Chapter 67 Hematologic Disease
Hematologic abnormalities are widely recognized and are of significant clinical importance in the management of patients with human immunodeficiency virus (HIV) infection. The etiology of these hematologic abnormalities is multiple. HIV infection of lymphocytes, monocytes, and macrophages is thought to induce abnormalities in cytokine production that in turn have marked effects on the regulation of both the hematopoietic and immune systems. Studies involving HIV infection of bone marrow progenitor cells have been conflicting.1–3 Although some early data suggested that CD34+ bone marrow progenitor cells might be the targets of HIV infection,4–7 other, more recent, findings have indicated that these cells are infrequently infected with HIV.8,9 Similarly, HIV RNA in protein products have been found in committed myeloid and erythroid progenitor cells,7 while another study failed to detect HIV in these cells.10 The presence or absence of accessory cells in the bone marrow can also influence hematopoiesis through the local production of growth factors.11 These accessory cells, such as T lymphocytes, monocytes, endothelial cells, and fibroblasts are targets for HIV and may serve as a reservoir for the virus in the bone marrow. HIV-infected accessory cells may be less able to produce local hematopoietic growth factors such as granulocyte colony-stimulating factor (G-CSF) and interleukin-3 (IL-3), which in turn may slow normal hematopoiesis or facilitate apoptosis.12–13 These accessory cells may also produce inhibitors of hematopoiesis such as transforming growth factor beta, interferon gamma, and tumor necrosis factor alpha (TNF-alpha) or other suppressive cyto-kines.11,14–18 HIV may also inhibit the hematopoietic process more directly, such as with antibodies to gp120 or through functioning of accessory genes.19,20
CYTOPENIAS
Cytopenias were some of the first recognized signs of HIV infection. The untreated natural history of HIV infection is associated with a relatively high prevalence of anemia, neutropenia, and thrombocytopenia. The prevalence of each of these cytopenias tends to increase as HIV infection progresses from asymptomatic infection to advanced disease and acquired immunodeficiency syndrome (AIDS).21–23 As will be discussed in subsequent sections, cytopenias also contribute to some of the morbidity associated with HIV and AIDS because they hinder therapy directed at HIV and the opportunistic infections and neoplasms of AIDS may increase the risk of bacterial infection, and affect quality of life.
Anemia
Anemia is the most common hematologic abnormality seen in patients with HIV infection (Fig. 67-1). Specific determination of the prevalence and incidence of anemia in HIV infection has been hampered by inconsistent definitions of anemia. Nevertheless, a number of studies have consistently demonstrated a prevalence of anemia of 10–20% in untreated asymptomatic HIV infection, and from 50% to 85% with overt AIDS.1,24–26 A large study of over 32 800 HIV-infected persons in care in nine US cities, the multistate Adult and Adolescent Spectrum of HIV Disease Surveillance study (AASHD), assessed anemia as defined by a hemoglobin level <10 g/dL or a physician’s diagnosis of anemia from 1990 to 1996, prior to the widespread availability of HAART. The annual incidence of anemia was 37% in those with clinical AIDS, 12% in immunologic AIDS (CD4+ T-cell count of >200 cells/mL) and 3% in HIV-infected individuals without AIDS.27 In this cohort and others, HIV-infected women have been found to have a higher prevalence and incidence of anemia than men.27–29 It has also been shown that HIV-infected African-Americans are more likely to have anemia than other racial/ethnic groups, and the HIV-infected persons with a history of injecting drug use are at higher risk for anemia than are other HIV transmission risk groups.26,30–32
There has been a consistent association between anemia and decreased survival in patients infected with HIV. This association has been demonstrated in observational studies and clinical trials since the mid-1980s.33 The strongest association with a decreased survival is for a hemoglobin level of less than 10 g/dL. In the AASHD, the risk of dying was 48% greater in those with anemia compared to those who did not have anemia.27 In another study using data from a clinical HIV cohort in Baltimore, the relative risk of dying for a patient with a hemoglobin level <9.5 g/dL was threefold that of those patients with higher hemoglobin levels.34 Data for both of these analyses preceded HAART, but did control for other prognostic variables such as the CD4+ T-cell count. However, an analysis from the multicountry EuroSIDA cohort performed using data collected after 1996, when HAART was available, showed that the highest risk of dying (RR = 7.1) was for a hemoglobin level <8 g/dL, but that the risk remained elevated in the 8–14 g/dL range (RR = 2.2).35 In the Human Immunodeficiency Virus Epidemiology Research Study (HERS), anemia was associated with a relative hazard of 1.64 for dying among HIV-infected women.29 Similarly in the Women’s Interagency HIV Study (WIHS), anemia was independently associated with decreased survival (relative hazard = 2.58).36 These studies of anemia and mortality were all conducted in high socioeconomic countries. An association has also been found between anemia and survival in Haiti37 and more recently, anemia was shown to be an independent predictor of mortality and immunologic progression of disease among HIV-infected women in Tanzania with a relative hazard of 2.06 for moderate anemia (hemoglobin <11 g/dL) and 3.19 for severe anemia (hemoglobin <8.5 g/dL).38
Anemia has also been shown to be associated with HIV progression to AIDS, and with the development of specific opportunistic illnesses. In data from the Multicenter AIDS Cohort Study (MACS), there was a 29% decrease in risk of progression to AIDS from HIV over 6 months for each one-point increase in the hemoglobin.39 Anemia has been associated with development of early dementia40 and the rapid progression of disseminated Mycobacterium avium complex.41 The importance of anemia as a prognostic factor for AIDS progression and death has led it to be incorporated into a prognostic algorithm developed using data from EuroSIDA. In this study, a multivariate analysis identified four factors; CD4+ T-cell count, HIV RNA, a history of clinical AIDS, and the hemoglobin level as independent predictors of HIV disease progression and death.42
It is important to note that this consistent association may not be causal, but instead may be an epiphenomenon of impaired erythropoiesis which is secondary to rapidly advancing HIV disease. Notably, some studies have suggested that resolving anemia is associated with improved survival in patients infected with HIV.17,34 Several studies have adjusted or otherwise controlled for a number of other prognostic factors such as CD4+ T-cell count, HIV RNA, AIDS diagnoses and antiretroviral and prophylaxis medications, yet found anemia to be an independent prognostic factor for survival. Nevertheless, short of a randomized clinical trial designed to assess treating versus not treating anemia in HIV infection, it will remain unknown whether anemia independently increases the risk of dying with HIV infection.
Anemia is also associated with a reduced quality of life, particularly as it relates to functional status. The association between anemia and a reduced functional status has been found in several studies of cancer treatment.43–45 Until recently, there were few data examining the association between anemia and quality of life in HIV infection. An earlier observational study showed that an increase in the hematocrit from <30% to >38% was associated with significant improvement in several quality of life measures.46 In a multicenter study of 221 patients receiving zidovudine who had a hemoglobin level <11 g/dL, a mean increase of 2.5 g/dL was associated with a significantly improved functional status.47 In another multicenter, prospective cohort study of 1406 individuals, a higher hemoglobin level was associated with higher energy scores and physical functioning scores, after adjusting for CD4+ T-cell count, sex, age, race, and HIV transmission risk factor.48 In a recent clinical trial of treatment of 650 patients who had anemia (hemoglobin <11 g/dL), there was a mean increase in hemoglobin of 2.5 g/dL, which corresponded to a 41% increase in the mean Linear Analog Scale Assessment (LASA) quality of life scale, and a 37% improvement in the (Medical Outcomes Study) MOS-HIV quality of life measure.49 In both cancer and HIV infection, improvements in the quality of life appear to occur until the hemoglobin reaches the 12–13 g/dL range, suggesting the need to achieve and maintain hemoglobin >12 g/dL in an effort to preserve and maximize quality of life benefits.50
Etiology
Impaired Erythropoiesis
The major cause of anemia in HIV-infected patients is impaired erythropoiesis (Table 67-1), either as a direct consequence of HIV infection of erythrocyte precursors or, more likely, as a result of inappropriate release of inflammatory cytokines such as TNF-alpha, a potent inhibitor of erythroid maturation in vitro.51,52 There is also a decreased production of erythropoietin, similar to that seen with other chronic inflammatory diseases.53 The anemia of HIV infection is typically classified as an anemia of chronic disease and is usually normochromic and normocytic, although it may be mildly hypochromic and microcytic. There is usually a low serum iron level and iron-binding capacity, but a normal to elevated ferritin level and adequate iron stores in the bone marrow. The reticulocyte count is inappropriately low. Macrocytosis is unusual except in patients treated with zidovudine, and less so with stavudine.54–56
Table 67-1 Causes of Anemia in HIV Infection
Some other common causes of anemia in patients with HIV infection are infiltrative diseases of the bone marrow, such as that caused by Mycobacterium avium complex (MAC) in patients with advanced HIV disease.57,58 Patients with MAC infections often have profound anemia with hematocrits as low as 15–20%. Fungal infections, cytomegalovirus, and neoplasms may also infiltrate bone marrow and cause profound anemia, often with associated neutropenia and thrombocytopenia.
Persistent infection with B19 parvovirus is also associated with intractable anemia in immunosuppressed patients.59–61 Parvovirus B19 can selectively infect actively replicating erythroid progenitors, resulting in loss of red blood cells and erythroid hypoplasia. Parvovirus B19 is common in childhood (fifth disease), and as many as 80% of adults have antibodies to the virus. Control of this infection is mediated by an intact humoral immune response; therefore immunocompromised patients may fail to clear this infection because they are unable to maintain an adequate antibody response to this virus.61–63 IgG and IgM antibodies are often absent, however, B19 parvovirus infection is reliably diagnosed by polymerase chain reaction (PCR) or by in situ hybridization using sequence-specific DNA probes for parvovirus B19. The presence of giant, abnormal pronormoblasts in the bone marrow is also pathognomonic for parvovirus B19 infection.
Nutritional deficiencies have been described in patients with HIV infection and may include disorders of iron metabolism or iron deficiency.1,32,64 Iron deficiency may result from chronic blood loss, from Kaposi sarcoma, lymphoma of the gastrointestinal tract, or various infectious enterocolitides. Recent data suggest that HIV-infected injecting drug users may be at higher risk for iron deficiency anemia (adjusted RR 5 6.65) than other HIV transmission risk groups.31 The prevalence of HIV infection in women has increased over the past decade, and iron deficiency is a common nutritional disorder in premenopausal women. Although advanced HIV disease and AIDS may be associated with diminished menses, women with effectively treated and asymptomatic HIV infection may have normal menstruation and be at risk for iron deficiency. Alcohol abuse is not uncommon in HIV infection, and hemorrhagic gastritis can be a complication. The causes of iron deficiency are myriad, and it is important to ensure that the patient has had a sufficient evaluation to determine the specific cause.
Vitamin B12 deficiency occurs in up to 30% of HIV-infected patients.1,32,64–66 Patients usually do not have other manifestations of vitamin B12 deficiency and often do not improve markedly with parenteral repletion.67 The low vitamin B12levels may result from abnormalities in vitamin B12-binding proteins and decreased serum transport of vitamin B12,68 however, abnormal absorption of vitamin B12 may also occur in patients with advanced HIV infection.69–71 Folate deficiency is not prevalent in this patient population, and would most likely occur in advanced HIV infection associated with poor food intake. Both vitamin B12 and folate deficiency may occur in the setting of alcohol abuse.
Finally, several drugs used to treat HIV (e.g., zidovudine) and the complications of HIV can cause anemia. The most common of these are listed in Table 67-2.
Table 67-2 Drugs Commonly Associated With Cytopenias in HIV Infection
| Anemia | Neutropenia | Thrombocytopenia |
|---|---|---|
Hemolysis
Hemolysis can occur in individuals with glucose 6-phosphate dehydrogenase (G6PD) deficiency who are exposed to antioxidant drugs (Table 67-1). In HIV infection, most commonly used antioxidant drugs are dapsone and primaquine, and less commonly, sulfonamides. Over 100 G6PD variants are inherited on the X-chromosome, but the most frequent are GdA- found in 10% of black men and in 1–2% of black women, and Gdmed, found predominantly in men from the Mediterranean area, India, and Southeast Asia. Rarer causes of hemoloysis include thrombotic thrombocytopenic purpura (TTP) a multisystem disease that is also associated with thrombocytopenia, renal impairment, fever and neurologic abnormalities, and disseminated intravascular coagulation (DIC).
Between 20% and 44% of asymptomatic HIV-infected patients are Coombs’ test-positive, with the numbers increasing up to 85% in those with frank AIDS,72,73 although it is usually nonspecific, and antibody-mediated hemolysis is rare in patients with HIV infection. Although these antibodies may be reactive with specific minor antigens on erythrocytes, in most cases nonspecific binding of antiphospholipid antibodies or deposition of immune complexes on erythrocytes are responsible for these positive Coombs’ tests.74
Treatment of Anemia
The hemoglobin level for which anemia should be treated may vary somewhat. The World Health Organization (WHO) recommends treatment in women with a hemoglobin <12 g/dL and in men with a hemoglobin <13 g/dL. Studies that demonstrated improvement in the quality of life in anemic HIV-infected women and men, found such improvements with treatment of hemoglobin <10 g/dL or <11 g/dL, respectively. Many HIV-treating physicians now would consider treatment of anemia to maintain the hemoglobin level above 12 g/dL for men and above 11 g/dL for women.75 The strongest association between anemia and adverse clinical outcomes appears to be for a hemoglobin level <10 g/dL, but it has not been shown whether treatment of anemia will actually alter the clinical prognosis of HIV infection. Finally, anemia may need to be treated if this allows for the use of antiretroviral drugs (e.g., zidovudine) or therapy for HIV complications that would otherwise be withheld or discontinued.
It is reasonable to assess the HIV-infected patient for possible treatment when the hemoglobin level meets the WHO thresholds. Simple assessments of functional status and other quality of life measures can be done with the LASA44 or using questions from the MOS-HIV.76 Although it may be difficult to determine when anemia is causing a decrease in quality of life in the setting of HIV infection and other complications, a decrease in functional status or other measures may clinically correlate with a decline in hemoglobin level.
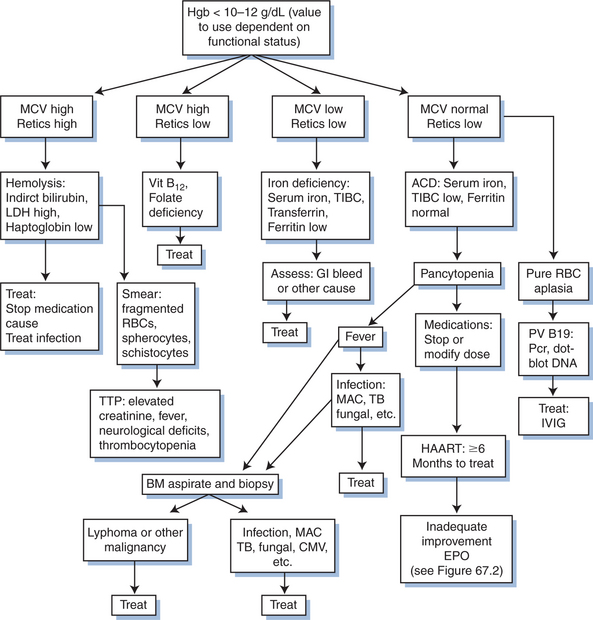
When anemia occurs, it is important to determine the reason for the decline in hemoglobin level. As noted previously, there are myriad causes of anemia and it is important that a co-morbidity that should be treated not be overlooked. The MCV and reticulocyte count can be particularly helpful in categorizing the potential etiology of the anemia, with additional laboratory testing as needed (Table 67-3).
Table 67-3 Diagnosing and Treating Causes of Cytopenia in HIV Infection
| Diagnosis | Diagnosis | Treatment |
|---|---|---|
| Anemia | ||
| Iron-deficiency anemia | Fe < 60 μg/dL, transferrin < 300 μg/dL, ferritin < 40 ng/mL, MCV < 80, reticulocytes > 2.0 | Ferrous sulfate 300 mg po tid |
| HIV-induced anemia (chronic disease) | Fe < 60 μg/dL, transferrin < 300 μg/dL, ferritin >80 ng/mL, normal BM iron, low erythropoietin level, MCV low-normal, retics < 2.0 | HAART (1–2 g/dL increase in hemoglobin over 6–12 months), epoetin-alpha |
| Vitamin B12 deficiency | MCV > 100, retics < 2.0, serum vitamin B12 (cyanocobalamin) < 125–175 pg/mL | Vitamin B12 1 mg IM daily × 7 days, then monthly |
| Folate deficiency | MCV > 100, retics < 2.0, serum folate < 2–3 ng/mL | Folate 1–5 mg po daily × 1 month |
| Parvovirus B19 | Severe pure RBC aplasia. Hemoglobin < 10, hematocrit < 24%, retics low-absent, positive IgG and IgM serology, positive PCR of dot-blot hybridization for PV B19 | HAART |
| IVIG 400 mg kg −1 day−1 × 5 days, repeat as necessary | ||
| Bone marrow infiltration by infection or tumor | MCV 80–100, retics < 2.0, possible pancytopenia, BM biopsy abnormal | Treat specific etiology |
| Drug induced | MCV > 100 (zidovudine and stavudine) | Discontinue drug, epoetin-alpha |
| G6PD deficiency and use of oxidant drugs, and other hemolysis | MCV 80–100, increased retics, indirect bilirubin > 1.2 mg/dL, LDH > 220 IU, haptoglobin <25 mg/dL, spherocytes and fragmented RBCs on smear | Discontinue drug, transfusion |
| Severe methemoglobinemia (G6PD deficiency) can be treated with IV methylene blue (1 mg/kg) | ||
| Thrombocytopenia | ||
| TTP | Hemolysis (see G6PD above), anemia, thrombocytopenia, increased creatinine, neurologic signs, fever | Plasma exchange with fresh frozen plasma (7–15 exchanges may be needed until normal platelets and LDH) |
| DIC | Hemolysis (see G6PD above), thrombocytopenia | Heparin |
| HIV-ITP | Platelets < 20 000, increased megakaryocytes in BM | Prednisone 30–60 mg daily, taper with response IVIG 1000 mg/kg × 2 days, then day 14, and every 2–4 weeks as needed |
| Anti-Rh (anti-D) 25–50 μg/dk, repeat day 3–4, then 3–4 week intervals as needed in Rh-positive, non splenectomized | ||
| Splenectomy (with prophylactic vaccination) RBC transfusion and platelet transfusion for active bleeding | ||
| Neutropenia | ||
| HIV induced, drug induced, others | ANC < 250–500 | G-CSF 5 μg/kg SQ daily, titrate as needed by adjusting dosing interval to qod, 3 × −2 × weekly, or reduce dose. Goal of ANC > 1000–2000 |
| GM-CSF 250 μg/m2 SQ daily, titrate by dosing interval or dose. Goal of ANC > 1000–2000 | ||
| Pegylated G-CSF (Pegfilgrastim) 6 mg SQ weekly or less | ||
Prior to the introduction of highly active antiretroviral therapy (HAART), there was no evidence that antiretroviral treatment of HIV infection would also improve anemia. However, several studies have now demonstrated that HAART is effective in improving hemoglobin levels within 6 months to 1 year of initiating therapy in both men and women, and in whites and blacks.35,36,77–81 These studies consistently showed improvements of 2–3 g/dL in the hemoglobin level over 1 year on HAART. However, the hemoglobin may not return completely to normal levels even when HAART results in HIV RNA suppression and there is effective immune restoration. Using zidovudine as part of an otherwise effective HAART regimen may decrease the likelihood that the hemoglobin level returns to normal.82
Administration of effective antiretroviral therapy may influence hematopoiesis by decreasing various inhibitory processes, and be critical to alleviating cytopenias in patients with HIV. In a study of the effects of antiretroviral therapy on cytokine and chemokine production by bone marrow and stromal cells in HIV-infected patients, there was decreased IL-2, elevated TNF-alpha, MIP-1 alpha, MIP-1 beta, and RANTES levels compared to uninfected controls. Antiretroviral therapy with protease inhibitors was associated with a restoration of stromal cell pattern and functions, and an increased IL-2 production and diminution of TNF-alpha levels. There was an increase in bone marrow colony growth in both HIV-infected patients and normal individuals in parallel with the normalization of functional and morphologic characteristics of stromal cells.83 In another study, increases in hemoglobin levels in the first 6 months on HAART were shown to be strongly correlated with a decrease in sTNFR II and neopterin concentrations, immune activation markers of proinflammatory cytokine activity.80
The hematopoietic growth factor, erythropoietin is a glycoprotein that regulates the production of hematopoietic cells and the process of terminal differentiation and maturation. It also suppresses apoptosis,84 and can enhance the function of terminally differentiated hematopoietic cells.85 Most HIV-infected patients with anemia have adequate erythropoietic capacity but are unable to augment it during periods of demand in large part because of relatively inadequate erythropoietin (EPO) levels.53,86 Inappropriately low endogenous levels (<500 mU/mL) of serum EPO are seen in most AIDS patients with anemia.86,87 In addition, cytokines that are produced in response to HIV infection, such as IL-1 and TNF-alpha, blunt the normal rise in hemoglobin with rising serum EPO levels. Circulating antierythropoietin antibodies have also been correlated with anemia in a subset of patients with HIV.88–89
Placebo-controlled trials have shown that small amounts of recombinant human erythropoietin (epoetin alpha) can increase hemoglobin and significantly reduce transfusion requirements of patients with HIV infection and anemia.90 In these trials, epoetin alpha was well tolerated and significantly reduced transfusion requirements, increased the hematocrit, and improved the quality of life of patients with HIV infection and anemia. Patients whose EPO levels were less than 500 mU/mL had statistically significant reductions in red blood cell transfusions and increases in hematocrit compared to placebo-treated patients after 6–8 weeks of therapy. Patients with endogenous epoetin alpha levels of more than 500 mU/mL did not experience significant increases in hematocrit or reduction in transfusion requirements when compared to placebo-treated patients.90
A randomized controlled trial of epoetin alpha (100 to 300 IU/kg subcutaneously three times per week) in anemic HIV-infected patients in the community setting also showed significant increases in hemogloblin levels that were independent of the baseline CD4+ T-lymphocyte counts. The mean quality of life score, as measured by the Functional Assessment of HIV Infection (FAHI) scale, improved significantly, with increases in hemoglobin after epoetin alpha use.91
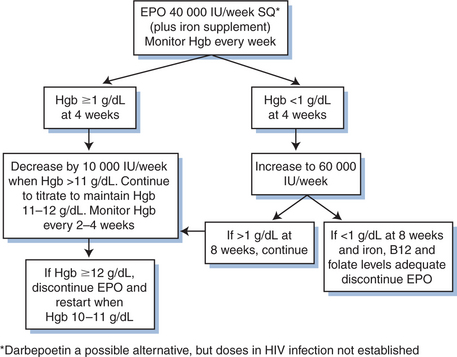
In a 16-week randomized trial of 285 HIV-infected adults with anemia (hemoglobin <12 g/dL) on stable antiretroviral therapy, once weekly epoetin alpha at 40 000 IU subcutaneously was as effective as three times weekly dosing in improving hemoglobin (2.9 g/dL for weekly vs 2.5 g/dL for thrice weekly).92 In still another randomized trial of 650 patients with a hemoglobin <11 g/dL, there was a significant increase in hemoglobin level of 2.5 g/dL after 16 weeks with the weekly use of epoetin alpha at 40 000 IU with dose escalation to 60 000 IU weekly if the hemoglobin increase was <1 g/dL after 4 weeks.49 Weekly use of epoetin alpha has also been shown to be effective for increasing the hemoglobin level in HIV-infected individuals in other studies, including in HIV/hepatitis C co-infected individuals being treated with interferon/ribavirin for the treatment of hepatitis C infection.93
The erythropoiesis stimulating protein, darbepoetin alpha, is a possible alternative to epoetin alpha for treating anemia. This drug has a longer half-life than epoetin alpha which may allow less frequent dosing. Currently, darbepoetin alpha has only been shown to effectively treat anemia associated with cancer chemotherapy and in renal failure, with limited data assessing its effectiveness in HIV infection.94,95 Currently, epoetin alpha is only approved for use in patients with anemia as a result of zidovudine therapy, and the initial recommended dose is 100 IU/kg three times weekly. Failure to respond to this treatment after 6–8 weeks would warrant increasing the dose and possibly performing a bone marrow aspiration and biopsy to exclude other possible causes of myelosuppression. A serum erythropoietin level >500 IU/mL is associated with a significantly decreased rate of response. Though not an FDA-approved dose, many HIV-treating physicians would use once-a-week epoetin alpha for convenience. Recent evidence indicates that epoetin alpha is associated with an increased risk of serious cardiovascular events and death when administered to a target hemoglobin >12 g/dL.96,97 Recommendations for the use of growth factors in HIV-infected patients are in Figure 67-2 and in Table 67-3.
Administration of a hematopoietic growth factor offers an alternative to transfusions and their potential morbidity and mortality. Transfusions may be immunosuppressive,96 and the time to progression to AIDS is shorter in those who are given transfusions.97 Use of transfusion may also be associated with a higher mortality rate in HIV infection.34,98 Data suggest an increase in HIV replication in those receiving red blood cell transfusions as well,99 and a possible increased risk in opportunistic infection.100 There is also a risk of exposure to new infectious agents with transfusions and a risk of transfusion reactions despite the immunodeficiency caused by HIV.101 If one does need to transfuse red blood cells in an HIV-infected patient to treat severe hemorrhage or to maintain a hemoglobin level that is not otherwise treatable by other means, results from the Viral Activation Transfusion Study did not show any advantage to using leukocyte-reduced red blood cells in a large prospective randomized controlled study.102
The use of iron and hematinics is recommended for patients on long-term hematopoietic growth factor therapy as functional iron deficiency can rapidly ensue unless individuals are iron-overloaded from prior transfusions. Oral iron given as ferrous sulfate at 300 mg three times daily or an equivalent oral preparation may be adequate. However, moderate to severe iron deficiency can develop and the use of parenteral iron (iron gluconate or iron sucrose) should be considered.103
In an observational study of 200 HIV-infected patients, low testosterone levels were associated with anemia, and the use of supplemental androgens was associated with a decreased odds of anemia.104 Supplemental testosterone or other androgens have not otherwise been assessed and cannot be recommended in the treatment of anemia.
Neutropenia
Infection with HIV affects lymphocytes, neutrophils, and macrophages/monocytes. The hallmark of HIV infection is progressive and profound depletion of CD4+ T-lymphocytes, presumably through direct viral invasion of these cells and enhanced cytolysis and apoptosis.51,105 Infection of macrophages and monocytes also occurs and may result in significant perturbation of the complex network of growth factor and cytokine generation seen with HIV infection.
Among patients with early symptomatic HIV infection, 10–30% are neutropenic, and this may progress to 75% of those with frank AIDS.21,22 Myelotoxic medications, including various antiretroviral drugs and drugs used to treat opportunistic infections and malignancies, are the major cause of neutropenia in patients with HIV infection. In addition, impaired myelopoiesis has been described in patients with leukopenia and HIV infection.106,107 Deficiencies in G-CSF production in response to neutropenia have been described in patients with HIV infection, although the G-CSF response to infection and febrile episodes appears to be normal.108 About one-third of patients have anti-neutrophil antibodies, but their presence does not appear to correlate with the incidence or severity of neutropenia.1,109 Accelerated neutrophil apoptosis has also been described in patients with HIV.110
Defects in qualitative functions of neutrophils, such as chemotaxis, deficient degranulation responses, inhibition of leukocyte migration, ineffective killing of pathogens, and deficient superoxide generation, have been described in neutrophils from patients with HIV infection.111,112 In vitro, decreased opsonization and intracellular killing of bacterial and fungal organisms by granulocytes have been seen.113 The clinical importance of these functional neutrophil defects in terms of host defense among HIV-infected individuals is unclear, although an increasing incidence of bacterial infections such as sinusitis and pneumonia does occur in patients with advanced HIV disease. Finally, several medications used to treat HIV and its complications are also associated with the development of neutropenia (Table 67-2).
Neutropenia and Infection
Consistent evidence supports an increased risk of bacterial infection in patients receiving chemotherapy for the treatment of malignancy when the absolute neutrophil count (ANC) falls below 1000 cells/dL. Several studies have also assessed the risk of infection associated with neutropenia in HIV-infected persons, although at a similar degree of neutropenia, the risk appears to be lower than in cancer patients.109–114 Another study showed that the risk of bacterial infection increased over twofold for an ANC <1000 cells/dL and over sevenfold for an ANC <500 cells/dL. However, the absolute risk of bacterial infection remained low at 3–5 infections per 100 person-months even for an ANC <500 cells/dL.115 In other studies, a nadir ANC of <500 cells/dL116 and an ANC <250 cells/dL117 were associated with an increased relative risk of bacterial infection.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree