Chapter 78 Hepatic and Hepatobiliary Diseases
Diseases of the liver are a major source of morbidity and mortality in patients with human immunodeficiency virus (HIV) infection. Liver-related conditions accounted for 13% of HIV-related hospitalizations in the year 2000, having increased from 8% in 1996.1 Although immune reconstitution and prevention of severe acquired immunodeficiency has decreased the prevalence of most opportunistic infections, the high prevalence of chronic hepatotropic viruses in this population has become increasingly troublesome.2 Furthermore, the very medications that improve life expectancy in HIV-infected patients may alter hepatic metabolism and produce features of direct and indirect hepatotoxicity.3 Finally, some of the more common metabolic disorders of the liver assume increased importance because of their contribution to chronic liver diseases. The evaluation and management of hepatic and hepatobiliary diseases has become a critical consideration for healthcare providers caring for patients with HIV infection.
CLINICAL EVALUATION
History
Obtaining a detailed history related to liver disease is perhaps the most crucial element of an evaluation of the patient with clinical or biochemical evidence of liver injury. Disease processes that must be considered include viral infections, medications, recreational drugs, toxic occupational exposures, vascular/thrombotic disorders, and inherited metabolic conditions. A detailed history of potential exposure to hepatotropic viruses is important for establishing a timeline for infection. This history should include the year of first exposure to intravenous drugs, nasal cocaine use, tattoos, body piercing, blood transfusion, and sexual exposures. Studies of injection drug users suggest that most of those with chronic hepatitis C virus (HCV) infection become infected during the first year of abuse. Risk of HCV was common in patients who received blood and blood products prior to 1991. Nearly all hemophilic men who required pooled factors prior to that time are HCV-infected. High-risk sexual behavior is a clear risk for hepatitis B infection and probably for HCV as well.4 Recent analysis of clusters of acute HCV infection reveals that a history of unprotected receptive and insertive anal intercourse and fisting was highly associated with disease transmission.5
Medication history must be detailed and include all drugs started or stopped within a 3-month period. It is critical to include medications used transiently. For example, the use of common antibiotics such as amoxicillin-clavulinic acid (Augmentin) can cause severe cholestasis 2–12 weeks after a short drug course and is often missed by healthcare providers as a potential cause of liver injury.6 Drug interactions must also be considered because some medications compete for active binding sites in hepatic mixed-function oxidase or cytochrome pathways, leading to toxic injury. Alcohol use should be estimated in terms of grams of ethanol ingested per day.
Family history is essential for establishing the possibility that the patient has an underlying inborn error of metabolism. A history of early cirrhosis (<45 years old) even in a relative should raise suspicion of a coexistent metabolic cause of injury. For instance, hereditary hemochromatosis and a1-antitrypsin (AAT) deficiency are quite common in certain ethnic groups.7
A review of the medical history for a patient with HIV infection must also include a detailed assessment of immunologic status. Patients with very low CD4+ T-lymphocyte counts (<50 cells/μL) are more susceptible to hepatic injury from infiltrative processes (Kaposi sarcoma, non-Hodgkin lymphoma, granulomatous disease) and less susceptible to injury from hepatitis B than those with a CD4+ T-lymphocyte count higher than 500 cells/μL. Patients who have undergone immune reconstitution may represent a special risk in terms of injury from hepatitis B8,9 and hepatitis C.10 Because immune reconstitution is generally associated with use of highly active antiretroviral agents, which may independently cause liver toxicity, the healthcare provider must carefully document the timing of immunologic recovery as related to the onset of liver injury.
Physical Examination
Neurologic evaluation should include observation for asterixis (a “flap” of the extended hands), which is seen in patients with late-stage liver disease and reflects the presence of hepatic encephalopathy. A number connection tests to evaluate concentration is a valuable tool for clinical management of patients with encephalopathy.11
BIOCHEMICAL TESTS
Liver Injury and Synthetic Function
Biochemical screening tests for liver disease generally include a panel of tests that help identify and categorize the type of liver injury. Common tests of liver injury and function can be divided into three groups: (1) tests of hepatocellular injury; (2) tests of cholestatic injury; and (3) tests of liver function capacity.12,13
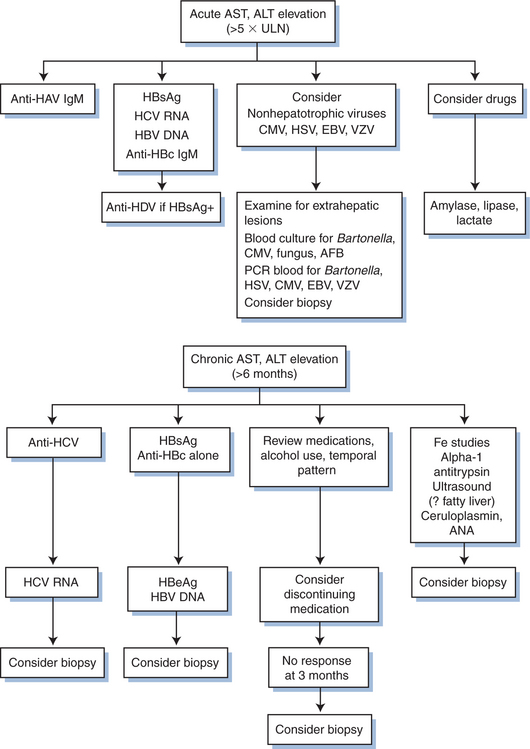
During the course of infection, up to 70% of patients with HIV infection have been reported to have abnormally high serum transaminase levels. This high prevalence has prompted a higher threshold for evaluating mild to moderately increased ALT/AST levels in HIV-infected patients. It is important to understand, though, that even mild chronic injury may result in fibrosis and eventual hepatic decompensation. An algorithm for evaluation of hepatocellular injury is shown in Figure 78-1.
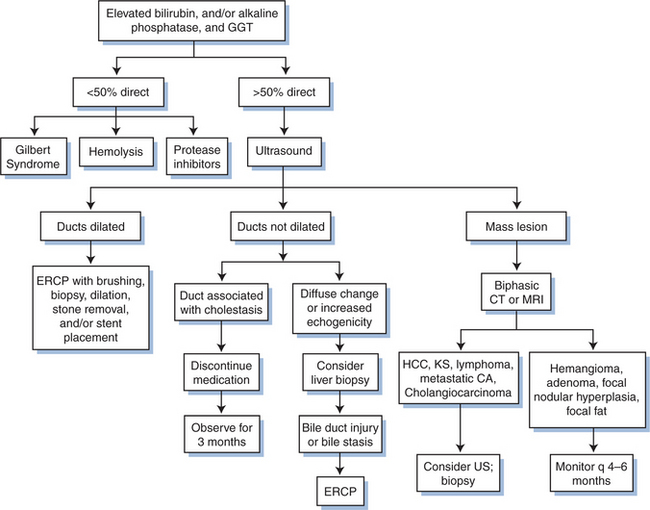
Cholestatic injury is indicated by an elevated alkaline phosphatase level, an inducible enzyme produced by hepatocytes. Failure to excrete bile acids (as is seen with cholestasis) leads to induction of alkaline phosphatase, which is excreted into the serum. Bile acid concentration may be increased by a variety of mechanisms including drug-induced canalicular transport dysfunction (e.g., chlorpromazine), small bile duct injury (e.g., primary biliary cirrhosis), large bile duct narrowing (e.g., AIDS cholangiopathy, cholangiocarcinoma), or following acute hepatocellular injury (posthepatitic cholestasis). Because alkaline phosphatase levels can also be due to abnormalities in bone, intestine, and salivary glands, confirmation of a liver source generally requires a concomitant elevation of g-glutamyl transpeptidase (GGT) or 59-nucleotidase. These liver enzymes are also induced by bile acids, and the GGT is induced by a variety of drugs and alcohol as well. It is important to note that increased GGT merely suggests cholestasis; it is not a marker of hepatocellular injury, as is often mistakenly thought. The complete evaluation of patients with a cholestatic injury is described in Figure 78-2.
Frequently, the term “liver function tests (LFTs)” is used to describe basic biochemical tests of the liver; but this is a misrepresentation, as none of the above named tests measures liver function. Typical chemical profiles often include albumin, which is a measure of liver synthetic function. However, the serum albumin level may be abnormally low in patients with nephrotic-range proteinuria or in those with malnutrition. The prothrombin time (PT) is a more reliable measure of the liver’s ability to manufacture clotting factors (II, VII, IX, X). Therefore, a prolonged PT is an indication of impaired synthetic function, although it may also be abnormal because of severe cholestasis and resultant malabsorption of fat-soluble vitamin K. Administration of parenteral vitamin K (10 mg SC or IV) corrects the PT within 3 days and therefore differentiates cholestasis from abnormal liver function due to a shortage of substrate. Specific measurements of liver function include studies of drug metabolism and clearance. Caffeine, bromsulphalein (BSP), and iodocyanine green clearance have all been utilized. Commercial test kits are available for evaluation of monoethylglycinexylidide (MEG-X), which is a metabolic by-product of liver metabolism of lidocaine. Recent studies by Shrestha et al have shown that calculation of a cholate shunt fraction may be a sensitive marker of liver function in patients with advanced liver disease.14 These tests are generally used only in the research setting.
Laboratory Evaluation for Disease Etiology
Although the tests described in the previous section define and classify liver injury, they do not necessarily identify specific etiologies. Some diagnoses are those of exclusion, combined with pertinent historical positives (e.g., drug injury), and others require specific biochemical or serologic tests to suggest or confirm the etiology. The range of diagnoses that must be considered is listed in Table 78-1.
Table 78-1 Diseases Affecting the Liver and Biliary Tree in HIV Infection
| Viral Hepatitis |
| Hepatotropic |
| Hepatitis A virus |
| Hepatitis B virus/hepatitis D virus |
| Hepatitis C virus |
| Nonhepatotropic |
| Cytomegalovirus |
| Herpes simplex virus |
| Epstein–Barr virus |
| Varicella-zoster virus |
| Human herpesvirus-6 |
| Adenovirus |
| HIV |
| Fungal liver disease |
| Candida albicans |
| Histoplasma capsulatum |
| Parasitic liver disease |
| Leishmania donovani |
| Pneumocystis carinii |
| Microsporidia |
| Bacterial liver disease |
| Bacillary angiomatosis |
| Granulomatous liver disease |
| Mycobacterium tuberculosis |
| Mycobacterium avium complex |
| Toxic/metabolic liver disease |
| Fatty liver, nonalcoholic steatohepatitis |
| Nucleoside reverse transcriptase inhibitors (NRTIs) |
| Protease inhibitors (PIs) |
| Nonnucleoside reverse transcriptase inhibitors (NNRTIs) |
| Alcohol |
| Other medications |
| Neoplastic liver disease |
| Kaposi sarcoma |
| Non-Hodgkin lymphoma |
| Hepatocellular carcinoma |
| AIDS cholangiopathy |
| Sclerosing cholangitis |
| Papillary stenosis |
| Long extrahepatic bile duct strictures |
| Gallbladder disease |
| Acalculous cholecystitis |
| Hepatic and hepatobiliary diseases |
Acute hepatitis A virus (HAV) infection is identified by the appearance of immunoglobulin M (IgM) class antibodies against HAV. The assay turns positive shortly after infection and is considered diagnostic of acute hepatitis A. Many clinical laboratories utilize a test that detects a combination of IgM and IgG antibodies, requiring further testing of IgM class antibody alone to distinguish between acute infection and past exposure. Therefore, the clinician should request fractionation in cases of suspected acute hepatitis A. Prevention of hepatitis A is based on administration of a commercially available hepatitis A vaccine. A sometimes difficult decision for the clinician is whether to test for naturally acquired antibodies prior to vaccine administration. Prevaccination anti-HAV testing greatly enhances the cost-effectiveness of vaccination among the general public.15 Although no formal analysis has demonstrated the cost-effectiveness of such testing in the HIV-infected population, many HIV-infected patients have a high pretest probability of prior hepatitis A exposure (low socioeconomic strata, homosexual men with multiple partners) and should therefore be tested for antibody prior to vaccine use.
Hepatitis B virus (HBV) infection is generally diagnosed by the presence of circulating hepatitis B surface antigen (HBsAg). The infection is classified as acute or chronic by distinguishing whether the antibodies directed against the virus core antigen (anti-HBc) are of the IgM or IgG class, respectively. Tests for IgG, IgM, and both (total) are available. As with HAV diagnosis, the clinician should select the correct test for the clinical setting. Patients with chronic hepatitis B should be evaluated for the degree of viral replication, which generally correlates with the degree of associated liver injury. In prior years, the replicative state has been determined by testing the level of HBV DNA in serum using a branched-chain-based assay (Quantiplex) or a hybridization-based assay. Whereas qualitative polymerase chain reaction (PCR) assays are not acceptable alternatives because their inordinate level of sensitivity fails to define the replicative state, quantitative PCR assays (Monitor) do provide useful information and have led to significant changes in prognostication and treatment paradigms. Hepatitis B “e” antigen (HBeAg) is generally associated with replicative levels of HBV DNA and may serve as a surrogate marker of replication. The presence of hepatitis B “e” antibody (HBeAb), conversely, is often a marker of a low or nonreplicative state. However, a large subset of persons will harbor HBeAg (−) replicative disease, a consequence of mutations in the HBV precore or core promoter region that results in failure to produce “e” antigen. These so-called precore mutants can be distinguished from nonreplicators by high levels of HBV DNA (>10 000 copies/mL). In patients with HIV infection, clinical scenarios uncommon in immunocompetent patients may occur. Some patients with IgG anti-HBc and without detectable HBsAg harbor active HBV replication with associated liver injury.16,17 These cases can be definitively diagnosed only by using a sensitive PCR-based HBV DNA assay. With chronic HBV infection treated with lamivudine, a high rate of mutant virus breakthrough can be identified by the presence of HBV DNA by branched-chain or hybridization assays.8 Testing for lamivudine-resistance mutations in an area called the YMDD motif is available in some clinical laboratories. Mutation is also observed with other HBV active agents including adefovir, tenofovir, and entecavir. A new generation of tests that permit evaluation of mutational patterns is now under development.
HCV infection can be detected by use of an enzyme-linked immunosorbent assay (ELISA or EIA). This test is highly sensitive, though false positives in patients with a low pretest probability of disease are common. False-negative reactions are sometimes observed in patients with HIV. The prevalence of this finding has been reported to range from 1.5% to ∼10.0% in some series. Seroreversion associated with worsening immune function has also been described.18 If HCV infection is strongly suspected and the HCV ELISA is negative, an HCV RNA test is indicated. Although HCV RNA detection represents the gold standard in terms of sensitivity and specificity, its accuracy and reliability have been low in some blind test panels of laboratories that routinely test for HCV RNA. Therefore, diagnosis and treatment decisions should not be based on a single HCV RNA assay alone. Recombinant immunoblot assays (RIBA) may also serve to confirm infection in patients with a low pretest probability of infection.
Hepatitis D virus (HDV) infection is uncommon in the United States but is much more prevalent in Mediterranean rim countries. Because it requires the presence of hepatitis B for its own replication, the diagnosis of HDV should be considered only in those patients who harbor HBsAg. The presence of HDV antibody or HDV RNA suggests active infection. HIV infection appears to increase the rates of hepatic decompensation and portal hypertension in patients with HDV.19
Hepatitis E virus (HEV) infection may be detected using commercial antibody assay kits. HEV infection is uncommon in the United States and Western Europe, but the prevalence of anti-HEV has been shown to be increased in HIV-infected patients in Argentina and Brazil. However, this association may result from common risk factors for the two infections, including low socioeconomic status.20,21 HEV seropositivity does not bear a clear relationship to HIV infection in most other epidemiologic studies.
LIVER/SPLEEN SCAN AND SPECT
Traditional planar imaging efficacy may be improved by the use of single-photon emission computed tomography (SPECT), which permits three-dimensional evaluation of the radionuclide uptake. However, current computed tomography (CT) scanning and magnetic resonance imaging (MRI) permit resolution at levels much higher than with SPECT. The role of SPECT in evaluation of fibrosis in HIV-infected patients with hepatitis C is under clinical investigation; a pilot study has shown that SPECT is 86–90% sensitive and 62–83% specific for fibrosis and predicts the histological fibrosis score with 80–85% accuracy.22
Biliary Scintigraphy
Using 99 mTc radiotracers attached to compounds that are taken up by hepatocytes and excreted into the biliary tract, biliary scintigraphy permits evaluation of bile excretion and creates images that define the biliary anatomy. A number of compounds have been studied, including N;N-(2,6-Dimethylphenyl)carbamoylmethyl iminodiacetic acid (HIDA) and other diacetic acid derivatives. Patients with severe liver disease lack the functional capacity for radiotracer uptake and fail to “light up” the liver during the early phases of scanning. Following uptake, scanning during the excretion phase might reveal blockage in the biliary tree evidenced by a discrete cutoff of photon emission in the areas distal to the obstruction. These studies may have some value for identifying biliary obstruction, but endoscopic retrograde cholangiopancreatography (ERCP) and MR cholangiography have largely supplanted reliance on these imaging studies. Small studies of hepatobiliary scintigraphy suggested a high correlation between biliary scintigraphy and ERCP in patients with AIDS-related cholangiopathy, but large clinical trials have not been reported.23–25
Ultrasonography and Doppler Ultrasonography
Sound pulses in the 2–5 MHz range are used to discern differences in soft tissues and fluids based on the “echo” return to a receiver probe. Transcutaneous ultrasonography has become an important noninvasive modality for assessing certain types of liver disease. Mass lesions can be discerned from normal liver tissue by the differences in the echogenicity of the lesion relative to its surroundings. A diffusely hyperechoic liver suggests the presence of an infiltrating process or a change in the water content of the hepatocytes. This finding may be observed in patients with diffuse fibrosis, fatty liver, or lymphoma. The bile ducts can be clearly visualized along with the gallbladder, and gallstones demonstrate a characteristic appearance with high echogenicity and shadowing. Ultrasonography is available in most clinical settings and is rapid and noninvasive–hence its popularity as a first-line choice for liver imaging, although the clinician must be aware of its limitations. It is extremely operator-dependent and is often performed by technicians, with only selected images reviewed by radiologists, and interpretation is highly subjective. Moreover, patients with truncal obesity may be too large to permit adequate penetration of the sound pulses to the liver below, and gas in the bowel may obscure the image. The limited resolution of ultrasound impedes detection of discrete lesions less than 1 cm in diameter. The bile ducts are well-visualized on ultrasound because of the interface between the liver parenchyma and each fluid-filled tube. Ultrasonography excels in defining the size and path of bile ducts as small as 3–5 mm and is the screening test of choice for patients with cholestatic patterns of liver disease. It is also excellent for identifying the presence or absence of ascites.
It is important to note that ultrasonography is not useful for defining the presence of fibrosis or cirrhosis, and it rarely helps define the etiology of noncholestatic disease processes. Beale et al found that 75% of 48 HIV-infected individuals had abnormal liver ultrasound scans. A diffusely hyperechoic liver was observed in 46% of patients, which was attributed mainly to steatosis. However, on biopsy, nearly half the patients had more than one histologic abnormality.26 Albisetti et al reported that significant hepatic steatosis on liver biopsy was suspected in only 70% of the pediatric HIV-infected cases reviewed.27 The portability and low cost of ultrasonography have resulted in its extensive use for evaluating HIV-infected patients in developing countries with a high prevalence of AIDS.28
Computed Tomography
Along with ultrasonography, CT imaging of the liver is the most important modality for evaluating masses, abscesses, cystic lesions, and infiltrative processes. Biphasic CT scans of the liver should generally be performed because identification and differentiation of mass lesions requires rapid imaging through the arterial and venous phases of contrast administration. Meta-analysis has shown that baseline CT without contrast was significantly inferior to biphasic or triphasic studies for identifying small mass lesions.29 Therefore, noncontrast CT scans are not recommended for hepatic imaging.
Knollmann et al described the results of abdominal imaging of 339 HIV-infected patients. This retrospective study reported that 82% of patients had abnormal imaging, including 11 patients with hepatic masses and seven with ascites. Both findings were associated with decreased survival relative to the studied cohort.30 In another group of 259 HIV-infected patients, hepatomegaly was reported in ∼39%, and focal hepatic lesions were seen in 19%.31
Magnetic Resonance Imaging
Magnetic resonance cholangiopancreatography (MRCP) is a variation of standard MRI that permits imaging of the biliary system. Studies comparing MRCP to ERCP, which is the gold standard for biliary duct imaging, demonstrate a high correlation.32 Sensitivities for detecting primary sclerosing cholangitis (PSC) have ranged from 86.5% to 97%, with specificities of 64–99%.33–35 There is no literature evaluating the efficacy of MRCP in HIV-infected patients with AIDS-related cholangiopathy, though it seems likely that a similar efficacy might be observed.
Endoscopic Retrograde Cholangiopancreatography
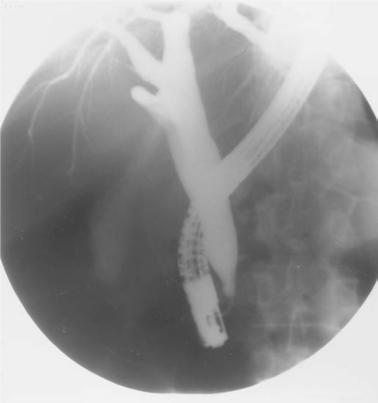
ERCP is regarded as the gold standard for evaluating the biliary duct system. Although somewhat invasive, the procedure is generally tolerable and, in the hands of experienced endoscopists, highly efficacious for diagnosing strictures, papillary stenosis and AIDS cholangiopathy, small stones, and masses in the biliary tree (Fig. 78-3). Somewhat better diagnostically than MRCP, it also offers the opportunity for immediate intervention, including stone removal, ductal dilation, sphincterotomy, and stent placement. Complications include pancreatitis in up to 5% of cases, though it is generally mild and self-limited. Some cases of severe pancreatitis occur, however.
Among patients with HIV infection, the literature supports use of ERCP for both diagnosis and intervention in patients with right upper quadrant pain and ultrasonographic evidence of ductal enlargement. Among 83 patients undergoing ERCP for abdominal pain or cholestatic liver enzyme abnormalities, 56 had AIDS-related cholangiopathy. Of the patients who underwent ampullary biopsy, 62.5% had evidence of opportunistic infection.36 A review of this subject by Walden suggested that patients with a prominent component of pain may benefit from ERCP with sphincterotomy, but those with asymptomatic cholestatic enzyme elevations are much less likely to benefit from intervention.37 Pancreatic ductal changes are common in patients diagnosed with AIDS cholangiopathy, and features suggestive of chronic pancreatitis may be observed in approximately half of these patients.38
Liver Biopsy
Histologic evaluation of liver tissue remains the definitive study for determination of liver disease activity and degree of fibrosis. It also helps confirm the presence of processes suspected during the biochemical and serologic workup phase. Occasionally, liver biopsy identifies previously unsuspected findings in terms of etiology or co-morbidity. Liver biopsies may also be obtained at the time of laparoscopy under direct liver visualization or via a transjugular route. One editorial suggested that radiologic guidance be restricted to patients with a suspected tumor or hepatic mass, obesity, or the rare case when physical examination fails to define the precise location of the liver. For cases of decompensated disease with coagulopathy, ascites, or significant thrombocytopenia, a transjugular approach is considered safer. All other cases may be performed by the percutaneous route, and physicians may or may not use ultrasound localization prior to biopsy.39 While one pediatric study found that ultrasound did not change morbidity or mortality,40 another showed that ultrasound improved the diagnostic yield and decreased the rate of minor complications.41 Riley suggested that use of ultrasound prior to biopsy changed the management in 15.1% of cases42; moreover, an economic analysis supported the use of ultrasound prior to liver biopsy.43
The risk of complications from liver biopsy is highly variable based on the experience of the clinician, number of passes required, type of biopsy needle used, platelet count, prothrombin time, and type of lesion to be biopsied.44 Serious complications are rare, although death has been reported to occur at a rate of nine per 100 000 cases and hemorrhage, pneumothorax, and biliary peritonitis at rates of one per 1000 to three per 1000 cases.45,46
There is little in the literature on the safety of liver biopsy in patients with HIV. A retrospective review of 248 liver biopsies in HIV antibody-positive patients reported a hemorrhage rate of 2.0%, which is higher than might be expected. The exceptionally high mortality rate (1.6%) was attributed to a high prevalence of thrombocytopenia and coagulation abnormalities in the cohort.47 The higher risk of bleeding and death may also be attributable to a high prevalence of disease etiologies that may increase the risk of postbiopsy bleeding, including malignancies such as Kaposi sarcoma and lymphoma and vascular abnormalities including peliosis due to bacillary angiomatosis. High-risk cases (e.g., patients with thrombocytopenia, coagulopathy, or liver lesions) with increased hemorrhage potential mandate screening and selection for safer biopsy approaches, such as transjugular liver biopsy.48
Deserving of special mention are patients with hemophilia who are frequently co-infected with HCV and HIV. There is often great reluctance to subject patients with heritable bleeding diatheses to liver biopsy, and the risk and cost of biopsy in hemophiliacs must be weighed against the value of the information obtained at liver biopsy. Current literature suggests that liver biopsy by the percutaneous or transjugular route can be performed safely in hemophiliacs following factor VIII replacement.49–51 A consensus review of this subject confirms this opinion and provides guidance for clinicians managing patients with hemophilia and liver disease.52
The role of liver biopsy in patients with HIV infection has been controversial. Cappell et al described the outcomes of liver biopsy in 36 patients with HIV. The leading indication was unexplained fever (83%) and abnormal liver enzymes (89%). Previously unidentified etiologies were found most frequently in patients with increased alkaline phosphatase, suggesting the presence of a cholestatic process. Most of these patients had granulomas associated with Mycobacterium infection. The patients in whom biopsy was helpful were those with leukocytosis, those in whom full-blown AIDS was suspected, and those who were not known to have HIV infection prior to the biopsy.53 Poles et al reported on the findings of liver biopsies from 501 HIV seropositive patients who underwent biopsy for indications similar to those described by Cappell et al. The most common diagnosis was mycobacterial infection (26.6% of the patients studied); 12% had chronic hepatitis. The study failed to determine if liver biopsy changed the treatment of the clinical diagnosis.54 Similarly, a European study reported that among 24 patients with unexplained fever, liver biopsy provided a microbiologic diagnosis in 54%.55 Among children with HIV infection, liver biopsy was found to be useful for determining a diagnosis when there was a strong suspicion of mycobacterial infection or the child was jaundiced (or both).56 In HIV-infected patients with hepatitis B and C co-infection, liver biopsy is useful for staging disease and planning treatment.
Noninvasive Markers of Hepatic Fibrosis
Because liver biopsy can be attended by occasionally serious complications, development of validated noninvasive tests for hepatic fibrosis will continue to be important. While a composite index of serum markers have been validated in HIV-negative patients, only one index has been studied in HIV-infected persons with liver disease. The serum hyaluronic acid, albumin, and AST (SHASTA) index has been studied in an HIV–HCV co-infected cohort.57 This index appeared to be sensitive for both moderate-to-severe fibrosis (Ishak fibrosis score 5 3) and low fibrosis stages. Noninvasive measurement of liver stiffness by transient elastography (FibroScan) has also shown promise. In a small study among HIV–HCV co-infected patients, this method was found to be more accurate for the prediction of cirrhosis than the SHASTA index.58 However, it remains unclear whether elastography will be less reliable among patients with hepatic steatosis or HIV-associated lipodystrophy/visceral adiposity. Elastography and biochemical testing may ultimately have complementary roles in the staging of hepatic fibrosis. These methods warrant further investigation in the HIV-infected population.
ETIOLOGIES OF LIVER DISEASE IN PATIENTS WITH HIV
Viral Liver Disease
Hepatotropic Viruses
In view of the markedly high rates of co-infection with hepatitis B and C viruses in HIV-infected persons, it has become increasingly clear that the enhanced survival during the era of HAART will make chronic liver disease with these pathogens important sources of morbidity and mortality. These infections are considered in more detail in Chapters 54 and 55.
Hepatitis A Virus
Seroepidemiologic surveys for HAV infection reveal an increased overall prevalence in the HIV-infected population; among risk groups, men who have sex with men and injection drug users are at increased risk for infection. Although most cases of acute hepatitis with this fecal-oral pathogen are self-limited episodes and are no more clinically severe in the HIV-infected host than in the general population,59 HIV-infected patients treated for hepatitis A do exhibit prolonged viremia compared with HIV negative controls.60 Individuals at particular risk for morbidity from hepatitis A are those who harbor chronic liver disease. Indeed, the risk of fatal acute hepatitis A is significantly increased in subjects with chronic HCV infection61; this has led to the recommendation that individuals with chronic HCV infection be actively immunized with an HAV vaccine.62 HIV-infected individuals at continued risk for HAV infection should also be vaccinated. Vaccine response rates in HIV-infected adults range from 50–95%, and lower CD4+ T-lymphocyte counts appear to be associated with reduced efficacy.63
Hepatitis B Virus
Co-infection with HBV occurs frequently in view of its shared routes of transmission with HIV. Up to 95% of persons with AIDS have serologic markers of prior HBV infection (anti-HBs1 or anti-HBc1).64,65 An estimated 7.6% of HIV-infected patients in the US are chronic HBV carriers (HBsAg1); co-infection rates may be higher than 20% in Asia, where HBV is endemic.66 Although there is little evidence for a direct virologic interaction between HIV and HBV, the critical role of the immune system in mediating cell injury in chronic hepatitis B suggests that HIV alters its natural history. In support of this concept, the incidence of acute icteric hepatitis B is lower in the presence of HIV, and the risk of developing chronic HBV infection is increased substantially in patients with preexisting HIV infection.67 Moreover, HIV appears to enhance and extend the period of HBV replication (HBeAg1, HBV DNA1) but without a concomitant increase in hepatic necroinflammatory activity. With advancing immunosuppression, markers of HBV replication appear to be reactivated. A 10-year cohort study of 5293 HIV positive men, including over 300 men with HBV–HIV co-infection, showed a 12.7-fold increase in mortality among HBsAg positive patients. Liver-related mortality was increased after 1996 (in the HAART era) and among those patients with low CD4 counts.68,69 There is little evidence that HBV worsens the course of HIV disease. One large-scale retrospective analysis demonstrated no influence on the mortality of co-infected patients compared with HBsAg negative patients with HIV infection.70
Irrespective of whether HBV disease is hastened by HIV, alteration of the HIV treatment picture by introducing HAART increases the likelihood that HBV liver disease will become more problematic with control of HIV. Indeed, immune-mediated flares of HBV with successful pharmacologic reconstitution of the immune system have been described.71,72 Furthermore, lamivudine withdrawal or YMDD-mutant breakthrough may precipitate acute liver injury in immunologically reconstituted patients who also have hepatitis B infection.8,73
The HIV–HBV co-infected patient should be treated for replicative HBV disease, defined by the following criteria: (1) presence of HBsAg, (2) abnormal serum ALT level, (3) HBV DNA level >100 000 copies/mL with HBeAg, or (4) HBV DNA level >10 000 copies/mL without HBeAg (indicating infection with a precore or core promoter mutant strain of HBV). Goals of therapy include normalization of ALT, suppression of HBV replication, and seroconversion from HBeAg to anti-HBe when applicable. Improvement in liver histology can indicate a response to HBV therapy, but serologic indices are quite reliable and obviate the need for biopsy in most cases. However, liver biopsy remains advisable in HBV–HIV co-infected patients prior to initiation of HAART, since a flare of liver enzymes could precipitate hepatic failure in patients with cirrhosis.
The mainstays of HBV therapy in co-infected patients are nucleoside and nucleotide analogs. Interferon is less effective in co-infected patients than in HBV-monoinfected patients and is infrequently used in the former group.74 Efficacy studies of pegylated interferon in co-infected subjects are underway, but no results have been published to date. Lamivudine, a nucleoside analog with activity against HIV reverse transcriptase and HBV DNA polymerase, lowers HBV DNA levels when used as a component of HAART in the short term but promotes emergence of YMDD mutants after years of treatment.75,76 If a patient’s HBV viral load increases during long-term HAART including lamivudine, a nucleotide analog should be added to the regimen. Lamivudine should not be stopped abruptly because a hepatic flare may occur.77 A co-infected patient not on HAART should not receive lamivudine monotherapy for HBV, as lamivudine-resistant HIV mutants may emerge.78 Other nucleoside analogs used to treat co-infected patients are emtricitabine, which, like lamivudine, has activity against both viruses but engenders YMDD resistance, and entecavir, which inhibits only HBV DNA polymerase and may be employed in the absence of HAART.79
Adefovir and tenofovir are nucleotide analogs that inhibit both HIV reverse transcriptase and HBV DNA polymerase. Unlike lamivudine, adefovir may be used as HBV monotherapy when HAART is not needed.80,81 Adefovir-resistant HBV strains occur in 18% of monoinfected patients after 96 weeks of therapy.82 However, an analysis of HIV–HBV co-infected patients treated with adefovir and lamivudine for up to 144 weeks did not reveal resistant HBV strains.83,84 Furthermore, adefovir may be used to treat lamivudine-resistant HBV in co-infected patients; emergence of adefovir-resistant HIV has not been demonstrated in this context.85 The major adverse effect of adefovir is nephrotoxicity, which is significantly less common in patients receiving the relatively low dose (10 mg/day) used to treat HBV than in those who receive higher doses for HIV.86,87
Tenofovir is a second-generation nucleotide analog that is effective against lamivudine-resistant HBV strains. This drug should therefore be included in HAART regimens for co-infected patients with lamivudine-resistant HBV. Tenofovir may reduce HBV viral load more rapidly than adefovir,88 and no tenofovir-resistant HBV strains have yet been reported in co-infected patients. Promising treatment results were also described in a retrospective study by Benhamou et al.89 This drug should not be co-administered with the antiretroviral didanosine (ddI) due to the increased risk of pancreatitis.90
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree