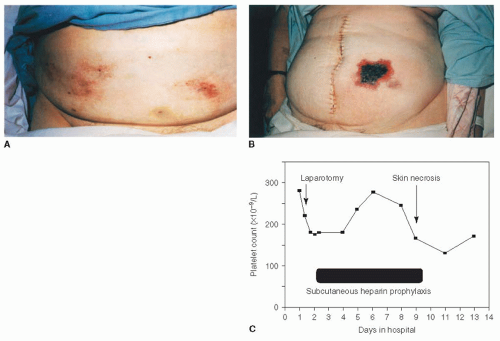
Heparin-induced thrombocytopenia (HIT) is an immune-mediated adverse drug reaction caused by heparin-dependent, platelet-activating immunoglobulin (Ig) G antibodies that recognize complexes of platelet factor 4 (PF4) bound to heparin.
1,
2,
3 HIT represents a strong, independent risk factor for venous and arterial thrombosis.
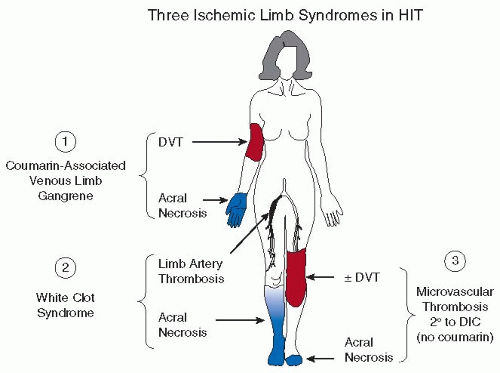
4HIT typically causes thrombosis in large veins and arteries; platelet-rich, intra-arterial “white thrombi” were first described in 1958.
5,
6,
7 However, HIT is also a risk factor for microvascular thrombosis in certain situations, such as in coumarin treatment of deep vein thrombosis (DVT) (i.e., coumarin-associated venous limb gangrene [see
Chapter 109]) or severe HIT-associated disseminated intravascular coagulation (DIC).
8,
9,
10Thrombosis typically occurs in anti-PF4/heparin antibodypositive patients who develop significant declines in platelet count,
11,
12,
13,
14 pointing to clinically relevant consequences of
in vivo platelet activation induced by these antibodies. A central role for
in vivo thrombin generation in HIT
8 provides a rationale for the use of anticoagulants that inhibit thrombin or its generation.
15
PATHOGENESIS
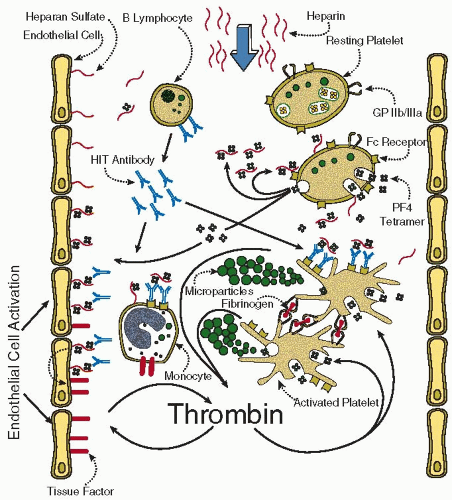
FIGURE 108.1 summarizes the pathogenesis of HIT.
17 The central concept is the formation of heparin-dependent IgG that activates platelets via their Fc
γIIa receptors.
18 It remains controversial—although in our view unlikely—whether IgA and IgM antibodies evince pathogenicity.
19,
20,
21,
22 The target antigen is a complex of heparin and the positively charged platelet
α-granule protein, PF4,
23,
24,
25,
26 a member of the CXC subfamily of chemokines. HIT antibodies are directed against one or more sites on PF4 that have been conformationally altered by binding to negatively charged heparin.
27,
28,
29,
30 This model is consistent with reports that other highly sulfated, nonheparin carbohydrates such as pentosan polysulfate
31 and polysulfated chondroitin sulfate
32 can trigger HIT. This possibly triggered additional adverse effects by oversulfated chondroitin sulfate within “contaminated” heparin.
33 It may also explain why low molecular weight heparin (LMWH) is less likely to trigger HIT,
11,
12 as only heparin molecules of at least 12 saccharides in length bind PF4 in such a way as to induce a large number of neoepitopes required for clinical breakthrough of HIT.
34 Rarely, a disorder mimicking HIT on clinical and serologic grounds can occur in the absence of preceding heparin exposure (“spontaneous HIT”); reported cases have featured preceding inflammatory events such as surgery or infection.
35,
36,
37 This indicates that the immune reaction to PF4/polyanion complexes can also be induced by endogeneous factors, a feature relevant to understanding the temporal features of antibody formation. Moreover, many HIT-positive sera
38,
39—but especially those obtained from patients whose HIT begins
40 or worsens after stopping heparin—are able to activate platelets
in vitro even in the absence of pharmacologic heparin, a phenomenon consistent with “autoimmune-like” features.
Anti-PF4/heparin seroconversion has certain atypical features
41,
42: Antibodies are formed relatively quickly—approximately 4 days (median) after even a first-time immunizing heparin exposure, and without evidence for more rapid formation with previous heparin exposure or even with previous HIT.
43 Anti-PF4/heparin antibodies of any of the three major Ig classes can develop and in any combination, in the frequency IgG > IgA > IgM.
41,
42 Moreover, when IgM antibodies are formed, these are detectable simultaneously with IgG and/or IgA, that is, there is no IgM precedence (as would be expected in a “classic” immune response). This indicates that the immune response induced by heparin is a secondary, rather than a primary, immune response; PF4 complexed to polyanions on bacterial surfaces might be the “preimmunizing” trigger.
44Several factors contribute to the prothrombotic nature of HIT
(FIGURE 108.1): (a) the potent platelet-activating
properties of the HIT antibodies, which also generate procoagulant, platelet-derived microparticles
45,
46; (b) pancellular activation (either directly or indirectly by HIT antibodies),
47,
48 manifesting as tissue factor expression on endothelium
25,
48,
49 and monocytes,
50,
51 and possibly platelet-leukocyte complex formation
52; and (c) neutralization of the heparin anticoagulant effects by PF4. Collectively, these factors result in increased thrombin generation in HIT.
8,
53,
54Women are more likely than men to develop HIT (˜ two-fold increase by odds ratio).
55 However, no HLA association or other genetic risk factor has been identified.
56 Although the platelets bearing Fc
γIIa receptors of the His131 phenotype (˜70% of the population) are more readily activated by HIT antibodies of IgG1 subclass (the predominant subclass in HIT),
57,
58 no clear association between HIT and Fc receptor genotype has been established.
59,
60 A strong, albeit indirect, argument against a genetic risk factor is the surprising observation that even patients with a previous history of HIT do not usually regenerate HIT antibodies upon repeat heparin exposure.
43,
61
RISK FACTORS FOR HIT
The frequency of HIT is variable and is influenced by the type of patient population (major surgery or trauma > minor surgery > medical > obstetrical), sex (female > male), the type of heparin used (bovine unfractionated heparin [UFH] > porcine UFH > porcine LMWH > fondaparinux), duration of heparin therapy (the risk increases until ˜10 to 14 days of heparin administration), and, possibly, the timing of the first
dose of anticoagulant in relation to surgery (postoperative > preoperative) and body mass index (BMI) (high BMI > low BMI).
55,
62,
63,
64 A stoichiometric model of optimally immunizing PF4:heparin ratios has been proposed to explain some of these observations.
64,
65A relatively high frequency (˜5%) of HIT occurs in postoperative orthopedic surgery patients receiving prophylactic-dose UFH for up to 14 days.
11,
12,
66 The frequency is even higher (8% to 10%) in postcardiac surgery patients who receive therapeuticdose UFH after implantation of a ventricular assist device.
67,
68,
69 In contrast, HIT was not observed in several large studies in which LMWH was administered for many weeks during pregnancy.
70,
71,
72 The frequency of HIT occurrence is estimated to be about 1% in patients receiving UFH for treatment of venous thromboembolism
62 and, perhaps, for prevention of thrombosis in medical patients,
73 and approximately 0.1% to 0.5% in postoperative patients receiving LMWH
62; case reports suggest a small risk of HIT (probably <0.01%) with fondaparinux thromboprophylaxis after surgery.
74,
75,
76 However, it might be that in these cases the antibodies (albeit triggered by fondaparinux) show the autoantibody-like behavior described above. Given the decreasing use of UFH after orthopedic surgery, one common scenario for HIT today is antithrombotic prophylaxis with UFH following cardiac surgery (risk: ˜1% if UFH is given for >1 week).
77Patient-dependent risk factors influence the type of HIT-associated thrombotic event. For example, venous thrombosis occurs in at least half of postorthopedic surgery patients who develop HIT, with relatively few arterial thrombi observed.
11,
12,
66 In contrast, arterial thrombosis occurs at least as often as venous thrombosis in patients with HIT after cardiac surgery, suggesting a predisposing role for atherosclerosis.
62,
78,
79 Risk of limb amputation is greater in patients who develop HIT in the setting of peripheral vascular surgery.
80 Patients with a central venous catheter who develop HIT have about a 10% chance of developing symptomatic upper limb DVT at the catheter site, reflecting the interaction of localized vessel injury with the systemic hypercoagulability of HIT.
81
SCORING SYSTEMS
Scoring systems have been developed for use in diagnosis of HIT, to evaluate new laboratory tests for HIT antibodies, or to estimate the pretest probability of HIT.
108,
109,
110,
111,
112,
113 Table 108.2 shows the 4Ts scoring system; depending on the clinical setting in which the 4Ts is used, the likelihood of HIT ranges from <2% (low score), to 10% to 30% (intermediate score), to 50% to 70% (high score).
111 Thus, the main utility of the 4Ts is in predicting a low likelihood of HIT when the score is low (≤3 points), that is, a high negative predictive value.
111 A recent scoring system developed using broad expert opinion, the HIT Expert Probability Score, exhibited favorable operating characteristics but requires prospective validation.
113