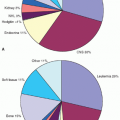
ALL—First Remission
Indications for transplant in patients in initial complete remission (CR1) are generally assigned on the basis of risk stratification. Definitions of high risk and therefore indications for transplant vary considerably; however, most agree that patients who have poor outcomes with chemotherapy alone and are at high risk of treatment failure or relapse with 5-year event-free survival (EFS) of less than 50% should be considered candidates for transplant. These very high-risk ALL patients include those who have failed induction, infants with MLL rearrangements, those with T-cell ALL who have a poor early response to therapy, and those with high-risk cytogenetic features such as hypodiploidy (chromosome number <44) or Philadelphia chromosome positive (Ph
+) ALL.
28Induction failure is associated with a very poor prognosis, with a reported survival of 30% or less.
29 Patients with induction failure have been considered candidates for transplant as early studies showed significant improvement in 5-year DFS for transplant compared with chemotherapy.
30 It is possible that not all patients with induction failure require transplant to achieve long-term remission. A recent analysis of 1,041 pediatric patients with induction failure treated between 1985 and 2000 showed that allogeneic transplant was beneficial over chemotherapy for patients with T-cell ALL and for older patients with precursor (pre-)B-cell ALL, while younger patients with pre-B-cell ALL fared better with chemotherapy.
29Induction failure has conventionally been defined by morphological assessment of disease. More sensitive methods, such as polymerase chain reaction (PCR) or flow cytometric-based assays can detect minimal residual disease (MRD) in as few as 1 in 100,000 malignant cells. These assays have become increasingly important as indicators for treatment failure.
31 End induction MRD has been shown to be the single most important prognostic factor able to discriminate outcomes for patients with pre-B-cell ALL and T-cell ALL.
32,33,34 Recent studies have incorporated MRD for risk stratification and assignment to transplant. This remains an active area of study.
35Despite the use of maximally intensified chemotherapy regimens, infants with ALL, in particular those <6 months of age with mutations involving 11q23, the MLL gene, and poor response to induction therapy, have very poor outcomes.
36 The role for upfront transplant has been evaluated, but several trials have failed to show benefit of transplant over chemotherapy (
Table 16.1).
37,38 The lack of benefit has been largely due to high transplant regimen-related mortality. For chemotherapy, relapse remains the principal cause of failure. A recent study from the Interfant-99 group showed a significant difference in DFS (adjusted for waiting time to transplant) between those who received transplant versus those who received chemotherapy (5-year DFS of 59% vs. 22%
p = 0.01). However, this advantage was restricted to the subgroup of infants with MLL gene rearrangements who were <6 months of age with poor response to steroids and/or presenting WBC > 300 × 10
9/L. For other infants with the MLL gene rearrangements but no other additional risk factors, there was no benefit to transplant over chemotherapy (5-year DFS of 60.9% vs. 53.8%,
p = 0.09).
39 One major drawback to transplant of infants is the concern for radiation-related growth failure and neurocognitive development. Reports have been mixed regarding the impact of radiation in these areas, but these concerns must be satisfactorily addressed.
40Hypodiploid ALL (<44 chromosomes) has a very poor outcome, and more than two-thirds of patients are likely to relapse. In a large multicenter study of patients with hypodiploid ALL, the 8-year EFS was significantly worse for patients with fewer than 44 chromosomes compared with patients with 44 chromosomes at 30.1% versus 52.2% (
p = 0.01).
41 In a retrospective review of the Center for International Blood and Marrow Transplant Research (CIBMTR) experience, the 5-year DFS was 51% in 78 patients who underwent transplant for hypodiploidy, suggesting that transplant may improve outcomes in these patients.
42Prior to the use of tyrosine kinase inhibitors (TKIs), for patients with Ph
+ ALL, there was a clear benefit for transplant.
43 With the incorporation of TKIs into upfront therapies for Ph
+ ALL, a recent report from the Children’s Oncology Group (COG) showed long-term leukemia-free survival (LFS) was comparable between patients receiving imatinib-based chemotherapy versus transplant (70% vs. 65% for matched related donor and 59% for unrelated donors).
44 While further studies are needed, current clinical trials do not utilize transplant for patients in CR1 unless there is evidence of persistent MRD.
Similarly, outcomes for T-cell ALL have improved with current therapies.
45 However, patients who have a poor response to initial therapy may achieve superior outcomes with transplant (5-year DFS 67%), compared with chemotherapy (5-year DFS 42%).
46 Therefore, transplant is recommended for T-cell ALL in CR1 when there is poor initial response to therapy. The recently described early T-cell precursor-ALL (ETP-ALL) is characterized by a distinct genetic and immunophenotypic profile, poor response to chemotherapy, and a very high risk of relapse. The overall survival for this subtype is significantly worse than for other patients with T-cell ALL.
47 Results from a recent study show that transplant was able to salvage patients with ETP-ALL who relapsed, suggesting there may be a role for upfront transplant for this resistant T-cell ALL.
48 Further studies will be needed to address this question.
Consortium studies have evaluated the role of transplant for patients with high-risk ALL in first remission, and these are summarized in
Table 16.1.
29,30,35,44,46,49,50 It is important to note that most studies comparing transplant with chemotherapy are not randomized controlled studies. To compare treatments, statistical analyses are used to adjust for treatment variables such as time to transplant. Outcome can be biased by the time and the logistics associated with finding a suitable allogeneic donor, with the result that children with high-risk ALL may relapse before transplantation can be performed. It is unclear whether this is a failure of chemotherapy or transplantation. To adjust for such biases, statisticians utilize “intent-to-treat” analysis, in which all transplant-eligible patients are considered as transplant, regardless of whether transplant is performed. “Time-adjusted” analysis excludes from the analysis any relapses that occur during the time interval to transplant. These statistical manipulations are never as satisfactory as randomized controlled studies.
Because of its diverse patient makeup and large patient numbers, registry data provide valuable perspective to transplant outcomes. For patients younger than 20 years with ALL in CR1, the CIBMTR reports a 65% overall probability of survival at 3 years after matched related donor transplant. After unrelated donor transplant, the overall probability of survival of patients aged 0 to 11 is 68% and of those aged 11 to 20 is lower at 48%.
The transplant conditioning regimen was originally intended to overcome leukemia cell resistance. Although graft versus leukemia (GVL) may be operative for some patients, much of the curative potential of transplantation for ALL comes from the conditioning therapy. It is reasonably well established that for patients with ALL, the ablative therapy, which includes total body irradiation (TBI), is superior to chemotherapy-only regimens.
51 Most centers use a backbone of fractionated TBI to which either cyclophosphamide or etoposide (in European centers) are added. Recent multicenter trials have evaluated the role of reduced intensity conditioning (RIC) regimens for patients who cannot undergo myeloablative conditioning, and while results appear promising, long-term studies are required before this strategy can be accepted.
52,53
ALL—Relapse
The principal role of stem cell transplantation in pediatric ALL therapy has been salvage after relapse. The decision to recommend transplant varies among investigative groups, but is generally based upon prognostic criteria such as length of remission, site of relapse, and leukemia immunophenotype. A number of studies have shown that the length of remission is the most important predictor of survival following relapse.
54,55,56Data from a collaborative study of the COG and CIBMTR showed that for children with early first relapse (<36 months), the risk of a second relapse was significantly lower after TBI-containing transplant regimens versus conventional chemotherapy (relative risk [RR] 0.49,
p < 0.001). For children with a late first relapse (≥36 months), the risks of second relapse were similar after TBI-containing regimens and conventional chemotherapy (RR 0.92,
p = 0.78).
51 The Dutch COG study found that for patients with early relapse (defined as <30 months after attaining remission), transplant led to superior 5-year EFS compared with conventional chemotherapy (25% vs. 0%), whereas for patients with late relapse (defined as relapse >30 months after attaining remission) the
reverse was true, with 5-year EFS of 16% for transplant versus 65% for conventional chemotherapy.
57Consensus is less as to the prognostic importance of the site of relapse. Several studies have reported that outcomes after isolated extramedullary relapse were superior to BM relapse or combined BM and CNS relapse,
55,58 whereas other reports showed no difference in outcome.
59 Leukemia immunophenotype also affects outcomes after relapse as patients with T-cell ALL have worse outcomes compared with those with B-cell ALL.
54 Thus, T-cell ALL patients are referred to transplant in second complete remission (CR2) regardless of time to relapse. Newer studies suggest that underlying cytogenetic changes also influence outcome after relapse. For example, the presence of ETV6/RUNX1 is associated with excellent prognosis, whereas patients with IKZF1 and P53 mutations have very poor outcomes after relapse.
60,61Several multicenter clinical trials have attempted to compare outcomes for children in CR2 treated with transplantation or chemotherapy (
Table 16.1).
55,57,62,63,64,65,66,67 Recent studies continue to show benefit for patients with high-risk features. In a recent study from the Berlin-Frankfurt-Münster (BFM) collaborative group, the 10-year EFS was significantly higher for transplant (33%) compared with chemotherapy (20%) for patients with early BM or combined relapse and for patients with T-cell ALL.
55 The findings of these and other studies
54 support the practice to recommend transplantation for children whose relapse occurs while on therapy or within 6 months of completing therapy and for patients with relapsed T-cell ALL as these patients are unlikely to be cured with chemotherapy alone. For patients with late relapse or when relapse is limited to extramedullary sites, chemotherapy alone can be used as salvage therapy.
More recently, it has been found that end of reinduction MRD identifies an additional subset of patients with late relapses of B-cell ALL who may benefit from transplant. These are patients who otherwise would not have met criteria for transplant.
68,69 Based on similar studies,
70,71 several cooperative groups are evaluating end-reinduction MRD to risk stratify patients with relapsed pre-B ALL to determine indication for transplant.
69,72Pretransplant MRD appears to adversely influence outcome. In patients with high pretransplant MRD (≥10
-4 leukemic cells) versus low MRD (≤10
-4 leukemic cells), the probability of EFS was significantly lower (0.27 vs. 0.60,
p = 0.04), and cumulative incidence of relapse was significantly higher (0.57 vs. 0.13,
p = 0.01).
73 For now, it is unclear how treatment should be tailored for patients with persistent detectable MRD before transplant. Additional aggressive therapy may not reduce persistent disease or may lead to complications, which ultimately are prohibitive for transplantation. Newer therapies such as the use of monoclonal antibodies directed against leukemic cells or T cells expressing chimeric antigen receptors offer novel approaches to reduce disease burden while minimizing toxicities.
74Data from a COG/Pediatric Bone Marrow Transplant Consortium (PBMTC) trial showed that in addition to detection of pretransplant MRD, the presence of posttransplant MRD is associated with a 14-fold increase in relapse rate (RR 14.3, 95% confidence interval [CI]: 6.1 to 33.6). Interestingly, while the effect of a GVL for ALL is controversial, patients who did not develop grade II-IV acute GVHD had higher levels of posttransplant MRD and relapsed nearly 5 times more often than those with acute GVHD (30% vs. 6.4% at 2 years).
75Data from the CIBMTR show that the 3-year probability of survival for children in CR2 aged 0 to 11 and 11 to 20 following matched related donor transplant is 61% and 46%, respectively. For similar patients of ages 0 to 11 and 11 to 20 following unrelated donor transplantation, the 3-year probability of survival is 49% and 43%, respectively. While these results indicate that stem cell source determines outcome, recent single-center studies report comparable outcome for patients undergoing transplant from matched related donors, and matched unrelated marrow and cord donors.
64,65 In addition, haploidentical donor transplants are also increasingly being used with newer graft engineering techniques successfully employed to maximize a GVL effect while minimizing GVHD.
23,76Similar to patients with BM or combined relapses, the prognosis for patients with isolated extramedullary relapse, most commonly CNS relapse, is correlated with the duration of initial remission. The United Kingdom (UK) ALL group reported 20% EFS for children with isolated CNS relapses when initial remission was <18 months.
59 The COG and CIBMTR retrospectively compared the outcome for children with CNS relapse and found EFS was similar, 66% and 58%, after chemotherapy/radiation and transplantation, respectively.
66 Regardless of therapy, initial remission lasting <18 months was associated with inferior EFS, as half the patients developed a subsequent occurrence. As the outcomes for chemotherapy and transplant are similar following isolated CNS relapse and data show that for patients with isolated late relapse (>18 months), treatment with chemotherapy alone may be sufficient,
77 transplant is generally not recommended for patients with isolated CNS relapse.
28 There are, however, insufficient data to make recommendations for patients with isolated CNS relapse of T-cell ALL.
Transplantation for children beyond second remission can be effective in selected cases. Data from the Nordic Society for Pediatric Hematology and Oncology (NOPHO) group reported a 10-year survival of 37% for patients receiving transplant in CR3. Patients who were treated less aggressively or who had longer durations of CR1 and CR2 fared better.
78 The CIBMTR reported 5-year estimates of LFS and NRM of 30% and 45%, respectively, for patients undergoing transplant in CR3. The risk of relapse was lowest when both first and second relapses were late. However, survival was impacted by high treatment-related mortality (TRM).
79 Therefore, although ALL in CR3 has a poor prognosis, transplant is usually recommended as it can improve survival.