Hepatocellular carcinoma (HCC) is the fifth most common cancer globally and accounts for the third most cancer deaths worldwide (Fig. 127-1). Although geographic variability exists in the incidence of the disease, occurring less commonly in the United States and Europe, its incidence is steadily rising, having tripled in the United States since the 1970s and projected to increase over the next 20 years (Table 127-1). The current incidence of HCC in the Western world is estimated at 3% to 4% with 5-year survival estimated to be 16% for all-comers.1
FIGURE 127-1
Estimated age-standardized rates of liver cancer per 100,000. (Reproduced with permission from Ferlay J, Soerjomataram I, Ervik M, et al. GLOBOCAN 2012 v1.0: Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11 [Internet]. Lyon: International Agency for Research on Cancer; 2013. Available at: http://globocan.iarc.fr. Accessed January 13, 2015.)

Estimated Incidence, Mortality, and Prevalence of Liver Cancer World-Widea
| Estimated No. (Thousands) | Men | Women | All | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Cases | Deaths | 5-Year Prevalence | Cases | Deaths | 5-Year Prevalence | Cases | Deaths | 5-Year Prevalence | |
| World | 554 | 521 | 453 | 228 | 224 | 180 | 782 | 746 | 633 |
| United States | 23 | 17 | 21 | 8 | 7 | 7 | 30 | 24 | 27 |
| China | 293 | 282 | 220 | 101 | 101 | 71 | 395 | 383 | 291 |
| India | 17 | 17 | 8 | 10 | 10 | 5 | 27 | 27 | 13 |
| EU | 36 | 32 | 33 | 16 | 17 | 14 | 52 | 48 | 47 |
Up to 90% of HCC develops in cirrhotic livers.2 Therefore, risk factors related to the development of chronic liver disease account for the majority of HCC cases worldwide. This chapter examines the risk factors for development of HCC, clinical manifestations of the disease, and therapeutic modalities available in the armamentarium of clinicians who encounter HCC, the most common primary liver cancer. Management of HCC, with a focus on indications and outcomes of common therapeutic modalities including liver transplantation, resection, and ablation, will be described. Upon reviewing this chapter, the reader should be able to suggest appropriate therapeutic modalities for treatment of HCC, which is individualized based on underlying liver functional status and extent of malignant disease in a given patient.
Risk factors for the development of HCC include hepatitis B virus (HBV), as the most common worldwide cause, hepatitis C virus (HCV), as the most common cause in the United States and other Western countries, alcoholic liver disease, and nonalcoholic fatty liver disease (NAFLD); less common causes include hemochromatosis, alpha-1-antitrypsin deficiency, primary sclerosing cholangitis, aflatoxin (B1) ingestion, history of hepatic adenoma, steroid use, exposure to pesticides, primary biliary cirrhosis, and Wilson’s disease. There is significant overlap between risk factors associated with liver parenchymal disease and HCC, since the risk of malignant transformation is significantly increased in underlying cirrhosis and rises to 1% to 8% upon the development of cirrhosis.3 The associated chronic inflammatory response leads to hepatocyte death and compensatory proliferation, and, similar to other inflammatory states, is thought to account for the oncogenic progression toward HCC in chronic liver disease states, where injury, repair, and high cellular turnover persist. Conversely, and unlike the RNA virus HCV, HBV is a double-stranded DNA virus that integrates into the host genome and additionally results in direct mutagenesis, providing a mechanism by which HBV may induce transformation to HCC without the development of underlying cirrhosis.4 Since the prevalence of HBV is geographically distributed and concentrated in the Far East, HBV-related HCC is also, therefore, associated with similar geographic distribution patterns (Fig. 127-2). Overall, however, the majority of patients with HCC suffer from underlying cirrhosis or fibrosis and inflammation.
FIGURE 127-2
A. Estimated age-standardized rates of liver cancer incidence per 100,000 in males. B. Estimated age-standardized rates of liver cancer Incidence per 100,000 in females. C. Estimated age-standardized rates of liver cancer mortality per 100,000 in males. D. Estimated age-standardized rates of liver cancer mortality per 100,000 in females. (Reproduced with permisson from Ferlay J, Soerjomataram I, Ervik M, et al. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11 [Internet]. Lyon, France: International Agency for Research on Cancer; 2013. Available at: http://globocan.iarc.fr. Accessed on January 13, 2015.)

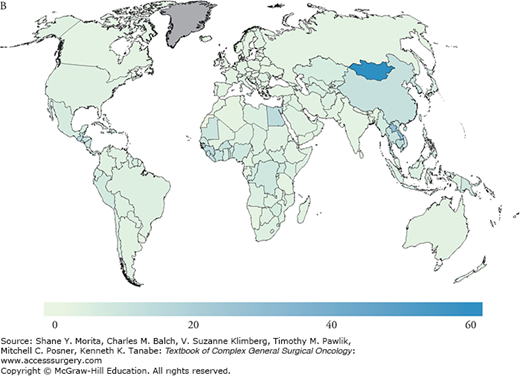
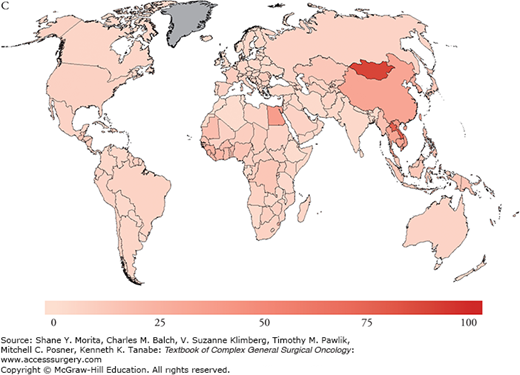
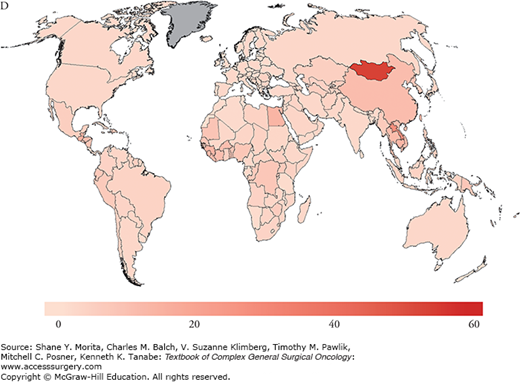
Globally, the disease is linked to chronic hepatitis infections (up to 80% of all HCC cases). In fact, vaccination programs in high-risk countries such as Taiwan have significantly impacted the incidence of HCC and are thought to have reduced the incidence of HCC by two-thirds in infants and young adults.5–7 As of 2008, 177 countries (91%) had introduced the HBV vaccination program into their infant immunization schedule.7 While HBV has historically accounted for the majority of HCC cases, the incidence of HCC in the Western world from poorly screened blood transfusions in the 1980s accounted for a significant rise in the rate of HCV chronic carriers. At this time, there are an estimated 4 million people with chronic hepatitis C in the United States alone, of whom approximately one-third would be expected to develop chronic liver disease. Programs to reduce infection rates through the improvement of screening of blood used for transfusion for HCV, HBV vaccination programs, strategies to reduce alcohol intake, and avoidance of aflatoxin food contamination through avoidance of grain storage in humid conditions are key in reducing HCC rates worldwide. Furthermore, the reduction of viral loads and replication rates may impede chronic hepatic inflammation, and, therefore, persistent injury and repair. Current improvements in therapy have accounted for secondary prevention against the development of cirrhosis and HCC. Once cirrhosis has occurred, however, the role of secondary preventative strategies may be limited. In a study by Dusheiko et al,8 the authors evaluated the use of weekly pegylated interferon alpha-2a for 3.5 years and showed no difference in the incidence of HCC in HCV cirrhotic patients. On a promising front, however, emerging therapies for HCV which include oral combinations of protease inhibitors and NS5A/B polymerase inhibitors appear to induce cure in the majority of chronic genotype 1 HCV carriers, which will likely, in turn, favorably alter future HCC incidence and prevalence rates.
Worthy of note, however, and while headway continues to be made in tackling chronic HCV disease, the rising incidence of obesity and diabetes appear to be accounting for an increasing incidence of NAFLD, which may be intimately related to cases of cryptogenic cirrhosis. Whether obesity as a risk factor will surpass current well-established risk factors as a leading cause of HCC is unknown. However, continued public health strategies to reduce the incidence of morbid obesity are warranted in this field too.
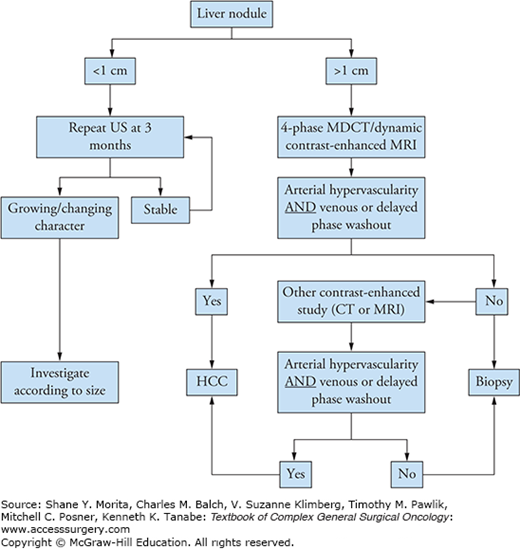
As with other cancers, the aim of screening is to enable detection of disease at an early stage, at a time when cure may still be possible and outcomes are improved with earlier therapy. The cost-effectiveness of screening in the United States has been debated.9 At this time, the European Association for the Study of Liver (EASL) panel of experts recommends 6-monthly abdominal ultrasound scanning with serum alpha-fetoprotein (AFP) testing for patients with documented liver cirrhosis. Upon discovery of an abnormal finding, a strategy proposed by the American Association for the Study of Liver Diseases (AASLD) panel is utilized, and is shown in Figure 127-3. Briefly, in the event of discovering a liver nodule on ultrasound, size criteria are used to determine further workup. If the lesion is deemed to be less than 1 cm in size, an ultrasound scan is performed again at 3 months. If stable, 3-monthly ultrasound scans are pursued. However, if the lesion is noted to have increased in size, additional investigations are required, as would be the case with lesions greater than 1 cm on initial imaging. At that point, four-phase multidetector computed tomography (CT) or dynamic contrast-enhanced magnetic resonance imaging (MRI) scans are recommended. Should either scan demonstrate arterial hypervascularity with venous or delayed phase washout, the lesion can safely be assumed to be HCC, and no further diagnostic workup of the lesion is required. In the event that both imaging techniques are exhausted without identifying the characteristic radiologic pattern of arterial hypervascularity with venous or delayed phase washout, a biopsy is indicated next in spite of the AFP level, which may also be elevated in cholangiocarcinoma, mixed HCC/cholangiocarcinoma, and secondary hepatic metastases.10 Interestingly, serum AFP measurement alone is less than 80% sensitive for diagnosing HCC. In combination with simple ultrasound, however, sensitivity for detection and diagnosis is increased to 85%, with 90% specificity. In a review of diagnostic modalities by Colli et al,11 the authors quote sensitivity and specificity values of 61% and 97% for ultrasound, 68% and 93% for CT, and 81% and 95% for MRI, respectively, based on a recent systematic review of available data.
In a study by Zhang et al,12 the authors compared two large HBV Chinese populations who underwent screening with 6-monthly ultrasound scans and AFP levels versus controls who were not screened and showed that screening resulted in significantly higher survival at 1, 3, and 5 years in the screened population compared with unscreened controls: 66%, 53%, and 46% in the screened group compared with 31%, 7%, and 0% in the unscreened group, respectively. Furthermore, screening allowed identification of 39 patients with tumors less than 5 cm, compared with 0 patients identified with tumors less than 5 cm in the unscreened population. In a separate report by Trinchet et al,13 screening more frequently at 3-monthly intervals did not appear to improve survival any further when compared with the 6-monthly protocol above. As a result, 6-monthly screening with serum AFP and abdominal ultrasonography appears to be beneficial and is recommended in high-risk populations.
While histopathologic assessment of HCC is not generally sought except in cases of diagnostic ambiguity as discussed above, histologic subtypes generally carry little significance in terms of workup, diagnosis, management, and prognosis. An interesting exception, however, is fibrolamellar HCC, a common variant that is found in younger patients and may carry an improved prognosis when matched to cases with conventional HCC.
Although the characteristic findings associated with HCC are described above, it is important that physicians are able to distinguish between HCC and other liver masses, including hepatic adenomas, focal nodular hyperplasia (FNH), hemangiomas, solitary cysts, and liver abscesses (parasitic and pyogenic). Briefly, hepatic adenomas do not contain Kupffer cells and do not exhibit uptake on sulfur colloid scans, while demonstrating hypervascularity on MRI, with a peripheral blood supply. FNH classically demonstrates a central stellate scar and exhibits sulfur colloid uptake on liver scans. FNH, however, also demonstrates hypervascularity on MRI and CT imaging. Hemangiomas show peripheral to central enhancement classically on MRI and CT imaging, and appear as highly hypervascular lesions. Other factors related to etiology, pathogenesis, and management of these lesions are beyond the scope of this chapter (see Chapters 123 and 136).
While the terms chronic liver disease, cirrhosis, portal hypertension, and liver failure are used interchangeably, it is important to accurately distinguish between these terms in order to establish a common language by which physicians can communicate. Cirrhosis is the chronic inflammatory and generally irreversible condition, which most commonly results in liver failure. The mechanism involves hepatocyte destruction by the identified insult secondary to the risk factors described above, followed by fibrosis and scarring, which subsequently results in increased hepatic sinusoidal pressures, portal venous congestion, lymphatic overload, and leakage of splanchnic and hepatic lymph into the peritoneum. Combined with an accumulation of ammonia in liver failure and failed decarboxylation of clotting factors, these mechanisms account for the development of the sequelae of cirrhosis, which specifically include ascites, bleeding, encephalopathy, and portal hypertension. Portal hypertension, in turn, results in worsening ascites and encephalopathy, development of varices, and splenomegaly. The degree of functional and anatomic splenomegaly is important, and is effectively evaluated with a platelet count, which inversely correlates with the degree of organ enlargement. Given that HCC predominantly develops in patients with significant cirrhosis and is often associated with liver failure, clinical findings of ascites, bleeding, encephalopathy, thrombocytopenia, and variceal bleeding often determine the management of HCC, which is based on the severity of these manifestations of chronic liver disease.
In patients in whom screening has not been undertaken, HCC often presents with vague abdominal symptoms such as abdominal pain, weight loss, loss of appetite, worsening distension, or palpable mass. Alternatively, the presentation may be related to underlying signs and symptoms of previously undiagnosed chronic liver disease, cirrhosis, and/or portal hypertension in whom an incidental diagnosis of HCC is often made. Paraneoplastic conditions, although rare, may be the presenting features in HCC, and include hypoglycemia, hypercalcemia, polycythemia, and hypertrophic osteoarthropathy. A complete history and physical to evaluate signs and symptoms of liver disease is imperative, and is used to guide investigations in diagnosing the presence of underlying hepatic dysfunction and its severity, as described below, along with the presence of malignancy.
The Child–Pugh classification, which is also known as the Child-Pugh-Turcotte classification, is a quantitative system used to evaluate the degree of liver dysfunction, and was initially devised to predict mortality related to surgical intervention in patients with chronic liver disease.14 It is currently applied more broadly to provide general prognostic information related to liver cirrhosis, using components that include the biochemical factors serum bilirubin, albumin, and international normalized ratio (INR), and the clinical factors encephalopathy and ascites. In one study that assessed survival related to liver disease stratified by Child–Pugh classification, 1-year mortality increased progressively from 0% to 20% to 55% in patients with class A, B, or C disease.15
Another scoring system is the Model for End-Stage Liver Disease (MELD) score, which also assesses the severity of chronic liver disease. This score was initially developed to predict the risk of death within 3 months of surgery specifically, and was subsequently also used to determine general prognosis. It is now widely used for prioritizing patients for liver transplantation and is the scoring system by which the United Network for Organ Sharing (UNOS) preferentially distributes donated cadaveric organs for transplantation. Components of the MELD score include serum bilirubin, creatinine, and INR, thereby incorporating renal failure as an important component of chronic liver disease. In addition to determining severity of liver disease, such scoring systems are used to guide therapies in order to optimize the treatment of HCC in individual patients based on the degree of underlying liver dysfunction. Other scoring systems exist, and notably include the Okuda classification and the Barcelona Clinic Liver Cancer (BCLC) system, among many more.
Hepatocellular carcinoma frequently spreads by local invasion to adjacent structures, including diaphragm, adjacent organs, and the liver vasculature. Metastatic spread generally occurs hematogenously, most frequently to lungs and bone. However, lymph node involvement, which remains relatively poorly studied, does occur and portends a worse prognosis due to higher local recurrence rates, compared with node-negative disease.
In order to determine therapeutic options suitable for the treatment of HCC, the initial assessment begins with careful assessment of liver function using objective tests (Child–Pugh classification or MELD scores) as described above. The assessment is continued to assess for the presence of discontiguous satellite nodules, vascular invasion, venous thrombosis, and extrahepatic disease. Available tests such as CT, MRI, and bone scans are preferentially used according to signs and symptoms elicited in the initial history and physical examination. The use of fluorodeoxyglucose (FDG) positron emission tomography (PET) plays little role in the workup of HCC and is not recommended. Additional testing of performance status, using measures such as the Eastern Cooperative Oncology Group (ECOG) status, is imperative and exists within a bundle of psychosocial factors that are rigorously assessed in accordance with practice guidelines in all patients who are about to undergo liver transplantation as a potential therapeutic option.
Table 127-2 shows the American Joint Commission on Cancer (AJCC) staging system for liver cancer, consisting of tumor (T), nodal (N), and metastatic (M) components. Grade (G) and fibrosis score (F) are excluded from the traditional stage aggregates, but their inclusion in defining the extent of disease and providing prognostic information is important.16 The most important information provided by the AJCC components pertains to vascular invasion, rather than tumor size, given that HCC has a strong predilection for vascular invasion, and which itself is associated with a poorer prognosis. Interestingly, bile duct invasion, although less common, is also associated with a poorer prognosis in HCC, particularly when the tumor involves more central branches of the biliary tree (Fig. 127-4). Although underlying liver function is important in determining survival in patients with HCC and provides information that is not incorporated into the AJCC staging system, the AJCC staging system has nonetheless been validated by multiple groups and for multiple HCC etiologies. Despite the presence of numerous staging systems to date, the current AJCC system appears to be reproducible and provides useful prognostic information. In the absence of fibrosis, 5-year survival rates range from 64% in stage I disease to 17% in stage III disease. The presence of fibrosis imparts a significantly worse 5-year survival at every disease stage.
AJCC Hepatocellular Carcinoma Staging Systema
| AJCC Hepatocellular Carcinoma TNM Staging | |
| Primary Tumor (T) | |
| TX | Primary tumor cannot be assessed |
| T0 | No evidence of primary tumor |
| T1 | Solitary tumor without vascular invasion |
| T2 | Solitary tumor with vascular invasion or multiple tumors none >5 cm |
| T3a | Multiple tumors >5 cm |
| T3b | Single or multiple tumors of any size involving a major branch of portal/hepatic vein |
| T4 | Tumor invades adjacent organs excluding gallbladder or visceral peritoneal perforation |
| Nodal Disease (N) | |
| NX | Regional lymph nodes cannot be assessed |
| N0 | No regional lymph node metastasis |
| N1 | Regional lymph node metastasis |
| Metastatic Disease (M) | |
| M0 | No distant metastasis |
| M1 | Distant metastasis |
FIGURE 127-4
A. Segment 8 hepatocellular carcinoma adjacent to middle hepatic vein with associated bile duct invasion (arrow). B. Resected extended right hepatectomy specimen with hepatocellular carcinoma and associated bile duct invasion (arrow). C. Pathologic representation of hepatocellular carcinoma with bile duct invasion (arrow).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree