STATUS
Although HER2/ERBB2 status has been highly concordant among assays of DNA, mRNA and protein in frozen tissues, the current clinical use of paraffin-embedded tissue limits the type of HER2/ERBB2 analyses that can be performed. The types of assay choices for formalin-fixed, paraffinembedded tissues include FISH, CISH, and SISH to determine gene amplification status; quantitative real-time reverse transcriptase-polymerase chain reaction (RQ-PCR) analysis and microarray-based RNA expression profiles (RNA-EP) to determine HER2/ERBB2 mRNA expression status (
34,
35); and IHC to determine HER2/ERBB2 protein overexpression status. The most popular of these methods is IHC, with approximately 85% or more (
36) primary HER2/ERBB2 assessments being performed with this method. The second most popular method for assessment is FISH, with 6% to 15% of assays performed with this assay method, predominantly for “reflex” FISH testing after primary IHC testing yields an immunostaining score of IHC 2+ (
36). The Genomic Health proprietary Oncotype DX 21-gene assay measures messenger RNA for a series of markers including ER, PR, and HER2/ERBB2 and proliferation markers in the clinical setting with ER, PR, and HER2/ERBB2 results now reported separately. A new biotechnology method, nanostring, also measures mRNA from formalin-fixed paraffin-embedded tissues (
37). These assay methods are briefly reviewed below.
IMMUNOHISTOCHEMISTRY
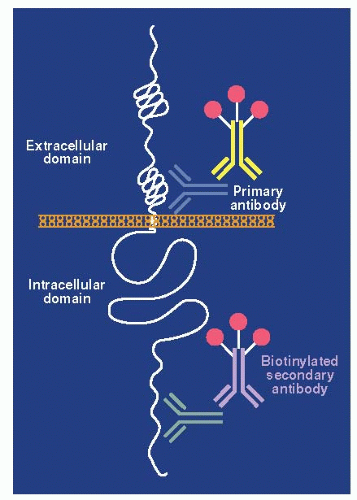
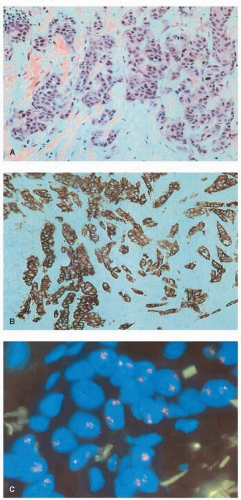
Immunohistochemistry uses antibodies that recognize antigenic determinants of the full-length HER2/ERBB2 protein to assess indirectly and qualitatively the overall level of HER2/ERBB2 protein expression in paraffin-embedded tumor samples (
Figs. 27-2 and
27-3). Overexpression of the HER2/ERBB2 protein by IHC is associated with a poor patient prognosis because of increased metastatic potential, and it is an independent predictor of disease-free and overall survival in patients with breast cancer containing this alteration (
12,
38,
39,
40,
41 and
42).
The Clinical Trials Assay (CTA) (
31) is the first clinical assay developed to select women for entry into the clinical trials investigating trastuzumab therapy in metastatic breast cancer patients (
7,
30,
32). The HercepTest (Dako, Carpenteria, California) and the CB11 IHC assays (Pathway, Ventana Medical Systems, Tucson, Arizona), two of the IHC assays currently approved by the FDA for determination of HER2/ERBB2 status, were modeled on the CTA and were approved by the FDA through concordance studies. The HercepTest was approved based on a direct comparison of the agreement rate, or concordance, with the CTA. As summarized above, the agreement rate between these assay methods was 79%. The Ventana Pathway assay used one of the two antibodies from the original CTA, the CB11 anti-ERBB2 antibody, as the detection antibody in the initial formulation of this assay method; however, the 4B5 monoclonal anti-HER2 antibody is used in the current kit. The Pathway IHC method was originally approved by the FDA based on a 92.4% concordance (95% CI, 89.6%-94.7%) with the HercepTest.
The HercepTest utilizes a rabbit anti-human HER2/ERBB2 polyclonal antibody to detect the HER2/ERBB2 protein, whereas the Pathway anti-HER2 (4B5) assay uses a rabbit antihuman monoclonal antibody. The scoring of immunostaining intensity for both of these assay methods is modeled after scoring in the original CTA. The microscopic appearance of the immunostained tumor cell membranes is subjectively graded by a pathologist on a scale of 0 to 3+, with 0 and 1+ considered low expression, 2+ considered indeterminate, and 3+ considered overexpression. To reduce the subjective variation in scoring by different pathologists, a series of cell line controls are used in parallel to provide a comparison with a negative control (0) (MDA-MB-231 cells), a slightly positive control (1+) (MDA-MB-175 cells), and a strongly positive control (3+) sample (SKBR3 cells) for Dako reagents. Alternately, a different set of cell lines is available for Ventana reagents, including a negative control (0) (MCF7 cells), a slightly positive control (1+) (T47D cells), a moderately positive control (2+) (MDA-MB-453), and a strongly positive control (3+) (BT474 cells). Previously in the CTA, IHC2+ immunostaining was considered to be over-expression, but these cases were found to have a highly variable proportion with HER2/ERBB2 gene amplification and it was decided by the College of American Pathologists (CAP) and, more recently, by a joint guideline from the ASCO and the CAP that these cases should be considered
indeterminate and
reflexed to FISH for evaluation of HER2/ERBB2 gene amplification to determine the status of the case (
43,
44). The original CTA and HercepTest scoring system approved by the U.S. Food and Drug Administration (FDA) required a minimum of 10% of tumor cells to be immunostained with a particular intensity for scoring at a particular level; however, initial ASCO-CAP guidelines required the use of 30% as the proportion of tumor cells stained to achieve a particular staining level (
43,
44) but has been reversed to the FDA-approved 10% proportional requirement in the current
ASCO-CAP guidelines (
45). No objective published data demonstrate the need for 10% as a suitable scoring minimum nor were objective data offered for a change from 10% to 30% as the minimum needed for assessment of a particular score such as IHC 3+. In fact, in frozen tissues nearly all tumor cells in a given breast cancer show the same level of immunostaining, either 1+, 2+, or 3+ (
12,
18), with substantial variability in staining intensity appreciated almost exclusively in the formalin-fixed, paraffin-embedded tissue samples (
12,
18,
19,
40). Nevertheless, both the HercepTest and Pathway anti-HER2 assays are approximately 90% accurate at assigning the known, molecularly determined status of breast cancer specimens (
20).
The use of IHC to determine HER2/ERBB2 status is appealing for several reasons. HER2/ERBB2 IHC tests are simple, rapid, inexpensive, and easily accommodated by existing surgical pathology laboratory practices. In addition, pathologists have an established familiarity with the IHC technique and reagents. However, application of IHC to assess HER2/ERBB2 status is problematic for a number of reasons. The clinical assays are performed on fixed, paraffin-embedded (FPE) tissues and HER2/ERBB2 IHC analyses of this type of material are associated with several problems (
12,
18,
19 and
20,
33,
40,
46,
47 and
48). Tissue handling, fixation, and
processing can greatly affect immunoreactivity of tissue antigens (
12,
18,
19,
49,
50). It has been proposed that loss or significant reduction of HER2/ERBB2 immunostaining may occur in approximately 10% of HER2/ERBB2-positive (amplified) samples, owing to formalin fixation (
46,
47,
49) or storage of unstained sections (
18,
51) before use for IHC. HER2/ERBB2 positivity by IHC in FFPE tissues is dependent on the HER2/ERBB2 antibody used for the protocol (
12,
19,
20). For example, 95% of the HER2/ERBB2-amplified tumors examined were HER2/ERBB2-positive using the CB11 test, but only 84% of these same tumor samples were HER2/ERBB2-positive using the HercepTest (
52).
Interpretation of IHC is inherently subjective and qualitative. This leads to observer variability and affects the accuracy of results using the IHC technique (
53), although relatively high concordance rates can be achieved among experienced observers using standardized scoring systems (
54). Considerable evidence indicates that IHC performance is poorly controlled “in the real world” (
43,
44,
47,
55,
56 and
57). The initial ASCO-CAP guidelines on HER2/ERBB2 testing draw attention to this with the claim that “20% of HER2/ERBB2 assays performed in the field were incorrect” (
43,
44). The United Kingdom National External Quality Assurance Scheme (UK-NEQAS; see http://www.ukneqasicc.ucl.ac.uk/neqasicc. shtml) documents performance of diagnostic laboratories within the United Kingdom and across Europe and Asia and includes participants from the United States. Data from this scheme shows a marked difference between the levels of acceptable performance for IHC-based assays. Although computerized image analysis could reduce the subjective nature of the pathologist scoring, it cannot address the preanalytic variability owing to tissue fixation and processing. Despite these issues, IHC remains the favored technique to determine the HER2/ERBB2 status of patients in the majority of laboratories. There are continuing efforts being made to standardize IHC testing (
45) and increasing numbers of laboratories are participating in the College of American Pathologists proficiency testing program for HER2/ERBB2 testing by IHC (
58). Even though the quality of HER2 testing appears to be improving, there is also concern that significant numbers of breast cancer patients who may be eligible for HER2/ERBB2-targeted therapy are either not being tested or the results of these tests are not being used in treatment decisions (
36).
The ASCO and the CAP have updated the 2007 Guideline recommendations. As in the original Guideline (
43,
44), immunohistochemistry remains acceptable as a primary test for HER2 status provided the clinical laboratory has demonstrated a high level of concordance, previously 95% (
43,
44) and currently 90% (
45), for each immunostaining category that will be used to determine eligibility for HER2-targeted therapies (i.e., IHC 0, 1+, and 3+ categories). All IHC2+ cases will continue to be reflexed to FISH for assessment of HER2 status. A review of the literature on the subject demonstrates that few laboratories (4/33) have attained the required 95% concordance between FISH and IHC for all three IHC categories (0, 1+, and 3+) either before or after the 2007 publication of the ASCO-CAP guidelines (
Table 27-3), while the majority of these laboratories (17/33) would now be able to achieve the prescribed concordance for all three IHC categories when the required percentage is lowered to 90% concordance.